-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
article has not abstract
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004252
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004252Summary
article has not abstract
Fas-Fas Ligand Signaling Induces Host Cell Death by Apoptosis
Fas ligand (FasL, CD95L) is a type-II membrane protein within the tumor necrosis factor (TNF) superfamily of death receptors [1]. FasL shares 25%–30% sequence homology with related family member proteins such as tumor necrosis factor alpha (TNFα) and TNF-related apoptosis-inducing ligand (TRAIL), with the most similarity present in the C-terminal homology ectodomain that extends into the extracellular space for receptor binding [2]. FasL engages and trimerizes the death receptor Fas (CD95) on cell surfaces to initiate the extrinsic apoptosis pathway [3]. The Fas-FasL interaction recruits the Fas-associated death domain adapter protein (FADD) via death domain binding, which interacts with dimerized procaspase-8 to form the death-inducing signaling complex (DISC) [4]. Caspase-8 catalyzes its autoactivation, followed by the proteolytic conversion of downstream effector caspases such as caspase-3 and -7 into their mature forms [5]. Effector caspases direct cell death by apoptosis, which results in nuclear and cytoplasmic condensation followed by cellular fragmentation into membrane-bound apoptotic bodies [6]. Caspase-activated DNase (CAD) cleaves genomic DNA between nucleosomes to generate short fragments prior to cellular condensation and membrane blebbing [7]. Membrane fragments are usually taken up by other cells and degraded in phagosomes via a process known as efferocytosis. Efferocytosis of apoptotic cells contributes to the resolution of inflammation by rapidly clearing cytotoxic cellular debris, and defects in this process can lead to inflammatory diseases such as acute lung injury [8].
Although FasL is expressed by many cell types, it is primarily recognized as associated with activated T lymphocytes and natural killer (NK) cells. FasL-dependent apoptosis plays important roles in tissue remodeling and the deletion of potentially autoreactive thymocytes to maintain immune tolerance during development, and it also contributes to tumor cell clearance by effector NK cells [9]–[11]. Though apoptosis has traditionally been defined as noninflammatory during these processes, FasL-induced cell death has been shown to be highly proinflammatory in the context of microbial infections [12].
Apoptosis Stimulates Inflammatory Host Defenses
In response to many bacterial pathogens, the host responds by triggering FasL-dependent cell death as an inflammatory innate immune response [13]. Fas-mediated apoptosis of epithelial cells induces the release of proinflammatory cytokines, including TNFα, interleukin 8 (IL-8), macrophage inflammatory protein 2 (MIP-2), monocyte chemotactic protein 1 (MCP-1), and interleukin-1 beta (IL-1β) [14], [15]. In addition to these cytokines, Fas signaling positively affects CXC chemokine production that leads to enhanced neutrophil infiltration [16]. This apoptotic response is usually a protective mechanism by the host during bacterial infections. Optimal levels of cell death may eliminate replicative niches for intracellular pathogens and enhance further immune cell recruitment through the secretion of cytokines and chemokines, while excessive cell death often leads to an exaggerated immune response, self-tissue damage, and possibly death of the host. Experimentally, the contribution of FasL to inflammatory diseases can be assessed using C57BL/6 FasLgld mice, which contain a single residue mutation (F275L) within FasL that prevents binding to the Fas receptor [17]. Similarly, Fas-FasL signaling may be studied using Faslpr mice, which lack a functional Fas receptor and thus cannot be activated by FasL [18]. In models of pulmonary inflammation, these mice exhibit reduced airway epithelial cell apoptosis, cytokine secretion, neutrophil influx, and tissue damage [19]. Similar results are obtained during knockdown of Fas by small interfering RNA (siRNA) [20].
During pneumonias caused by infection with Pseudomonas aeruginosa, FasL has been identified as a central regulator of innate defenses and inflammation. The presence of P. aeruginosa in the lungs leads to FasL-dependent apoptosis of host cells; the experimental use of FasLgld mice with an intranasal P. aeruginosa infection model shows increased disease severity with more rapid sepsis as compared to wild-type controls [21]. Adoptive transfer of bone marrow cells between wild-type and FasLgld mice determined that the protection conferred by FasL-dependent apoptosis was due to FasL interactions among lung epithelial cells during infection [21]. Epithelial cell apoptosis may consequently result in the secretion of defensins and cytokines that promote a stronger immune response. A similar role for FasL in host defenses is seen during infection with the stomach pathogen Helicobacter pylori: infection leads to Fas-dependent apoptosis of gastric epithelial cells [22]. Consistent with the role of Fas-FasL in enhancing immunity to bacterial infections, FasLgld mice produce lower levels of interferon gamma (IFNγ) from splenocytes and have enhanced disease severity compared to wild-type mice [22].
Some bacterial pathogens have developed virulence strategies to alter apoptosis during infection. For instance, following inhalation, macrophages phagocytose Chlamydia pneumoniae as a normal host defense mechanism. To evade killing of infected macrophages and to enhance pathogenesis, C. pneumoniae blocks cytochrome C release by the cell, thus inhibiting apoptosis via the intrinsic pathway [23]. Similarly, the virulence factors SidF of Legionella pneumophila and AvrA of Salmonella Typhimurium suppress apoptosis by inhibiting Bcl2 family proteins and by blocking c-Jun N-terminal kinase (JNK) signaling, respectively [24], [25]. Recently, several studies have described previously unknown mechanisms by which two bacterial pathogens overcome cell death–mediated host defenses by directly targeting the Fas-FasL signaling pathway. Yersinia pestis, the causative agent of the disease plague, prevents Fas induction by cleaving and inactivating FasL on the surface of effector cells, while enteropathogenic Escherichia coli (EPEC), a cause of gastrointestinal infections, post-translationally modifies FADD within target cells to arrest Fas-induced apoptosis (Figure 1).
Fig. 1. Disruption of Fas-FasL signaling by Pla of Y. pestis and NleB of EPEC. 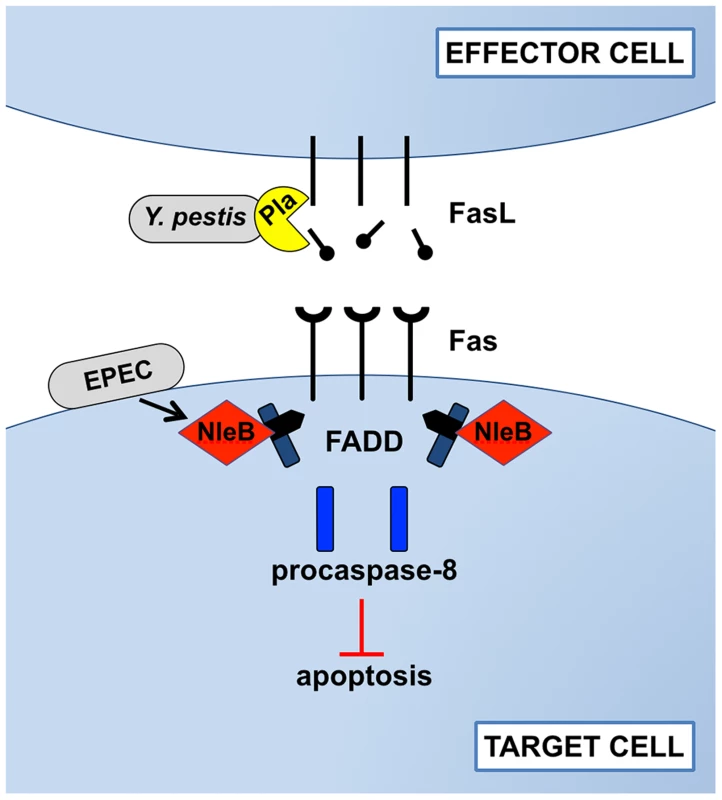
In response to bacterial infections, the host attempts to induce Fas/FasL-dependent cell apoptosis. During pneumonic plague, however, the Pla protease of Y. pestis directly cleaves FasL on effector cells to prevent the initiation of Fas signaling, blocking the activation of the initiator caspase-8, effector caspases -3 and -7, and cell death by apoptosis. As an alternative strategy during gastrointestinal infection, EPEC injects the type-III-secreted effector NleB into the cytoplasm of target cells, where it modifies FADD with N-acetylglucosamine to prevent death domain binding and downstream signaling following the engagement of Fas by FasL. While the mechanisms by which these bacteria target Fas-FasL signaling are distinct, the end result is the same: inhibition of apoptosis. Y. pestis Prevents Apoptotic Cell Death by Cleaving FasL
We recently reported that Y. pestis prevents Fas-FasL signaling as a distinct pathogenic strategy to reduce apoptosis and enhance disease during mammalian infection [26]. Y. pestis produces the plasminogen activator Pla, a surface-exposed protease that is critical for the progression of the pneumonic (respiratory) form of plague and is required for bacterial outgrowth in the lungs [27]. While cell death via apoptosis predominates during the early, anti-inflammatory stage of pneumonic plague, as the infection progresses, cellular apoptosis is reduced as the synthesis of Pla increases and the bacterial burden rises [28], [29].
Our study showed that the Pla protease alters pulmonary apoptosis by directly cleaving both soluble and membrane-bound FasL to block the activation of Fas [26]. Through the extracellular degradation of FasL on the surface of effector cells, Pla prevents the induction of Fas-dependent caspase-3/7 activation in target cells, thereby actively manipulating host innate defense responses (Figure 1). In a murine model of primary pneumonic plague, the loss of active FasL in FasLgld mice reduces caspase-3 activation in recruited immune cells, including neutrophils, and allows for the enhanced outgrowth of Y. pestis Δpla bacteria in the lungs. As a consequence of FasL cleavage by Pla, immune cell recruitment and inflammatory cytokine production are altered, while local tissue damage accumulates, resulting in increased pulmonary edema. In the absence of Pla, however, increased caspase-3/7 activation is observed in multiple cell types recruited to inflammatory lesions within the lungs, demonstrating a direct role for Pla in preventing host cell apoptosis during pneumonic plague. Specific inhibition of caspase-3/7 with the peptide inhibitor DEVD recapitulates the loss of FasL by enhancing bacterial outgrowth and reducing cytokine secretion, indicating that the cleavage of FasL by Pla overcomes these caspase-3/7-dependent host defenses. This work describes a previously unknown mechanism by which Y. pestis controls host cell death pathways and the activation of Fas signaling through the proteolytic degradation of FasL.
While a role for Pla in altering caspase activation was not previously known, the manipulation of host cell apoptosis by pathogenic Yersinia species via the Yop-Ysc type III secretion system (T3SS) is well established. The conserved Yersinia T3SS effector protein YopJ (also known as YopP in Y. enterocolitica) has been shown to enhance apoptosis in vitro under artificial secretion conditions [30]. During its evolutionary divergence from Y. pseudotuberculosis, however, Y. pestis acquired mutations within YopJ that reduce the efficiency of its translocation, which results in decreased caspase activation and diminished apoptotic activity [31]. This evolutionary loss of YopJ cytotoxicity led to the increased virulence of the plague bacillus [32], [33]. Additionally, neutrophils are resistant to YopJ-induced apoptosis, which is particularly relevant for plague pneumonia as neutrophils are the primary immune cell type recruited to the lungs [34]. Indeed, we showed that YopJ has no impact on caspase-3/7 activation during pneumonic plague, regardless of the presence or absence of Pla. Therefore, as opposed to the gastrointestinal Yersiniae that lack Pla and have a more cytotoxic variant of YopJ, the virulence of Y. pestis is enhanced by coordinate efforts to reduce apoptosis via the direct inactivation of FasL by Pla and the concomitant suppression of YopJ function, particularly in the lungs.
Over the past 1,500–20,000 years, Y. pestis has evolved away from a gastrointestinal lifestyle towards one that favors extraintestinal environments. Therefore, a shift in virulence strategy to prevent apoptosis during the later stages of disease may reflect an adaptation to the host response of the organs and tissues that the plague bacillus now infects. In the lungs, it is possible that cells injected and reprogrammed by the Yersinia T3SS may be recognized by the host and thus targeted for clearance via caspase-3/7-dependent mechanisms, with associated activation of the innate immune response. Indeed, injection of the T3SS effector YopK is known to stimulate apoptosis of pulmonary macrophages, and the inhibition of Fas-FasL signaling by Pla may therefore act to limit the recognition and clearance of these cells, resulting in an altered cytokine response in the lungs.
Enteropathogenic E. coli Antagonizes Fas Signaling through Effector Secretion
As with pathogenic Yersinia species, enteric bacterial pathogens have also been shown to manipulate apoptotic signaling to successfully colonize the gut [35], [36]; however, direct interactions with the Fas-FasL signaling pathway were only discovered recently. Two independent groups showed that the EPEC T3SS effector NleB disrupts FADD-mediated apoptosis downstream of Fas-FasL engagement within target cells to counteract host defenses and enhance colonization [37], [38]. After injection into host cells, the N-acetylglucosamine (GlcNAc) transferase activity of NleB post-translationally modifies FADD at a single arginine residue (Figure 1). This residue is conserved among the related proteins TNF receptor type-1 associated death domain protein (TRADD) and receptor-interacting serine/threonine protein kinase 1 (RIPK1), which are also modified by NleB. GlcNAcylation of these proteins prevents death domain oligomerization and thus aborts apoptotic signaling downstream of the TNF family death receptors TNFR1, Fas, and TRAIL. The GlcNAc transferase activity of NleB is specifically required for bacterial gut colonization in a mouse model of EPEC, suggesting that EPEC and related pathogens disrupt Fas-induced apoptosis to overcome the otherwise protective host response conferred by this signaling pathway.
Therapeutic Potential of Cell Death Modulators
It is becoming clear that the manipulation of cell death is a major strategy by which bacterial pathogens enhance virulence, although the specific mechanisms through which this occurs appear to be different from species to species. Some pathogens actively promote host apoptosis, while others inhibit Fas-FasL signaling. Understanding the in vivo effects of FasL on the virulence of a pathogen is made even more complex since different microbes stimulate varying levels of cell death (and by different pathways) and are likely to produce factors that both induce and abrogate apoptosis, with fine-tuning of cell death pathways for maximal virulence.
Despite our limited mechanistic understanding of cell death manipulation by pathogens, therapeutics that prevent bacterial virulence factors from modulating apoptotic cell death may have broad implications. Since both Pla of Y. pestis and NleB of EPEC disable Fas-FasL signaling to promote virulence, the administration of proapoptotic compounds, exogenous FasL protein, or agonistic antibodies that trimerize Fas may serve as possible therapeutics [39]. These treatments may help to overcome the manipulation of innate immunity by specific pathogens or to enhance the immune response more generally. Inhibitors to specific bacterial virulence factors such as Pla of Y. pestis or NleB of EPEC may also confer more targeted protection.
Preliminary studies have been conducted using the related apoptotic ligand TRAIL. When exogenous TRAIL is administered during pneumococcal pneumonia, apoptosis levels are restored, Streptococcus pneumoniae colonization is reduced, and mouse survival is enhanced, demonstrating the viability of increased apoptosis to bolster host defenses [40]. As the role of apoptosis in modulating host defenses becomes more clear, it will be important to validate the use of cell death manipulators as therapeutics for each infection model, since apoptosis may be detrimental to the host under certain circumstances, such as during systemic bacterial sepsis [41]. Continued investigation of the mechanisms by which pathogens manipulate apoptosis to alter host responses, via a combination of intracellular or extracellular activities on either effector cells or target cells, will provide insight for the development of future therapeutics.
Zdroje
1. SudaT, TakahashiT, GolsteinP, NagataS (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75 : 1169–1178.
2. LocksleyRM, KilleenN, LenardoMJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104 : 487–501.
3. StrasserA, JostPJ, NagataS (2009) The many roles of FAS receptor signaling in the immune system. Immunity 30 : 180–192.
4. WangL, YangJK, KabaleeswaranV, RiceAJ, CruzAC, et al. (2010) The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat Struct Mol Biol 17 : 1324–1329.
5. NagataS (1997) Apoptosis by death factor. Cell 88 : 355–365.
6. BoatrightKM, SalvesenGS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15 : 725–731.
7. EnariM, SakahiraH, YokoyamaH, OkawaK, IwamatsuA, et al. (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391 : 43–50.
8. HensonPM, TuderRM (2008) Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol 294: L601–611.
9. CastroJE, ListmanJA, JacobsonBA, WangY, LopezPA, et al. (1996) Fas modulation of apoptosis during negative selection of thymocytes. Immunity 5 : 617–627.
10. XerriL, DevilardE, HassounJ, MawasC, BirgF (1997) Fas ligand is not only expressed in immune privileged human organs but is also coexpressed with Fas in various epithelial tissues. Mol Pathol 50 : 87–91.
11. HamannKJ, DorscheidDR, KoFD, ConfortiAE, SperlingAI, et al. (1998) Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol 19 : 537–542.
12. AshidaH, MimuroH, OgawaM, KobayashiT, SanadaT, et al. (2011) Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol 195 : 931–942.
13. LabbeK, SalehM (2008) Cell death in the host response to infection. Cell Death Differ 15 : 1339–1349.
14. HagimotoN, KuwanoK, KawasakiM, YoshimiM, KanekoY, et al. (1999) Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation. Am J Respir Cell Mol Biol 21 : 436–445.
15. ParkDR, ThomsenAR, FrevertCW, PhamU, SkerrettSJ, et al. (2003) Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol 170 : 6209–6216.
16. FarnandAW, EastmanAJ, HerreroR, HansonJF, MongovinS, et al. (2011) Fas activation in alveolar epithelial cells induces KC (CXCL1) release by a MyD88-dependent mechanism. Am J Respir Cell Mol Biol 45 : 650–658.
17. TakahashiT, TanakaM, BrannanCI, JenkinsNA, CopelandNG, et al. (1994) Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76 : 969–976.
18. NagataS (1994) Mutations in the Fas antigen gene in lpr mice. Semin Immunol 6 : 3–8.
19. PerlM, ChungCS, PerlU, Lomas-NeiraJ, de PaepeM, et al. (2007) Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med 176 : 591–601.
20. PerlM, ChungCS, Lomas-NeiraJ, RachelTM, BifflWL, et al. (2005) Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol 167 : 1545–1559.
21. GrassmeH, KirschnekS, RiethmuellerJ, RiehleA, von KurthyG, et al. (2000) CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290 : 527–530.
22. JonesNL, DayAS, JenningsH, ShannonPT, Galindo-MataE, et al. (2002) Enhanced disease severity in Helicobacter pylori-infected mice deficient in Fas signaling. Infect Immun 70 : 2591–2597.
23. FanT, LuH, HuH, ShiL, McClartyGA, et al. (1998) Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187 : 487–496.
24. BangaS, GaoP, ShenX, FiscusV, ZongWX, et al. (2007) Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci U S A 104 : 5121–5126.
25. JonesRM, WuH, WentworthC, LuoL, Collier-HyamsL, et al. (2008) Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe 3 : 233–244.
26. CaulfieldAJ, WalkerME, GieldaLM, LathemWW (2014) The Pla protease of Yersinia pestis degrades Fas ligand to manipulate host cell death and inflammation. Cell Host Microbe 15 : 424–434.
27. LathemWW, PricePA, MillerVL, GoldmanWE (2007) A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science 315 : 509–513.
28. BergsbakenT, CooksonBT (2007) Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog 3: e161.
29. LathemWW, SchroederJA, BellowsLE, RitzertJT, KooJT, et al. (2014) Posttranscriptional regulation of the Yersinia pestis cyclic AMP receptor protein Crp and impact on virulence. mBio 5: e01038–01013.
30. PhilipNH, BrodskyIE (2012) Cell death programs in Yersinia immunity and pathogenesis. Front Cell Infect Microbiol 2 : 149.
31. ZaubermanA, CohenS, MamroudE, FlashnerY, TidharA, et al. (2006) Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect Immun 74 : 3239–3250.
32. BrodskyIE, MedzhitovR (2008) Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog 4: e1000067.
33. ZaubermanA, TidharA, LevyY, Bar-HaimE, HalperinG, et al. (2009) Yersinia pestis endowed with increased cytotoxicity is avirulent in a bubonic plague model and induces rapid protection against pneumonic plague. PLoS ONE 4: e5938.
34. SpinnerJL, SeoKS, O'LoughlinJL, CundiffJA, MinnichSA, et al. (2010) Neutrophils are resistant to Yersinia YopJ/P-induced apoptosis and are protected from ROS-mediated cell death by the type III secretion system. PLoS ONE 5: e9279.
35. NewtonHJ, PearsonJS, BadeaL, KellyM, LucasM, et al. (2010) The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-kappaB p65. PLoS Pathog 6: e1000898.
36. NadlerC, BaruchK, KobiS, MillsE, HavivG, et al. (2010) The type III secretion effector NleE inhibits NF-kappaB activation. PLoS Pathog 6: e1000743.
37. LiS, ZhangL, YaoQ, LiL, DongN, et al. (2013) Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature 501 : 242–246.
38. PearsonJS, GioghaC, OngSY, KennedyCL, KellyM, et al. (2013) A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature 501 : 247–251.
39. LinkermannA, QianJ, LettauM, KabelitzD, JanssenO (2005) Considering Fas ligand as a target for therapy. Expert Opin Ther Targets 9 : 119–134.
40. SteinwedeK, HenkenS, BohlingJ, MausR, UeberbergB, et al. (2012) TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med 209 : 1937–1952.
41. HotchkissRS, NicholsonDW (2006) Apoptosis and caspases regulate death and inflammation in sepsis. Nature Rev Immunol 6 : 813–822.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



