-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Evaluation of water absorption rate of tablets by using an Enslin-Neff device
Authors: Jan Stoniš; Zdenka Šklubalová; Pavel Ondrejček; Petra Svačinová; Hana Hurychová
Authors place of work: Department of Pharmaceutical Technology, Faculty of Pharmacy Hradec Králové, Charles University in Prague, Hradec Králové, Czech Republic
Published in the journal: Čes. slov. Farm., 2015; 64, 197-198
Category: Pracovní den sekce technologie léků
Introduction
Tablets are the most popular dosage form. To ensure their quality, safety and efficiency, tablets are tested using wide spectra of methods. Most of these methods are included in the Pharmacopoeia; however, there are other useful methods that are not included.
In this research paper, the focus was placed on water absorption rate. The method proposed by Bi et al.2) is commonly used. Here, an Enslin-Neff device, generally used in testing of clays and soils in geology3), is used to check water absorption rate of tablets. Tablets were prepared by direct compression of the granules performed by fluid-bed granulation process from corn starch and lactose.
Experimental methods
Granulation
For dying and granulation, a fluid-bed granulator (a Fluid bed dryer and a granulator UNI-GLATT, Glatt GmbH, Binzen, Germany) was used. At first, corn starch was dyed in fluid-bed using a Wurster adaptor. Consequently, dyed corn starch was mixed with lactose in a ratio of 1 : 1 and the mixture was granulated in the fluid-bed granulator using a 10% aqueous solution of Kollidon 25 as the binder.
Tablet production
Tableting mixture was prepared from 250 g of granules and 1.25 g of magnesium stearate (used as the glidant) and pressed into tablets with a diameter of 7 mm (ZWICK ROELL Press, Zwick Roell GmbH, Germany). The compression force was set at 4 kN. Three batches of tablets were prepared using the same approach – CORNLAC 1, CORNLAC 2, CORNLAC 3.
Quality control of granules and tablets
Granules were evaluated for the angle of repose (a Granulate Tester ERWEKA GTB, Erweka GmbH, Germany), Hausner ratio and compressibility index (a Tapped Density Tester ERWEKA SVM 102, Erweka GmbH, Germany), the particle size distribution (a Vibratory Sieve Shaker RETSCH AS200 basic, Retsch GmbH, Germany) and the water content by loss on drying (a Moisture Analyzer PRECISA XM 60, precision 0.001 g, Precisa, Switzerland). The mixture of granules with magnesium stearate was tested for the angle of repose, Hausner ratio and compressibility index again. The mass (an Analytical Balance, precision 0.1 mg, HELAGO SR 120, Helago s.r.o., Czech Republic), the diameter and the height of tablets were estimated as well as the strength (a Tablet Tester SCHLEUNIGER 8M, Pharmatron AG, Switzerland) and the disintegration time (a Disintegration Time Tester ERWEKA ZT 301, Erweka GmbH, Germany).
Water absorption test with an Enslin-Neff device
Water absorption rate was measured with an Enslin-Neff device. The device was filled with a coloured aqueous medium the level of which was kept carefully at the surface of a glass filter. Then, the tablet was laid down and the time to achieve the complete uptake of the liquid was registered (water absorption time in seconds, WAT). The volume of water was measured in the equipment capillary and the tablet was weighted again to express the mass difference. In Fig. 1, the experimental design using the Enslin-Neff device can be seen.
Fig. 1. Water absorption rate test of tablet 
Results and Discussion
Water absorption rate (ml/s) was calculated using the volume of absorbed water (ml) and WAT (s). The absorbed volume correlates well with the mass difference between a dry and wet tablet. The results of tablet properties evaluation (the average values of 10 values, and the standard deviations) are presented in Table 1.
Tab. 1. Properties of tablets of batches of CORNLAC 1, CORNLAC 2, CORNLAC 3 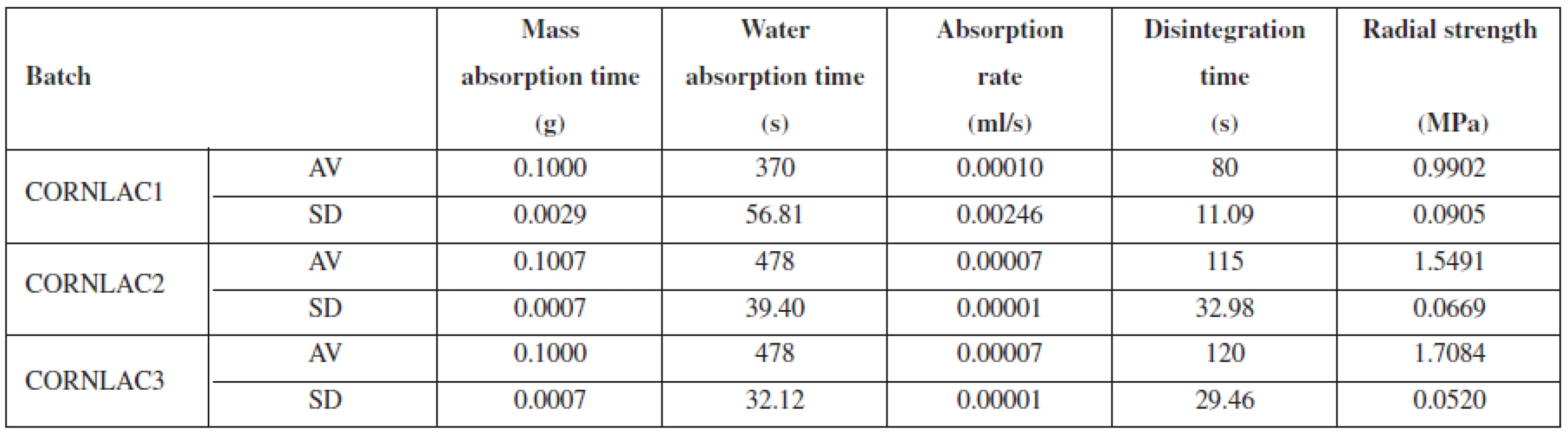
AV – average, SD – standard deviation Out of three compared batches, CORNLAC 1 absorbed the highest amount of water during the experiment with the shortest water absorption time. As can be seen from Table 1, it has also the lowest radial strength and the shortest disintegration time. On the other hand, CORNLAC 2 and CORNLAC 3 possess a much higher radial strength, a longer disintegration time, and a longer water absorption time resulting in a lower absorption rate than that of CORNLAC 1.
Conclusions
An Enslin-Neff device can be used for measuring the water uptake rate of the tablets compressed from granules prepared in a fluid-bed granulator. From the volume difference of the absorbed liquid and the wetting time, the water absorption rate for the tablet can be calculated. Preliminary results show that the radial strength and the water absorption time correlate with each other. This relationship would be further studied.
Research work and poster publication was financially supported by the specific scientific research programme SVV 260 183.
Conflicts of interest: none.
Mgr. Jan Stoniš
Univerzita Karlova v Praze, Farmaceutická fakulta v Hradci Králové
Katedra farmaceutické technologie
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: stonisj@faf.cuni.cz
Zdroje
1. The Council of Europe. European Pharmacopoeia. 8th ed. Strasbourg: 2013.
2. Bi X. Y., Sunada H., Yonezawa Y., Danjo K. Evaluation of rapidly disintegrating tablets prepared by direct compression method. Drug Dev. Ind. Pharm. 1999; 25(5), 571–581.
3. Kaufhold S., Dohrmann R. Comparison of the traditional Enslin-Neff method and modified Dieng method for measuring water-uptake capacity. Clays Clay Min. 2008; 56(6), 686–692.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 5- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Metody používané ve farmaceutické technologii ke zvyšování biologické dostupnosti špatně rozpustných léčiv po perorálním podání
- Výdaje na léky a péči pro stárnoucí populaci
- Úroveň a faktory ovplyvňujúce pacientsku spokojnosť s lekárenskou starostlivosťou na Slovensku
-
Pracovní den sekce technologie léků České farmaceutické společnosti ČLS JEP
Pokroky v lékových formách - Drug bioavailability increasing by formulation of liquisolid systems
- Hodnocení sypných a konsolidačních vlastností prášků ve farmaceutické technologii
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Formulační aspekty orodispergovatelných tablet
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Prof. RNDr. Jaroslav Květina, DrSc. dr.h.c. FCMA – 85letý
- Farmakochemie
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Metody používané ve farmaceutické technologii ke zvyšování biologické dostupnosti špatně rozpustných léčiv po perorálním podání
- Formulační aspekty orodispergovatelných tablet
- Prof. RNDr. Jaroslav Květina, DrSc. dr.h.c. FCMA – 85letý
- Hodnocení sypných a konsolidačních vlastností prášků ve farmaceutické technologii
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



