-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
TREATMENT OF PARTIAL-THICKNESS SCALDS BY SKIN XENOGRAFTS – A RETROSPECTIVE STUDY OF 109 CASES IN A THREE-YEAR PERIOD
Autoři: P. Bukovčan; J. Koller
Působiště autorů: Department of Burns and Reconstructive Surgery, University Hospital Bratislava, Ružinov Hospital Bratislava, Slovak Republic
Vyšlo v časopise: ACTA CHIRURGIAE PLASTICAE, 52, 1, 2010, pp. 7-12
INTRODUCTION
Burns primarily damage the skin and nearby structures. However, on a secondary level they can endanger all the systems in the human body. From this point of view, the management of burns represents a sector of medicine where a multidisciplinary approach is essential. Nevertheless, the basic and inevitable prerequisite of successful treatment of burns represents the treatment of the burn wound, where skin substitutes are still paramount.
According to epidemiological studies (9, 14), in the Slovak Republic scald injuries are the most frequent, mostly afflicting patients in lower age groups. Partial-thickness scald burns in particular constitute one of the most frequent indications at our workplace for the utilisation of a biological skin substitute: porcine skin xenograft. Considering the high incidence of this type of injury – amounting to 43% of all burned patients admitted in the years 1989–1993 in Bratislava (9) – their use in reasonable and efficacious treatment is of great importance.
MATERIAL AND METHODS
The retrospective study considered data from 109 patients with fresh superficial and deeper partial-thickness burns who were hospitalized at the Teaching Department of Burns and Reconstructive Surgery of the University Hospital, Bratislava, Ružinov Hospital, Slovakia during the years 2005–2007. The study was approved by the Ethics Committee of Ružinov Hospital.
Xenograft preparation method
Skin xenografts (SXG) have been used as biological skin substitutes at the Department of Burns and Reconstructive Surgery since 1987. They have been prepared for clinical use in the Central Tissue Bank (CTB), part of the Teaching Department of Burns and Reconstructive Surgery Bratislava, Slovakia where SXG preparation method has been performed according to the original method of Moserová (1974) with some minor modifications. Porcine skin removed from veterinary certified slaughtered pigs, stored at the temperature of 4°C, is transported from the slaughter-house to CTB. Within 24 hours after slaughter, under sterile conditions, the retrieval of the dermoepidermal grafts is performed by electrical dermatome. After washing the grafts retrieved in a solution of chloramine, lavage in antibiotic solution and lavage in cryoprotective agent, four small samples (0.5 x 0.5cm each) are taken from each graft for bacteriological checks. Then each graft is placed on sterile gauze with the dermal side up, folded to create a maximum of four layers and sealed in a sterile plastic bag. Prepared SXGs are placed in a deep-freezer appliance and stored at a temperature of -80°C. If the sterility tests are negative, according to Czech-Slovak Pharmacopoeia 4th Ed., the SXGs can be released for clinical use.
Wound management protocol
During patient admission initial assessment of the depth and extent of burns is performed. To estimate the extent of the burns (BSAB) the Lund-Browder chart is used. The depth of the burn is expressed in degrees. When indicated for the xenograft coverage, after disinfection of the wounds with Betadine all patients undergo complete debridement of non-viable epidermis using blunt debridement (gauze) under systemic analgesia; in the case of children with greater extent of burns general anaesthesia is used. After obtaining a clean wound surface with the dermis exposed (Fig. 1) the cryopreserved porcine xenografts (defrosted rapidly in saline immediately before usage) are applied (Fig. 2). Treated areas are then covered with tulle dressing impregnated with Vaseline, gauze with silver sulphadiasine, dry gauze layers and elastic bandage. When xenografts are placed over the joints of limbs, in particular with children, Kramer splints are used. Systemic antibiotic prophylaxis is provided by intravenous administration of penicillin in standard doses in cases with negative history of penicillin allergy. Wound cultures are taken during initial treatment and also regularly during the hospital stay. If the wound dressings are not displaced and no complications occur, dressings are changed every 48 hours. The xenografts are left to adhere to the wound surface until they are separated by means of transepithelial elimination (Fig. 3). The residual defects (if present) are treated by application of topical antimicrobial agents within the scope of the methods for the wound healing during the hospital stay or on an outpatient basis.
Fig. 1. IIa degree scald burn suffered by a 13-month-old child 
Fig. 2. The same child after immediate coverage with skin xenografts 
Fig. 3. Separation of the xenografts by means of transepithelial elimination on the 8<sup>th</sup> day post injury 
Inclusion criteria
The inclusion criteria during this retrospective study were as follows:
- patients were hospitalized during the three-year period (from the beginning of the 2005 to the end of the 2007)
- all patients sustained partial-thickness (superficial and deep dermal) scald burns
- all patients were admitted and treated within the first 24 hours after injury
- all patients were indicated for wound coverage by biological skin substitutes
- patients of every age were included, with a wide range of burn extent and associated illness
Evaluation criteria
- Total number of patients healed within 14 days postburn and their mean healing time
- Mean healing time of all patients included in the study (total healing time)
- Mean discharge time to outpatient management
- Total number of patients whose treatment required operation - wound closure by skin autografts
Total healing time was defined as the time necessary to reach 95% of re-epithelialization by each patient. All patients were discharged from hospital discharge if outpatient management could be performed safely and comfortably.
RESULTS
In total, 109 patients were included in the study. Of these patients 59 were males (54%) and 50 females (46%). The male/female ratio was 1.16 : 1 for adults and 1.2 : 1 for children. The mean age of the patients was 7.6 years (min.: 6 months, max: 80 years). The majority of the 109 patients were children (96 cases). The mean BSAB was 13% (min.: 3%, max: 43%). All patients suffered burns caused by hot liquids (water, tea, coffee, soup).
Number of patients, sex, mean age, mean BSAB, minimal and maximal values (min/max.) and standard deviations (S.D.) are shown in Table 1.
Tab. 1. Patients included in the study 
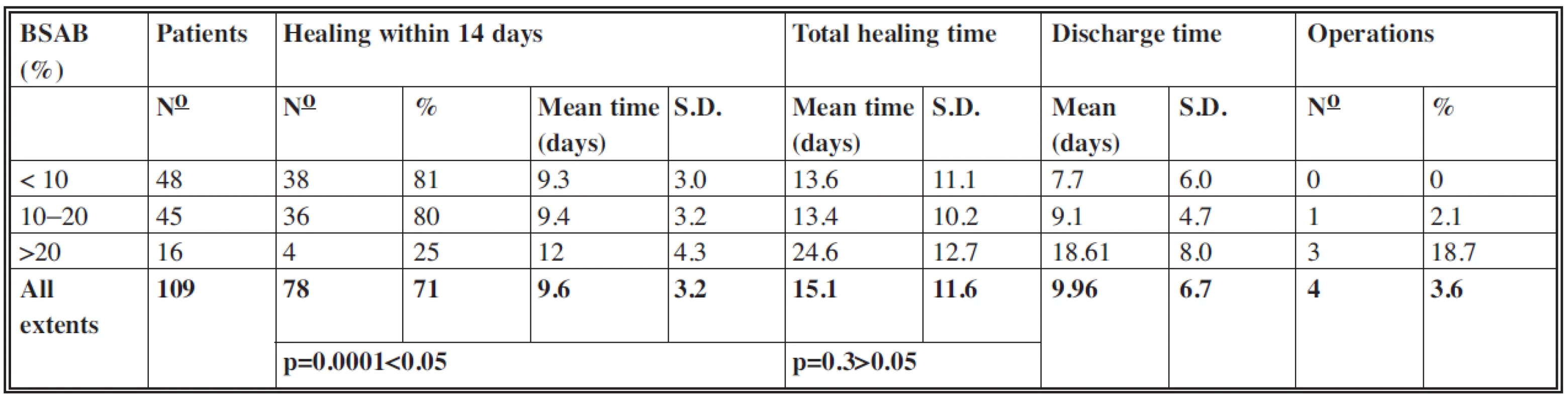
According to BSAB the patients were divided into three groups:
- below 10% (48 patients with mean extent 7.6%)
- 11–20% (45 patients with mean extent 13.4%)
- more than 20% (16 patients with mean extent 29.4%)
The results are displayed in Table 2.
Healing within 14 days
Of the 109 patients, 78 (71%) patients healed within 14 days with a mean time of 9.6 days (S.D.: 3.2). One sample t-test which compared mean healing times achieved within 14 days with the value of 14 days established the significant difference (p=0.0001<0.05) with a level of significance of 0.05.
In the group with extents larger than 20% the lowest proportion of patients healed within 14 days. This shows the correlation between the larger extents of burns and increased healing times in this group.
Healing within 14 days serves as an expression of the healing dynamics and as a parameter for comparison with other studies focused on alternative treatment options.
Total healing time
In this category too the values in groups with extents up to 10% and from 10 to 20% were almost identical and within 14 days. In the group with extents more than 20% the mean value of total healing time was 24.6 days, which is in accordance with the increased value of mean healing time within 14 days in this group. In all the 109 cases the mean total healing time was 15.1 days (S.D.: 11.6) with no significant difference between mean total healing times and the value of 14 days (p=0.3>0.05).
Discharge time
As the treatment of residual defects could have been performed, in many cases, on an outpatient basis, the mean values of discharge time in each group were lower than mean values of total healing time. Of the 109 patients in total the mean discharge time was up to 10 days.
Operations
In four cases it was necessary to perform tangential excision of the deep parts of the wounds and coverage with split-thickness skin autografts or coverage of residual defects of a larger extent, the spontaneous healing of which would require much more time. In the group with extents up to 10% there were no operations. In the group with extents from 10 to 20% one case of a seven-year-old girl with BSAB of 13.5% was treated by surgery. Because of the wound deepening of BSAB 3.5%, the tangential excision and temporary coverage with porcine skin xenografts were performed on day 10 postburn. Skin xenografts were replaced by split-thickness skin autografts in the second stage. The wound cultures at the day of admission were sterile, on day 4 postburn sterile – after multiplication Staphylococcus aureus. Of the 16 patients in the group with BSAB above 20% three cases underwent surgery, which represented 18.7% of this group. In two cases (one girl and one boy, both aged two with BSAB 22% and 23% respectively), tangential excision and coverage with split-thickness skin autografts of BSAB 6% and 6.5% respectively were performed. In one case (a two-year-old girl, BSAB: 21.5%) it was necessary to cover the residual defects, which represented BSAB 2%, with split-thickness autografts.
Of the 109 patients four underwent surgery, which represents 3.6%. The mean surface operated on was 4.5% TBSA (total body surface area).
DISCUSSION
We tried to compare our results with those obtained at other workplaces, where different types of skin substitutes have been used in this indication.
In Beverwijk, the Netherlands, a retrospective study of the treatment of scalds by glycerolised skin allografts was performed by Brans (3) and published in 1994. Over a period of four years 45 patients with a mean age of 23 months, mean BSAB 10.2% (from 3% to 23%) were treated during the first 24 hours post burn. Of these patients 21 (47%) healed within 14 days. The better outcome of patients in this study, who were given treatment for scalds involving skin xenografts (71%) can only partially be explained by the lower mean age of the patients in the Dutch study. The decisive factor may be the difference in the viability of these skin substitutes.
The results of other studies indicate that it is necessary for a bioactive surface to be present, in order to accelerate re-epithelialisation through contact orientation and stimulation of cell proliferation (4, 8). From this point of view, and with regard to the results achieved, the partially retained bioactivity of skin xenografts seems more advantageous.
It is also possible to preserve skin allografts by deep freezing, which retains their viability. However, utilisation is recommended in cases where integration of the dermal component as a part of permanent wound closure is desired (5), because the glycerol preserved allograft provides no viable coverage material and lacks the beneficial effect of integration and vascularisation of viable allogeneic grafts (2).
In Copenhagen, Denmark, a prospective randomised study was performed by Leicht (10) based on a comparison of treatment of scalds by lyophilised alografts (LA) and by open technique. Of 50 patients with scalds, 25 – with the mean age of 1.4 years and mean BSAB 8.35% – received LA within the first 24 hours post burn. Of these 25 patients, 15 (60%) healed within 14 days. Better results achieved in the study with xenografts (71% healed within 14 days) could be partially explained by the higher mean age (7.6 years), but as mentioned previously, it is also the case that lyophilised skin allografts do not retain viability in comparison with cryopreserved skin xenografts.
The parameters compared are shown in Table 3.
Tab. 3. Comparison of the results 
For the coverage of partial-thickness burns amnion obtained from the placentas of selected donors is also being used. In 2007 Singh (13) compared the burn wound healing rate between radiation sterilized amniotic membranes and glycerol preserved amniotic membranes. Fifty patients with partial-thickness burns (41 of them with scalds, the rest caused by flame) up to 70% of BSAB were selected in this study. The wounds of each patient were divided into halves, one half treated with glycerol preserved membranes and the second half with irradiated membranes. There were no significant differences in the rate of healing between the gamma-irradiated amniotic membranes and glycerol preserved membranes, indicating that there is no adverse effect of gamma irradiation on the efficacy of the membranes. According to the authors, in all the patients the membranes dessicated and separated in 10–14 days, leaving behind an epithelialized surface. The exact results of the healing were not stated.
The use of allogeneic cultivated keratinocytes as a temporary wound coverage is advantageous mainly with IIb degree burns, where the capability of keratinocytes to promote epithelialisation by releasing growth factors and mediators of wound healing is combined with the ability of epithelialization from the adnexal structures of the viable parts of dermis. The “Viennese concept” is based on this approach, as published by Rab in 2005 (12):
- early tangential excision of partial and full thickness scalds (4–7 days after trauma)
- coverage of partial thickness burns by cryopreserved allogeneic cultivated keratinocytes
- in scalded areas which have to be excised to the subcutaneous tissue the coverage of autologous split skin grafts is still the method of choice
The authors compared 22 scalded children with wounds tangentially excised and covered with cultivated allogeneic keratinocytes on days 4–7 after trauma with 14 children who underwent the same procedure on days 4–7 after trauma but using split skin autografts. They observed significantly lower volume of blood transfusion and significantly better long-term results pertaining to hypertrophic scar formation in the group of patients covered by cultivated allogeneic keratinocytes. Bearing in mind the results achieved, the authors feel that the higher costs resulting from the usage of keratinocytes are justified. The “Viennese concept” is promising mainly for management of scalds in the period after the first 24 hours subsequent to trauma, when the usage of temporary skin substitutes is more risky because of possible infectious complications, which can in many cases disable their application.
The lower incidence of hypertrophic scars in the group of patients covered with cultivated allogeneic keratinocytes as a long term result corresponds with the finding of Ghaffari (7), who observed significantly lower amount of collagen produced by dermal fibroblasts during cultivation with keratinocytes. He explains this with reference to the presence of keratinocyte-derived collagen-inhibitory factors (KD-CIFs) with the molecular weight 30–50 kDa, which are regulating type I collagen expression and synthesis in dermal fibroblasts.
There is a reason to suppose that this regulation also takes place with other types of biological skin substitutes with preserved viability of keratinocytes, which could be the scope of further investigation.
In addition to biological skin substitutes, other types of skin substitutes of different origin and constitution are also used for coverage of partial-thickness burns.
In 1998 Ou (11) published a retrospective study of the treatment of scalds by Biobrane. Over a period of 2.5 years 106 patients with scalds with an average BSAB of 12.5% were treated within 24 hours post burn. With 24 of these patients coverage was aborted because of the low adherence; 14 of them healed spontaneously. For the 10 remaining patients it was necessary to perform coverage with split skin autografts. The cases of low adherence and accumulation of exudate below Biobrane was explained by the authors with reference to the presence of devitalised upper dermis. Therefore they emphasised accurate initial diagnosis of the depth of burns because, in their view, the best results can be obtained only on superficial partial-thickness burns. Patients undergoing surgery represented 9.4% of all patients in the study, while in the study with skin xenografts the figure was 3.6%. Neither proportion of healing within 14 days nor total healing times were stated. The mean time of separation of the skin substitute was 11.1days (between 3 and 18 days).
In the period immediately after an injury, during initial treatment, it is very difficult to make an exact differentiation between IIa and IIb degree burns. Therefore it appears more suitable to employ skin substitutes with adherence capacity which is not influenced by this depth range.
On partial thickness face burns a biosynthetic skin substitute, Transcyte, has been used to comply with the changing irregular surfaces of the face. In a comparative study (6) the usage of Transcyte was compared with open technique (Bacitracine ointment). The authors observed significantly shorter healing times, shorter time for wound-care and lower pain in the group treated with biosynthetic skin substitute.
Zajíček, 2008 (16), reported on the usage of a new sterile commercially-available biological cover derived from acellular pig dermis. It is distributed in dry form and can therefore be kept in storage for a long time. Hydrated cover can be used, among other indications, for the treatment of superficial and deep dermal burns.
At present research is still ongoing into a permanent skin substitute with a quality closest to the ideal skin substitute (15):
- inexpensive
- long shell life
- used off the shelf
- non-antigenic
- durable
- flexible
- prevents water loss
- bacterial barrier
- drapes well
- easy to secure
- grows with child
- applied in one operation
- does not become hypertrophic
- does not yet exist.
Resulting from these ideal properties, the characteristics of a good wound dressing which can be used to cover all burns in primary care can be derived (1):
Considered essential
- maintain moist wound environment
- contours easily
- non-adherent but retains close contact with the wound
- easy to apply and remove
- painless on application and removal
- cost-effective
- protects against infection
Considered desirable
- lasts for 10 days (one application)
- minimal dressing changes
- waterproof to allow for washing and bathing
Many biological skin substitutes comply with some of the above criteria. The appropriate choice should depend on careful considering of the characteristics, indications for use and also the price of each available skin substitute. Only with this approach it is possible to achieve the goal – early wound closure of the best quality.
The retrospective study proved the clinical efficiency of using skin xenograft for the treatment of partial thickness scald burns. This method, with its treatment effect, is fully comparable with others, realized at other clinical workplaces, which use biological or other types of skin substitutes for the given indication.
Address for correspondence:
Peter Bukovčan, M.D., Ph.D.
Department of Burns and Reconstructive Surgery
University Hospital Bratislava
Ružinov Hospital
Ružinovská 6, 826 06 Bratislava
Slovak Republic
E-mail: bukovcanmed@hotmail.com
Zdroje
1. Alsbjörn B., Gilbert P., Hartmann B., Kaźmiersky M., Monstrey S., Palao R., Roberto MA., Van Trier A., Voinchet V. Guidelines for the management of partial-thickness burns in a general hospital or community setting – Recommendations of a European working party. Burns, 33, 2007, p. 155-160.
2. Atiyech BS., Hayek SN., Gunn SW. New technologies for burn wound closure and healing – Review of the literarture. Burns, 31, 2005, p. 944-956.
3. Brans TA., Hoekstra MJ., Vloemans AFPM., Kreis RW. Long-term results of treatment of scalds in children with glycerol-preserved allografts. Burns, 20, 1994, p. 10-13.
4. Brown R., Kinsty D. et al. Strategies for cell engineering in tissue repair. Wound Rep. Reg., 5, 1997, p. 212-221.
5. Brož L., Vogtová D., Königová R. Experience with banked skin in the Prague burn centre. Acta Chir. Plast., 41, 1999, p. 54-58.
6. Demling R.H., DeSanti L. Management of partial thickness facial burns (comparison of topical antibiotics and bio-engineered skin substitutes). Burns, 25, 1999, p. 256-261.
7. Ghaffari A., Kilani RT., Ghahary A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. Journal of Investigative Dermatology advance online publication, 11. 9. 2008, www.jidonline.org.
8. Git M., Toda K., Grinell F. Activation of human keratinocyte migration on type 1 collagen and fibronectin. J. Cell. Sci., 96, 1990, p. 197-205.
9. Koller J., Orság M., Ondriašová E., Gräffinger I., Bukovčan P. Analysis of 1119 burn injuries treated at The Bratislava Burn Departement during a five-year period. Acta Chir. Plast., 36, 1994, p. 67-70.
10. Leicht P., Muchardt O., Jensen M., Alsbjörn BA., SŅrensen B. Allograft vs. exposure in the treatment of scalds – a randomized controlled clinical study. Burns, 15, 1989, p. 1-3.
11. Ou LF., Lee SY., Chen YC., Yang RS., Tang YW. Use of Biobrane in pediatric scald burns – experience in 106 children. Burns, 24, 1998, p. 49-53.
12. Rab M., Koller R., Ruzicka M., Burda G., Kamolz LP., Bierochs B., Meissl G., Frey M. Should dermal scald burns in children be covered with autologous skin grafts or with allogeneic cultivated keratinocytes?—“The Viennese concept”. Burns, 31, 2005, p. 578-586.
13. Singh R., Purohit S., Chacharkar MP., Bhandari PS., Bath AS. Microbiological safety and clinical efficacy of radiation sterilized amniotic membranes for treatment of second-degree burns. Burns, 33, 2007, p. 505-510.
14. Šimko Š., Koller J. Popáleniny. Martin: Osveta 1992, p. 24.
15. Tompkins RG., Burke JF. Burn wound closure using permanent skin replacement materials. World J.Surg., 16, 1992, p. 47-52.
16. Zajíček R., Brož L., Klein L., Bláha J., Königová R., Jirkovská A., Dubský M., Bureš I., Matoušková E. Xe-Derma: A new biological cover for treatment of acute and chronic wounds. Hojení ran, 2, 2008, p. 18-27.
Štítky
Chirurgie plastická Ortopedie Popáleninová medicína Traumatologie
Článek vyšel v časopiseActa chirurgiae plasticae
Nejčtenější tento týden
2010 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
- Neodolpasse je bezpečný přípravek v krátkodobé léčbě bolesti
- Léčba akutní pooperační bolesti z pohledu ortopeda
-
Všechny články tohoto čísla
- NASAL DERMOIDS WITHOUT INTRACRANIAL EXTENSION IN TEENAGERS
- DIAGNOSTIC DILEMMAS OF INFANTILE SARCOMA OF THE FOREARM
- MINIABDOMINOPLASTY
- ČESKÉ A SLOVENSKÉ SOUHRNY
- NASAL RECONSTRUCTION IN CHILDREN WITH THE COMBINATION OF NASOLABIAL AND ISLAND FLAPS
- TREATMENT OF PARTIAL-THICKNESS SCALDS BY SKIN XENOGRAFTS – A RETROSPECTIVE STUDY OF 109 CASES IN A THREE-YEAR PERIOD
- Acta chirurgiae plasticae
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- NASAL DERMOIDS WITHOUT INTRACRANIAL EXTENSION IN TEENAGERS
- TREATMENT OF PARTIAL-THICKNESS SCALDS BY SKIN XENOGRAFTS – A RETROSPECTIVE STUDY OF 109 CASES IN A THREE-YEAR PERIOD
- MINIABDOMINOPLASTY
- NASAL RECONSTRUCTION IN CHILDREN WITH THE COMBINATION OF NASOLABIAL AND ISLAND FLAPS
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání