-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Study of local anaesthetics: Part 201*
Determination of the critical micellar concentration of pentacaine hydrochloride from the measurements of UV absorption of pyrene in methanol solutions
Štúdium lokálnych anestetík: Časť 201*
Stanovenie kritickej micelovej koncentrácie pentakaíniumchloridu pomocou merania UV absorpcie pyrénu v metanolovom prostredíProces tvorby miciel lokálneho anestetika pentakaínium-chloridu (K 1902) v dvoch rôznych koncentráciách metanolu sa študoval pomocou merania absorbancie pyrénu v roztoku surfaktantu v UV-VIS oblasti spektra. Závislosť absorbancie všetkých hlavných píkov pyrénu od koncentrácie surfaktantu má charakteristický sigmoidálny priebeh. Hodnoty cmc študovaných systémov sa získali fitovaním Sigmoidálnou-Boltzmannovou funkciou. Študoval sa vplyv metanolu na hodnoty kritickej micelovej koncentrácie. Zistilo sa, že kritická micelová koncentrácia stúpa so vzrastajúcou koncentráciou alkoholu v roztoku.
Kľúčové slová:
lokálne anestetikum • pentakaínium-chlorid • kritická micelová koncentrácia • pyrénová absorpcia • Sigmoidálna-Boltzmannova rovnica • metanol
Authors: Jana Gališinová • Fils Andriamainty • Ivan Malík • Jozef Čižmárik • Ľubica Sichrovská
Authors place of work: Comenius University in Bratislava, Faculty of Pharmacy Department of Pharmaceutical Chemistry, Bratislava
Published in the journal: Čes. slov. Farm., 2013; 62, 132-135
Category: Původní práce
Summary
The formation of micelles of the local anaesthetic pentacaine hydrochloride (K 1902) in methanol solutions at two concentrations was investigated by measuring the absorbance of pyrene in surfactant solution. The absorbance vs. surfactant concentration profiles for all the major UV spectral peaks of pyrene have been found to be sigmoidal in nature which were analyzed according to Sigmoidal-Boltzmann equation to evaluate the cmcs values of the studied systems. The influence of the methanol concentration on the critical micellar concentration was studied. The observed critical micellar concentration rises with an increasing of alcohol.
Keywords:
local anaesthetic • pentacaine hydrochloride • critical micellar concentration • pyrene absorption • Sigmoidal-Boltzmann equation • methanolIntroduction
Surfactants are molecules made up of two distinct particles, one with a strong affinity for oils and the other for water. When dissolved in water, they lower the surface tension of the water and increase the solubility of organic compounds. It is known that surfactants can assemble in solution and critical micellar concentration (cmc) is an important solution property of surfactants1).
The process of self-association of surfactants into micelles, vesicles and membranes play a very important role in many areas, ranging from biological systems to technical applications2).
Several techniques are used, such as NMR, surface tension, conductometry, light scattering and calorimetry, to determine cmc3-8) and bulk thermodynamic properties such as the aggregation number9-11). In addition, spectral methods like UV-VIS and fluorescence using other compounds as probes are also used for the evaluation of cmc1, 12).
The effect of the presence of additives on the critical micelle concentration of surfactants has been widely studied13-15). Increasing attention is being devoted to the study of the incorporation or solubilization of neutral molecules into micelles in aqueous solution. Some of the most widely studied solubilizates are alcohols because of the important role they have in the preparation of microemulsions16-19). It is generally accepted that the alcohol binds to the micelle in the surface region leading to three principal effects19):1. The alcohol molecules intercalate between the surface ionic head groups to decrease the micelle surface area per head groups and increase of ionization. 2. The dielectric constant at the micellar interface decrease probably due to the replacement of water molecules in the interface region by alcohol molecules. 3. The molecular order of the interface region of the micelle changes.
In this article, we have found that the UV-VIS spectroscopy technique (pyrene absorption spectra) is a suitable method for the determination of the cmc of the methanol solutions containing surfactant pentacaine hydrochloride.
Experimental part
Materials and Methods
The surfactant of the cationic local anaesthetic pentacaine hydrochloride (K 1902) was synthesized according to Beneš et al.20). Methanol and ethanol used in the present study were obtained from Merck. Pyrene was purchased from Sigma-Aldrich, Switzerland. Laboratory temperature throughout the experiment was 25 °C. The critical micellar concentrations were determined by a method of Basu Ray et al.1).
Preparation of pyrene solution
The pyrene stock solution with a concentration of 0.0012 mol/l was prepared by adding a known weight of the compound in 20 wt% ethanol in water. The mixture was sonicated in order to yield a clear solution. The experimental 2 ∝mol/l solution of pyrene was prepared from it by dilution wherein the ethanol concentration was 0.25%. Such a small concentration of the ethanol was considered unable to affect the spectral and self-aggregation behavior of amphiphiles.
Absorbance study
Absorbance measurements were taken in a UV-VIS spectrophotometer Spekol 1300 Analytic Jena AG (Germany) using 10 mm path length quartz cuvette. The spectra were recorded in the 200–400 nm wavelength range.
Results and discussion
The absorption spectrum of pyrene (2 μmol/l) in water-methanol solution is illustrated in Figure 1. The absorption spectra have evidenced eight21) peaks strong (s) and weak (w) at 232w, 242w, 252w, 260w, 272s, 308w, 320s and 336s nm as depicted in Figure 1. The concentration of used pyrene was 2 μmol/l, which was within its solubility limit of 2–3 μmol/l22). At this concentration excimer formation was expected to be absent22).
Fig. 1. The absorption spectrum of pyrene (2 μmol/l) in water-methanol solution 
In water-methanol solution, the pyrene absorption peaks 232, 242, 252, 260, 272 and 308 nm of pyrene were not observed. Probably, this was due to strong absorption of K 1902 in the near UV region, which masked the peaks of pyrene in question. Figs. 2 and 3 show the pyrene plots of the sum of absorbances of two peaks (320 and 336 nm) AT versus the concentration of surfactant K 1902 in solvent systems. The shapes were all of sigmoidal character. Fitting them to the Sigmoidal-Boltzmann equation (SBE) was herein used for cmc calculation.
Fig. 2. A<sub>T</sub> vs. concentration of (K 1902) in 0.2 mol/l methanol solution profile for pyrene (0.2 μmol/l) at t = 25 ºC. Inset: the pyrene plots of absorbances of two peaks (320 and 336 nm) A versus concentration of surfactant K 1902 in solvent systems 
Fig. 3. A<sub>T</sub> vs. concentration of (K 1902) in 0.3 mol/l methanol solution profile for pyrene(0.2 μmol/l) at t = 25 ºC. Inset: the pyrene plots of absorbances of two peaks (320 and 336 nm) A versus concentration of surfactant K 1902 in solvent systems 
Hence, where x is the total concentration of surfactant, ai and af are the initial and final asymptotes of the sigmoid, respectively, x0 is the center of the sigmoid, and Δx is the interval of the independent variable x. They have designed that the sigmoidal plot can create two cmcs, one at x0 and the second at x0 + 2 Δx. Farther, the ratio x0 /Δx can be a guide to decide upon for the choice of the right cmc between the two. The surfactant systems that provide Δx0 /Δx < 10 produce cmc1 = x0 those which yield Δx0 /Δx > 10 by the SBE process produce cmc2 = (Δx0 + 2Δx). The cmc1 and cmc2 values thus determined for two concentrations of methanol (0.2 and 0.3 mol/l) and the most significant fit parameters for surfactant systems studied, including the number of points used in the fit (n), regression-square (r2) and the chi-square (χ2) are presented in the Table 1. The absorption data treated by the above equation and analyzed in compliance with the suggestion of Aguiar et al.12) have produced x0 /Δx values much lower than 10, so that the cmc values were taken equal to x0 as discussed above. The concentrations of methanol solutions (0.2 and 0.3 mol/l) were chosen because substance K 1902 is practically insoluble in water, respectively in 0.1 mol/l methanol. Experimental results show (Table 1) that, with increasing concentration of methanol solution from 0.2 to 0.3 mol/l, we observed an increase in the value of cmc. We assume that with increasing alcohol concentration higher than 0.3 mol/l, we would find increased cmc values as found by other authors13, 15, 23).
Tab. 1. Fitting parameters of the absorption spectra of pyrene to SBE for the investigated surfactant (K 1902) 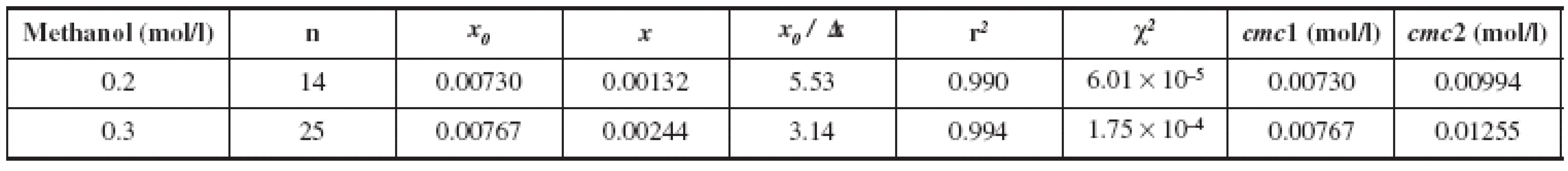
Conclusion
- UV absorption of pyrene in surfactant solution can be an easy method to establish their cmc as described in the work1);
- cmc value increases with increasing concentration of methanol.
Conflicts of interest: none.
Received 31 Januar 2013 / Accepted 19 March 2013
PharmDr. Jana Gališinová (∗) • F. Andriamainty • I. Malík • J. Čižmárik • Ľ. Sichrovská
Comenius University in Bratislava, Faculty of Pharmacy
Department of Pharmaceutical Chemistry
Odbojárov 10, 832 32 Bratislava, Slovak Republic
e-mail: galisinova@fpharm.uniba.sk
Zdroje
1. Basu Ray G., Chakraborty I., Moulik, S. P. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Coll. Interf. Sci. 2006; 294, 248–254.
2. Tanford C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes. Second ed. New York: Wiley-Interscience Publication 1980.
3. Zakharova L. Y., Gaysin N. K., Gnezdilov O. I., Bashirov F. I., Kashapov R. R., Zhiltsova E. P., Pashirova T. N., Lukashenko S. S. Micellization of alkylated 1.4-diazabicyclo[2.2.2]octane by nuclear magnetic resonance technique using pulsed gradient of static magnetic field. J. Mol. Liq. 2012; 167, 89–93.
4. Nyuta K., Yoshimura T., Esumi K. Surface tension and micellization properties of heterogemini surfactants containing quaternary ammonium salt and sulfobetaine moiety. J. Coll. Interf. Sci. 2006; 301, 267–273.
5. Sajid Ali M., Abdul Rub M., Khan F., Al-Lohedan H. A., Kabir-ud-Din. Interaction of amphiphilic drug amitriptyline hydrochloride with β-cyclodextrin as studied by conductometry, surface tensiometry and viscometry. J. Mol. Liq. 2012; 167, 115–118.
6. Bai G., Lopes A., Bastos M. Thermodynamics of micellization of alkylimidazolium surfactants in aqueous solution. J. Chem. Therm. 2008; 40, 1509–1516.
7. Moulik S. P., Mitra D. Amphiphile self-aggregation: An attempt to reconcile the agreement–disagreement between the enthalpies of micellization determined by the van’t Hoff and Calorimetry methods. J. Coll. Interf. Sci. 2009; 337, 569–578.
8. Šarac B., Bešter-Rogač M. Temperature and salt-induced micellization of dodecyltrimethylammonium chloride in aqueous solution: A thermodynamic study. J. Coll. Interf. Sci. 2009; 338, 216–221.
9. Das D., Ismail K. Aggregation and adsorption properties of sodium dodecyl sulfate in water–acetamide mixtures. J. Coll. Interf. Sci. 2008; 327, 198–203.
10. Javadian S., Gharibi H., Sohrabi B., Bijanzadeh H., Safarpour M. A., Behjatmanesh - Ardakani R. Determination of the physico-chemical parameters and aggregation number of surfactant in micelles in binary alcohol–water mixtures. J. Mol. Liq. 2008; 137, 74–79.
11. Dong B., Zhao X., Zheng L., Zhang J., Li N., Inoue T. Aggregation behavior of long-chain imidazolium ionic liquids in aqueous solution: Micellization and characterization of micelle microenvironment. Colloids and Surfaces A: Physicochem. Eng. Aspects. 2008; 317, 666–672.
12. Aguiar J., Carpena P., Molina-Bolívar J. A., Carnero Ruiz C. J. On the determination of the critical micelle concentration by the pyrene 1 : 3 ratio method. J. Coll. Interf. Sci. 2003; 258, 116–122.
13. Akhter M. S., Alawi M. S. The effect of organic additives on critical micelle concentration of non-aqueous micellar solutions. Colloids and Surfaces A: Physicochem. Eng. Aspects. 2000; 175, 311–320.
14. Kroflič A., Šarac B., Bešter-Rogač M. Influence of the alkyl chain length, temperature, and added salt on the thermodynamics of micellization: Alkyltrimethylammonium chlorides in NaCl aqueous solutions. J. Chem. Therm. 2011; 43, 1557–1563.
15. Akhter M. S. Effect of solubilization of alcohols on critical micelle concentration of non-aqueous micellar solutions. Colloids and Surfaces A: Physicochem. Eng. Aspects. 1999; 157, 203–210.
16. Høiland H., Ljosland E., Backlund S. Solubilization of alcohols and alkanes in aqueous solution of sodium dodecyl sulfate. J. Coll. Interf. Sci. 1984; 101, 467–471.
17. Zana R. Effect of medium chain-length alcohols on the micelles of tetradecyltrimethylammonium bromide. J. Coll. Interf. Sci. 1984; 101, 587–590.
18. Roux-Desgranges G., Grolier J. P. E., Villamañan M. A., Casanova C. Role of alcohol in microemulsions. III. Volumes and heat capacities in the continuous phase water-n-butanol-toluene of reverse micelles. Fluid Phase Eq. 1986; 25, 209–230.
19. Førland G. M., Sameth J., Høiland H., Mortensen K. The Effect of Medium Chain Length Alcohols on the Micellar Properties of Sodium Dodecyl Sulfate in Sodium Chloride Solutions. J. Coll. Interf. Sci. 1994; 164, 163–167.
20. Beneš L., Borovanský A., Kopáčová L. Alkoxycarbanilic Acid Esters with High Local Anaesthetic Activity. Arzneim. Forsch. 1969; 19, 1902–1903.
21. Khan Z. H., Khanna B. N. Electronic absorption spectra of pyrene and its monopositive ion. J. Chem. Phys. 1973; 59, 3015.
22. Kalyansundaram K., Thomas J. K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977; 99, 2039–2044.
23. Čižmárik J., Andriamainty F., Malík I., Sedlárová E. Study of local anesthetics. Part 176. Study of the micellization and thermodynamic parameters of heptacainium chloride in aqueous and alcoholic solutions. Farm. Obzor 2007; 56, 133–137.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2013 Číslo 3- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- A study of a new co-processed dry binder based on spray-dried lactose and microcrystalline cellulose
-
Study of local anaesthetics: Part 201*
Determination of the critical micellar concentration of pentacaine hydrochloride from the measurements of UV absorption of pyrene in methanol solutions - HPLC determination of saccharides after pre-column derivatization in honey samples
- Use of human medicinal preparations in veterinary medicine
- Analysis of the consumption of sedative and hypnotic drugs in Yemen and the Czech Republic
- Prof. RNDr. Milan Melník, DrSc. – 75-ročný
- Štátne vyznamenanie – Dr.h.c., prof. RNDr. Jozefovi Čižmárikovi, PhD.
- Nové knihy
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Use of human medicinal preparations in veterinary medicine
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- HPLC determination of saccharides after pre-column derivatization in honey samples
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




