-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
Authors: Maciej Kapkowski; Monika Słota; Jarosław Polański
Authors place of work: University of Silesia, Institute of Chemistry, Katowice, Poland
Published in the journal: Čes. slov. Farm., 2015; 64, 209-211
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
We tested for the first time the efficiency of SiO2-, Cu-, and Ni-supported Au in deep glycerol oxidation in a diluted and viscous H2O2/H2O liquid phase. Acetic acid (AA), the C2 oxidate, was preferentially formed in such a system. High conversion (100%) and AA yields (90%) were observed for the sol-gel SiO2-suppported Au in diluted solutions. Although with the increase of glycerol concentration in the viscous liquid phase these values decreased to ca. 40% (conversion) and 20% (AA yield), the addition of acetonitrile improved the AA yield to ca. 40%, while the surfactants were found to be capable of a many-fold enhancement of the catalyst activity at the room temperature highly-viscous liquid phase. High performances were also observed for the bimetallic Au/Cu and Au/Ni catalysts obtained by nano-Au transfer; however, these catalysts were destroyed during the reaction by the Cu or Ni leaching effect.
Experimental methods
Nano-Au catalyst (20 mg, 0.1–10.0 μμmol Au) was suspended in a mixture of 1.0 mL of 30% hydrogen peroxide (10 mmol H2O2) and 0.5 mL (0.5–13.6 mol/L) glycerol (Fisher BioReagents® – Glycerol For Molecular Biology) by sonication at room temperature for 10 min (RK 52 H, Bandolin Electronics, 35 kHz). Reagents were stirred at 770 rpm in a sealed tube (septa system) placed in a thermostated oil bath at 80 °C for 24 h. The resulted reaction mixture was centrifuged and decantated. The supernatant was dissolved into deuterated water and analyzed using 1H and 13C NMR. For quantitative determination of the reaction products we used an external standard procedure with a (NMR Coaxial Small Volume NMR Insert tubes from ARMAR Chemicals) hydroquinone as the reference substance. Additionally, the 2D COSY and HMQC methods were used to identify and quantify products. The spectra were recorded on Bruker Avance 400 or 500 spectrometers with TMS as the internal standard (400 MHz, 1H, 101 MHz 13C or 500 MHz, 1H, 126 MHz 13C) at room temperature. The signal from water was suppressed using 90 water-selective pulses (zggpwg). Optionally this oxidation procedure was modified by the addition of 1.0 mL acetonitrile (19.10 mmol) or surfactants: Sulforkanol (sodium laureth sulfate – SLES), Triton X-100, PEG 400, ca. (0.05 wt. %).
The results had an error of ± 2% throughout the experiments. The formation of acetic acid was confirmed using spectroscopic techniques. Eqs. [1] to [3] were used to calculate conversion, product selectivity and yield, respectively.
conversion (mol%) = (initial moles of glycerol – final moles of glycerol)/initial moles of glycerol ⋅ 100 [1]
selectivity of products (mol%) = percentage amount of product formed/the total percentage of all product formed ⋅ 100 [2]
yield (%) = conversion of glycerol × selectivity of desired product/100 [3]
Results and discussion
We assumed that treatment of the concentrated glycerol solutions under mild conditions would potentially be a major advantage for processing waste glycerol. Intuitively, the glycerol concentration that influences the viscosity of a system should strongly control the glycerol contact with the catalyst surface. In practice, the catalytic reactions of glycerol in water solutions have been performed previously in a relatively diluted solutions, e.g., in the liquid phase: 0.6 mol/L (mol of glycerol per litre of the reaction mixture, if recalculated using data from the literature; glycerol to H2O2 molar ratio amounted to 1 : 4)1) or in the vapor phase: Ar/glycerol/H2O = 5 : 1 : 212) or N2/H2O/Gly = 46/48/63).
In Table 1, we specified the performance of catalytic Au/SiO2 systems in the oxidation of glycerol in the diluted liquid phase; 0.2 mol/L glycerol, whereas glycerol to H2O2 molar ratio amounted to 1 : 37. For the new catalysts, i.e., SiO2-supported Au, both the conversion and selectivity of the process reached as high as 100% and 90 to 99% (Table 1, entries 1 and 3), respectively, while in these conditions, the Au/C system (Table 1, entry 7) provided much lower conversion (ca. 35%). AA (Au/SiO2) or glycolic acid (GA) and aldehyde (Au/C) were the main products observed for the reactions, respectively. The performance of the Au/C system, typically used for catalytic glycerol oxidation4), compares well to the published data for the 1% Au/C (0.6 mol/L glycerol to H2O2 molar ratio 1 : 4), which provides only slightly higher conversions of ca. 40%1).
Tab. 1. Catalytic performance of SiO2 and C supported Au NPs in diluted glycerol solutions at 80 °Ca 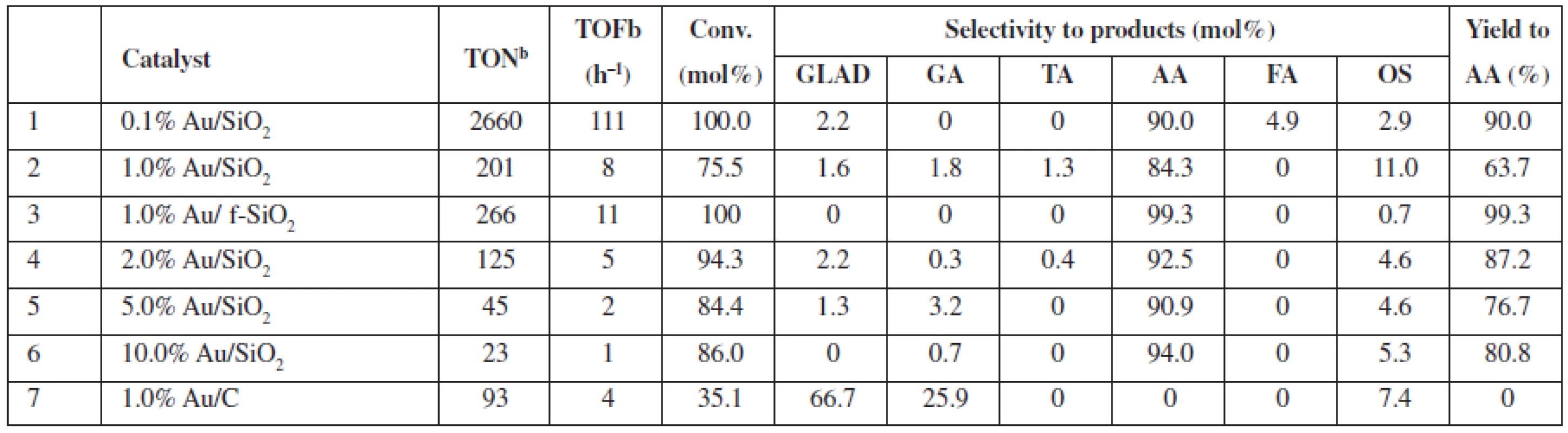
a0.2 mol/L of glycerol in the reaction mixture (glycerol/H2O2 molar ratio 1 : 37), 20 mg of catalyst (0.1–10.0 μmol Au), 80 °C, 24 h, 770 rmp bturnover number (TON) or turnover frequency (TOF) based on the total gold content in the material GLAD – glycolaldehyde, GA – glycolic acid, TA – taratronic acid, AA – acetic acid, FA – formic acid, OS – others In non-diluted glycerol solutions, the selectivity to AA can be increased (up to 99.5% selectivity at 40% conversion, 1.0% Au/SiO2) under the addition of acetonitrile (Table 2). It is worth mentioning that the addition of acetonitrile allowed us to decrease the reaction temperature to 60 °C vs. 80 °C in acetonitrile-free conditions; however, a nominal glycerol concentration after the addition of acetonitrile was the lower 2.7 mol/L vs. ca. 4.5 mol/L in the acetonitrile-free conditions. Moreover, the addition of acetonitrile (Table 2, entries 2 (+) and 4 (–)) also increases the selectivity of the AA formation for the f-SiO2-supported Au system (AA selectivity ca. 88% and AA yield ca. 21% with acetonitrile, vs. AA selectivity 0.7% and AA yield 0.3% without acetonitrile). Thus, we observed a decrease in conversion but an increase in AA selectivity for the reaction in the acetonitrile/water solution. We proved that no changes occurred for the blind experiment with the catalysts heated in acetonitrile without glycerol (results not shown).
Tab. 2. Catalytic performance of SiO2 supported Au NPs in undiluted glycerol solutions at 60 °C with (+) and without (–) acetonitrile 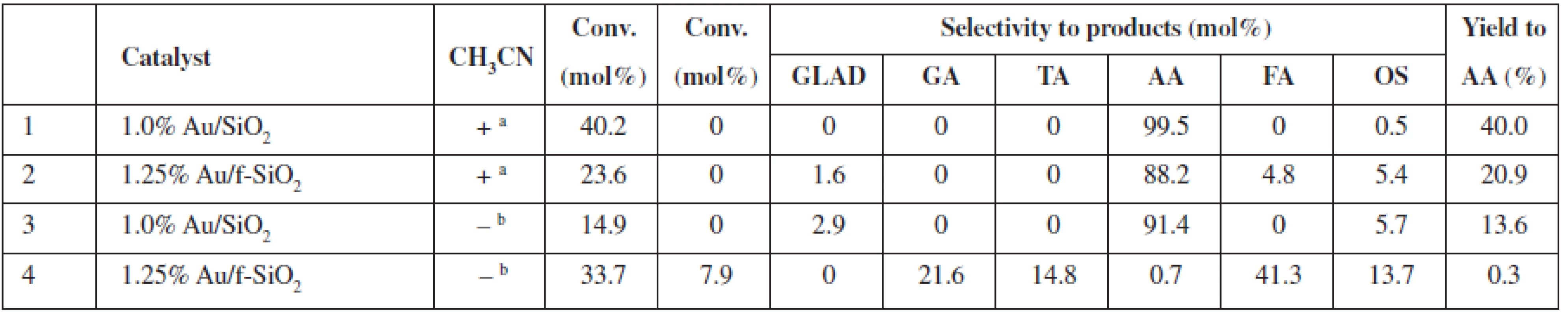
a2,7 mol/L of glycerol in the reaction mixture (glycerol/H2O2/acetonitrile molar ratio 1 : 1.5 : 2.8), 20 mg catalyst (1.0–1.25 µmol Au), 60 °C, 24 h, 770 rmp bglycerol: 4,5 mol/L (glycerol/ H2O2 molar ratio 1 : 1.5) GLA – glyceric acid, GA – glycolic acid, GLAD – glycolaldehyde, HPA – hydroxypyruvinic acid, AA – acetic acid, FA – formic acid, OS – others Thus far, the results of the exhaustive oxidation of glycerol indicated that catalyst availability is of major importance for the observed performance of the system. Below, we report the results of the experiments in which we tested possible applications of the surfactants to improve this feature (Table 3). We observed that a conversion and the yield of AA can be almost doubled at room temperature when Sulforoktanol, PEG 400, or Triton X-100 was used as surfactants.
Tab. 3. Catalytic performance of 0.1% Au/SiO2 at room temperaturea, if enhanced by surfactant addition 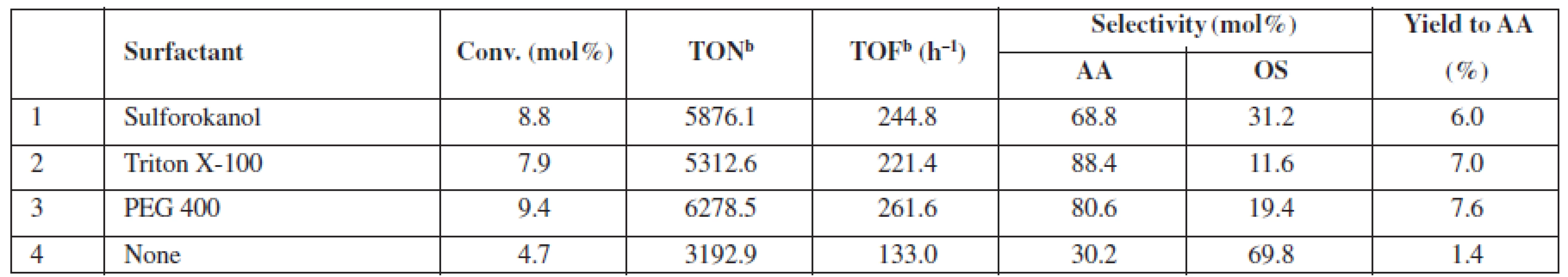
a4.5 mol/L of glycerol in the reaction mixture (glycerol/ H2O2 molar ratio 1 : 1.5) surfactant (0.05 wt.%), 25 °C, 24 h, 770 rpm bturnover number (TON) or turnover frequency (TOF) based on the total gold content in the material AA – acetic acid, OS – others Conclusions
We observed that, within the complex network of possible reactions, a process of deep glycerol oxidation proceeded on Au/SiO2 catalysts preferentially to acetic acid. The selectivity of glycerol oxidation to C3 products was investigated thoroughly. It is not a coincidence, because only C3 oxygenates can form the single carbonous products of glycerol processing, while any oxidation to C2 must also yield the C1 oxygenate (C3 → C2 + C1). This means that, although the process can be fully selective, we obtain at least two products.
Thus, the reactivity to C3 products has been well described, in contrast to the C1 and C2 products. Herein, we described the systems providing high selectivity to acetic acid, the C2 glycerol oxygenate. High conversions (100%) and acetic acid yields (90–99%) were observed for the best catalysts in the diluted aqueous glycerol solutions. Although in a relatively viscous liquid phase these values decreased to ca. 40% and 20%, the addition of acetonitrile could improve the acetic acid yield to ca. 40%, while surfactants were found to be capable of a many-fold enhancement of the catalyst activity. However, this was relatively low at the room temperature highly-viscous liquid phase.
In summary, SiO2-supported Au NPs can form an interesting catalytic system for deep selective glycerol oxidation to acetic acid in undiluted viscous liquid solutions. This seems especially interesting for the processing of glycerol wastes.
The research was co-financed by the National Research and Development Center (NCBiR) under Grant ORGANOMET No: PBS2/A5/40/2014. Author Maciej Kapkowski expresses his appreciation for the support of the DoktoRIS – Scholarship program for innovative Silesia, which is co-financed by the European Union within the framework of the ESF.
Conflicts of interest: none.
*This is a short conference communication version of a publication which appeared in a full form in Kapkowski M, et al, J. Catalysis 2014; 319, 110–118.
prof. Jarosław Polański
University of Silesia, Institute of Chemistry
Szkolna 9 Street, 40-007 Katowice, Poland
e-mail: polanski@us.edu.pl
Zdroje
1. Sankar M., Dimitratos N., Knight D. W., Carley A. F., Tiruvalam R., Kiely C. J., Thomas D., Hutchings G. J. Oxidation of glycerol to glycolate by using supported gold and palladium nanoparticles. ChemSusChem. 2009; 2, 1145–1151.
2. Massa M., Andersson A., Finocchio E., Busca G. Gas-phase dehydration of glycerol to acrolein over Al2O3–, SiO2–, and TiO2-supported Nb - and W-oxide catalysts. J. Catal. 2013; 307, 170–184.
3. Deleplanque J., Dubois J. L., Devaux J. F., Ueda W. Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts. Catalysis Today 2010; 157, 351–358.
4. Besson M., Gallezot P., Pinel C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014; 114, 1827–1870.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 5- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Metody používané ve farmaceutické technologii ke zvyšování biologické dostupnosti špatně rozpustných léčiv po perorálním podání
- Výdaje na léky a péči pro stárnoucí populaci
- Úroveň a faktory ovplyvňujúce pacientsku spokojnosť s lekárenskou starostlivosťou na Slovensku
-
Pracovní den sekce technologie léků České farmaceutické společnosti ČLS JEP
Pokroky v lékových formách - Drug bioavailability increasing by formulation of liquisolid systems
- Hodnocení sypných a konsolidačních vlastností prášků ve farmaceutické technologii
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Formulační aspekty orodispergovatelných tablet
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Prof. RNDr. Jaroslav Květina, DrSc. dr.h.c. FCMA – 85letý
- Farmakochemie
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Metody používané ve farmaceutické technologii ke zvyšování biologické dostupnosti špatně rozpustných léčiv po perorálním podání
- Formulační aspekty orodispergovatelných tablet
- Prof. RNDr. Jaroslav Květina, DrSc. dr.h.c. FCMA – 85letý
- Hodnocení sypných a konsolidačních vlastností prášků ve farmaceutické technologii
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



