-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Cross-Seeding of Misfolded Proteins: Implications for Etiology and Pathogenesis of Protein Misfolding Diseases
article has not abstract
Published in the journal: . PLoS Pathog 9(9): e32767. doi:10.1371/journal.ppat.1003537
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003537Summary
article has not abstract
Introduction
Accumulation of misfolded protein aggregates is a hallmark event in diverse diseases. These structures are able to seed their own polymerization by acting as aggregation nuclei both in vitro and in vivo. Recent studies in animal models suggest that misfolded proteins associated with different diseases can synergize in a heterologous fashion, potentiating pathological mechanisms and accelerating disease progression. The coexistence of misfolded protein aggregates has been described in patients affected by several protein misfolding disorders, suggesting a possible molecular cross-talk between pathological processes associated with different diseases. One putative mechanism for this cross-talk is a direct interaction between misfolded proteins, leading to cross-seeding of protein aggregation. This article summarizes the evidence for the cross-seeding phenomenon recently obtained in studies performed in vitro, in animal models, and in human patients, as well as the potential contribution of this mechanism to our understanding of the still elusive etiology and progression of maladies such as Alzheimer's disease, where no effective diagnostic or therapeutic strategies exist.
Misfolded Proteins, Amyloids, and the Seeding-Nucleation Model of Aggregation
Disease-associated misfolded proteins usually organize in β-sheet conformations that tend to form large aggregates and accumulate in different organs in the form of amyloid deposits, becoming toxic and leading to diverse protein misfolding diseases (PMDs), such as Alzheimer's disease (AD), transmissible spongiform encephalopathies (TSEs), Parkinson's disease (PD), and type 2 diabetes (T2D), among many others [1]. The seeding-nucleation polymerization model explains how amyloid aggregates are formed (Figure 1) [2]. Oligomeric, misfolded protein “seeds” are thought to be produced during the nucleation or lag phase, a thermodynamically unfavorable process involving various intermediates including partially denatured or mutated monomeric proteins as well as small unstable oligomers of diverse size [2], [3]. During the second step of this process, termed polymerization or exponential phase, these seeds induce a fast and exponential recruitment of the normally folded monomeric protein. As a result, a wide variety of misfolded structures are formed, ranging from small soluble oligomers to large fibrillar deposits. In vitro studies show that the additional new seeds act as templates for further polymerization, reducing the length of the lag phase (Figure 1, panel A) [2]–[4]. This seeding process could be homologous or heterologous (Figure 1, panel B) [5]. Homologous seeding has been widely documented for many of the proteins implicated in PMDs [5]. Seeding is likely the basis by which the misfolded prion protein acts as an infectious agent to propagate prion diseases [3], [6]. Interestingly, several recent exciting reports have shown that under defined experimental conditions, various other misfolded proteins can spread the pathology between cells and tissues and even induce the disease features when administered into animal models [6]–[10].
Fig. 1. Seeding-nucleation model of protein aggregation and the cross-seeding phenomenon. 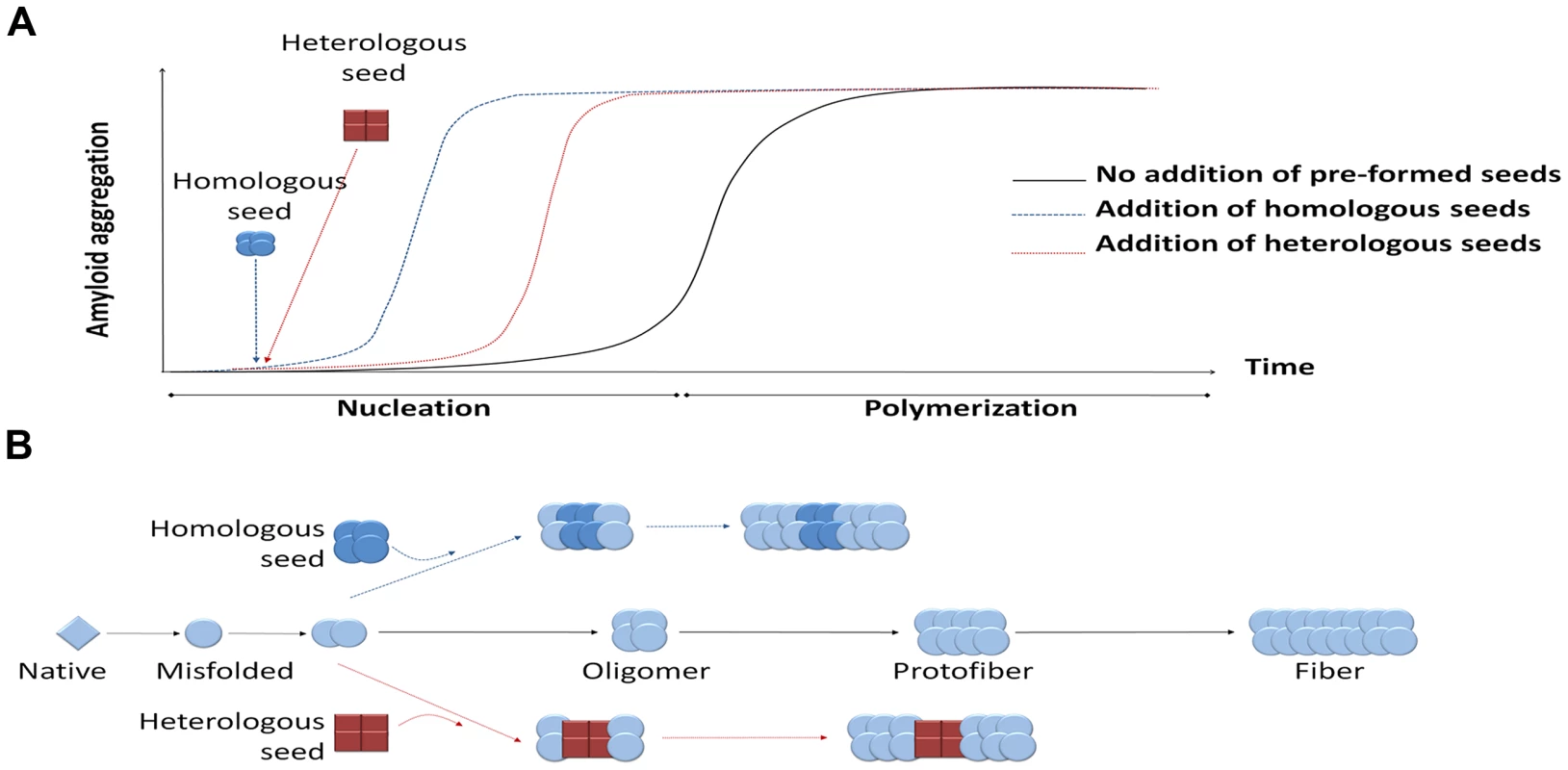
(A) Amyloid aggregates are formed following the seeding-nucleation polymerization model. This aggregation process is divided into two phases, the so-called nucleation/lag phase and the polymerization/elongation phase (solid lines). Since nuclei are formed, the aggregation increases in an exponential manner from small oligomers to fibers. The addition of preformed seeds leads to a shorter lag phase and a faster aggregation (dashed lines). (B) Seeding can occur by adding a previously formed seed, facilitating and speeding up the polymerization process. These seeds can have the same chemical nature as the nuclei, leading to a homologous seeding, or be made from a different protein, inducing a heterologous seeding or cross-seeding. Heterologous seeding, also known as “cross-seeding,” occurs when oligomers composed by one misfolded protein can promote the polymerization of a different protein [5]. Cross-seeding processes may provide a mechanistic explanation for various observations in distinct diseases, including: i) the simultaneous presence of different misfolded proteins in one disease; ii) the coexistence of more than one PMD in the same individual; iii) the epidemiological observation that one PMD may be a risk factor for development of a second PMD; and iv) the exacerbation of clinical features when various misfolded protein aggregates accumulate simultaneously. Furthermore, since many proteins form amyloid-like misfolded aggregates as part of their normal biological function [6], cross-seeding with functional amyloids may play an important and yet uncovered role in the origin of PMDs.
Cross-Seeding between Misfolded Proteins: The In Vitro Evidence
The direct interaction of misfolded proteins, a subject poorly explored so far, could play a major role in the genesis and progression of several pathological conditions. Although, not extensively studied, there are various reports showing cross-seeding interaction among several amyloidogenic proteins in vitro [11]–[17]. Some studies have suggested that the ability of certain proteins to cross-seed may depend upon the specific conformation that the misfolded seed acquires, but also on the sequence/conformation of the monomeric-soluble protein to be seeded. Experiments using hen lysozyme showed that the capability of this protein to be seeded was directly proportional to the sequence homology with the misfolded seed [11]. However, experiments in the test tube demonstrated that amyloidogenic proteins substantially differing in their sequences were able to interact and potentiate their aggregation processes, albeit with a lower efficiency than the homologous protein [5], [12]. Due to its capital involvement in AD pathogenesis, one of the most studied proteins in cross-seeding experiments is Amyloid-β (Aβ). Several studies have documented an interaction between Aβ and other misfolded proteins, including prion protein [13], tau [14], and α-synuclein [15]–[17]. From these studies an important feature is revealed: the interaction between amyloidogenic proteins may work in both directions. For example, Aβ aggregates can seed the polymerization of prions and α-synuclein, and aggregates of those proteins can also accelerate the polymerization rate of soluble Aβ. Similar findings have been observed for other proteins implicated in PMDs [5]. However, sometimes the cross-seeding effect is unidirectional, and other times the interaction results in cross-inhibition of protein aggregation. An example of the former involves the interaction between Aβ and Islet Amyloid Polypeptide (IAPP, a protein that forms amyloid aggregates in the pancreas and is involved in T2D). In vitro results showed that Aβ acts as a good seed on IAPP polymerization; however, IAPP aggregates have little or no effect over soluble Aβ oligomerization [12]. A more complex result was reported for the interaction between apolipoprotein A2 and serum amyloid A; depending upon the experimental conditions, these proteins can both cross-seed and cross-inhibit amyloid formation [18].
Interaction of Pathogenic Proteins in Animal Models
Although the putative biological consequences of cross-seeding have not been investigated in detail, there are several studies suggesting a molecular cross-talk between misfolded proteins in vivo. Perhaps the most emblematic study of cross-seeding in animal models is the one involving Aβ and tau proteins. The simultaneous brain accumulation of these proteins is the main hallmark of AD. Studies in animal models using various transgenic mice revealed that Aβ is able to accelerate the aggregation of tau; however, it seems that tau aggregates do not have the same effect over Aβ [19], [20]. These findings suggest a possibly unidirectional cross-seeding. However, it is also possible that this outcome may be mediated by indirect processes, for example, Aβ aggregates may activate certain kinases responsible for tau phosphorylation, leading to higher misfolding and aggregation of this protein [21].
Another interaction that has been studied in some detail is between α-synuclein and the misfolded proteins associated with AD. The presence of α-synuclein aggregates has been observed in approximately 50% of the AD patients, and this has been associated with more severe pathological outcomes [22]. Transgenic mice developing both Aβ and α-synuclein aggregates in their brains recapitulate some of the pathological features observed in these patients, including accelerated cognitive decline, and higher accumulation of misfolded aggregates compared to single transgenics [23]. In vitro studies involving pure α-synuclein and Aβ support the interaction between these proteins [17]. Experiments in murine models combining α-synuclein, Aβ, and tau showed that these animals display an accelerated cognitive decline as measured by loss of spatial memory [16]. As expected, this is positively correlated with the deposition of all three amyloidogenic proteins.
Another study analyzing the cross-seeding effect between misfolded proteins was done at our lab taking Aβ and infectious prions (PrPSc) as models [13]. The leading role of Aβ in AD, along with the clear phenotype of prion disease in animal models (clinical signs followed by death), led us to analyze the extent of interaction of these proteins in an in vivo situation. After intraperitoneal injection of RML prions in transgenic models of AD, we observed a significant acceleration of the prion pathology compared to wild-type littermates injected with the same agent [13]. The acceleration of the disease phenotype was dependent on the amount of Aβ aggregated in the brain. Interestingly, the deposition of Aβ plaques was higher in animals injected with prions compared to the ones receiving just buffer, suggesting that the presence of misfolded prions is also able to enhance AD features. Moreover, we found localized presence of prion protein within Aβ aggregates in addition to the diffuse PrP aggregation typically found in wild-type animals injected with this particular prion strain. These findings were complemented by cell-free assays showing a bidirectional interaction of prions and Aβ in terms of seeding and aggregation [13].
An interesting earlier study by Westermark and colleagues showed convincing evidence for exogenous induction of pathology through cross-seeding in a mouse model of AA amyloidosis [24]. In this model, deposition of AA amyloid develops spontaneously after subjecting the animals to an inflammatory challenge. The time for appearance of deposits can be shortened dramatically by administration of a small amount of tissue extract containing amyloid aggregates from a sick animal. Intravenous injection of preformed amyloid fibrils made from synthetic peptides corresponding to parts of several different misfolded proteins resulted also in acceleration of AA amyloid accumulation [24]. Remarkably, using radiolabeled heterologous, synthetic fibrils, these authors found that the injected materials deposited in various organs and that new AA-amyloid fibrils developed onto the exogenously administered seeds. The coexistence of the two heterologous proteins in the same fibrillar aggregate was confirmed by immunogold electron microscopy studies of the tissue [24]. These results provide direct evidence for cross-seeding as a putative disease mechanism.
Evidence of Cross-Talk between Protein Misfolding Disorders in Human Studies
The fact that misfolded proteins can interact and accelerate their rate of aggregation in vitro and in animal models suggests that the simultaneous occurrence of different misfolded proteins may enhance the onset and progression of certain pathological conditions. Remarkably, several epidemiological studies support this idea. One interesting and well-supported case is the interaction between AD and T2D. It has been described that about 81% of AD patients are also affected by either T2D or impaired fasting glucose [7]. In addition, and comparing with age - matched nondemented individuals, AD patients show a higher incidence of islet amyloidosis than healthy individuals. Conversely, multiple epidemiological studies have shown that T2D patients exhibit an increased risk of developing AD compared with nondiabetic individuals matched for age and sex (for reviews see [25], [26]). However, it is important to highlight that the linkage between these two diseases has not always been observed or in some studies occurs in only one direction. The mechanism for the risk association between AD and T2D is unknown and several hypotheses have been proposed, including: alterations in insulin signaling, oxidative stress, abnormal clearance capacity, hypercholesterolemia, and interactions at the level of protein misfolding [5], [27]. Although the presence of misfolded aggregates at different organs throughout the body complicates the possibility of a cross-seeding in this specific case, it is important to highlight that there is evidence for soluble oligomers circulating in biological fluids [28], [29].
Protein aggregates associated with various diseases often show the presence of other misfolded proteins within the deposits, as determined by immunostaining and co-immunoprecipitation analyses. For example, amyloid plaques in several cases of AD have been reported to contain aggregated PrP [30], [31]. Similar observations have been made in patients affected with either sporadic Creutzfeldt-Jakob disease or Gerstmann-Sträussler-Scheinker syndrome, where the presence of Aβ within the typical prion plaques observed in these diseases is clear [32], [33]. Maybe more important is the case of patients affected with the Lewy-body variant of AD [22]. Patients suffering from this “triple brain amyloidosis” show a more aggressive disease progression compared to individuals affected by classical AD. As mentioned earlier, the interaction of Aβ, α-synuclein, and tau has been also studied in vitro and in animal models.
Cross-Seeding and Alternative Pathways to the Cross-Talk between Misfolded Proteins
Although a direct interaction between misfolded proteins through ordered events of cooperative misfolding is appealing and is supported by in vitro experiments, there are various other alternative explanations for the synergistic interaction between diverse PMDs observed in animal models and human studies. The alternative pathways to the cross-seeding mechanism include among others: enhancement of cellular vulnerability, impairments in clearance machinery, excessive tissue inflammation, increase of oxidative stress, and triggering of other signal transduction pathways resulting in increase of protein misfolding. Among them, the possibility that the simultaneous presence of various protein aggregates produce defects in clearance mechanisms seems quite feasible. It is well established that the net accumulation of misfolded protein aggregates depends on the relative balance between the rate of formation of new aggregates and their removal [34], [35]. It is likely that the clearance machinery already impaired by one misfolded protein may be further diminished by a second misfolding event, leading to the faster and higher accumulation of both type of aggregates and the subsequent cellular and tissue damage [13], [36].
Exploring further the molecular cross-talk between PMDs and its underlying mechanism seems essential to understand how these complex processes participate in the origin and progression of PMDs. It is important to highlight that a large number of the PMDs cases are etiologically classified as “sporadic.” The term sporadic is used since no clear cause for the pathogenesis is known. In order to explain the sporadic origin of these diseases, several hypothesis have been proposed, mostly arguing that various processes act as risk factors, including aging, inflammation, oxidative stress, and environmental factors, etc. [37]. In addition, genetic predisposition by mutation or polymorphism in accessory proteins has been identified [38]. All these events, although well characterized, do not account for the majority of sporadic cases. The cross-seeding phenomenon could contribute to explaining, at least in part, the still unknown origin of several of these pathological conditions.
Zdroje
1. SotoC (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4 : 49–60.
2. JarrettJT, LansburyPTJr (1993) Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73 : 1055–1058.
3. SotoC, EstradaL, CastillaJ (2006) Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci 31 : 150–155.
4. KociskoDA, ComeJH, PriolaSA, ChesebroB, RaymondGJ, et al. (1994) Cell-free formation of protease-resistant prion protein. Nature 370 : 471–474.
5. MoralesR, GreenKM, SotoC (2009) Cross currents in protein misfolding disorders: interactions and therapy. CNS Neurol Disord Drug Targets 8 : 363–371.
6. SotoC (2012) Transmissible proteins: expanding the prion heresy. Cell 149 : 968–977.
7. JuckerM, WalkerLC (2011) Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol 70 : 532–540.
8. BrundinP, MelkiR, KopitoR (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11 : 301–307.
9. AguzziA (2009) Cell biology: beyond the prion principle. Nature 459 : 924–925.
10. WestermarkGT, WestermarkP (2010) Prion-like aggregates: infectious agents in human disease. Trends Mol Med 16 : 501–507.
11. KrebsMR, Morozova-RocheLA, DanielK, RobinsonCV, DobsonCM (2004) Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci 13 : 1933–1938.
12. O'NuallainB, WilliamsAD, WestermarkP, WetzelR (2004) Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem 279 : 17490–17499.
13. MoralesR, EstradaLD, Diaz-EspinozaR, Morales-ScheihingD, JaraMC, et al. (2010) Molecular cross talk between misfolded proteins in animal models of Alzheimer's and prion diseases. J Neurosci 30 : 4528–4535.
14. GuoJP, AraiT, MiklossyJ, McGeerPL (2006) Abeta and tau form soluble complexes that may promote self aggregation of both into the insoluble forms observed in Alzheimer's disease. Proc Natl Acad Sci U S A 103 : 1953–1958.
15. TsigelnyIF, CrewsL, DesplatsP, ShakedGM, SharikovY, et al. (2008) Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer's and Parkinson's diseases. PLoS ONE 3: e3135 doi:10.1371/journal.pone.0003135
16. ClintonLK, Blurton-JonesM, MyczekK, TrojanowskiJQ, LaFerlaFM (2010) Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 30 : 7281–7289.
17. MandalPK, PettegrewJW, MasliahE, HamiltonRL, MandalR (2006) Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer's and Parkinson's in dementia with Lewy body disease. Neurochem Res 31 : 1153–1162.
18. YanJ, FuX, GeF, ZhangB, YaoJ, et al. (2007) Cross-seeding and cross-competition in mouse apolipoprotein A-II amyloid fibrils and protein A amyloid fibrils. Am J Pathol 171 : 172–180.
19. GotzJ, ChenF, Van DorpeJ, NitschRM (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293 : 1491–1495.
20. LewisJ, DicksonDW, LinWL, ChisholmL, CorralA, et al. (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293 : 1487–1491.
21. FerrerI (2004) Stress kinases involved in tau phosphorylation in Alzheimer's disease, tauopathies and APP transgenic mice. Neurotox Res 6 : 469–475.
22. HamiltonRL (2000) Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 10 : 378–384.
23. MasliahE, RockensteinE, VeinbergsI, SagaraY, MalloryM, et al. (2001) beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A 98 : 12245–12250.
24. JohanK, WestermarkG, EngstromU, GustavssonA, HultmanP, et al. (1998) Acceleration of amyloid protein A amyloidosis by amyloid-like synthetic fibrils. Proc Natl Acad Sci U S A 95 : 2558–2563.
25. BiesselsGJ, StaekenborgS, BrunnerE, BrayneC, ScheltensP (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5 : 64–74.
26. Sims-RobinsonC, KimB, RoskoA, FeldmanEL (2010) How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol 6 : 551–559.
27. BiesselsGJ, KappelleLJ (2005) Increased risk of Alzheimer's disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans 33 : 1041–1044.
28. FukumotoH, TokudaT, KasaiT, IshigamiN, HidakaH, et al. (2010) High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J 24 : 2716–2726.
29. GaoCM, YamAY, WangX, MagdangalE, SalisburyC, et al. (2010) Aβ40 oligomers identified as a potential biomarker for the diagnosis of Alzheimer's disease. PLoS ONE 5: e15725 doi:10.1371/journal.pone.0015725
30. FerrerI, BlancoR, CarmonaM, PuigB, RiberaR, et al. (2001) Prion protein expression in senile plaques in Alzheimer's disease. Acta Neuropathol (Berl) 101 : 49–56.
31. DebatinL, StrefferJ, GeissenM, MatschkeJ, AguzziA, et al. (2008) Association between deposition of beta-amyloid and pathological prion protein in sporadic Creutzfeldt-Jakob disease. Neurodegener Dis 5 : 347–354.
32. TsuchiyaK, YagishitaS, IkedaK, SanoM, TakiK, et al. (2004) Coexistence of CJD and Alzheimer's disease: an autopsy case showing typical clinical features of CJD. Neuropathology 24 : 46–55.
33. MiyazonoM, KitamotoT, IwakiT, TateishiJ (1992) Colocalization of prion protein and beta protein in the same amyloid plaques in patients with Gerstmann-Straussler syndrome. Acta Neuropathol (Berl) 83 : 333–339.
34. MawuenyegaKG, SigurdsonW, OvodV, MunsellL, KastenT, et al. (2010) Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science 330 : 1774.
35. TanziRE, MoirRD, WagnerSL (2004) Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron 43 : 605–608.
36. GidalevitzT, KikisEA, MorimotoRI (2010) A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr Opin Struct Biol 20 : 23–32.
37. BarnesDE, YaffeK (2011) The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 10 : 819–828.
38. BertramL, LillCM, TanziRE (2010) The genetics of Alzheimer disease: back to the future. Neuron 68 : 270–281.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Cross-Serotype Immunity Induced by Immunization with a Conserved Rhinovirus Capsid Protein
- The CLIP-Domain Serine Protease Homolog SPCLIP1 Regulates Complement Recruitment to Microbial Surfaces in the Malaria Mosquito
- Aggressive Chemotherapy and the Selection of Drug Resistant Pathogens
- Host Adaptation Is Contingent upon the Infection Route Taken by Pathogens
- Acute Neonatal Infections ‘Lock-In’ a Suboptimal CD8+ T Cell Repertoire with Impaired Recall Responses
- Lymph Node Colonization Dynamics after Oral Typhimurium Infection in Mice
- Highly Significant Antiviral Activity of HIV-1 LTR-Specific Tre-Recombinase in Humanized Mice
- Emerging and Emerged Pathogenic Species: Beyond the Paradigm
- Cross-Seeding of Misfolded Proteins: Implications for Etiology and Pathogenesis of Protein Misfolding Diseases
- Emergence of the Middle East Respiratory Syndrome Coronavirus
- Memory of Infections: An Emerging Role for Natural Killer Cells
- Death Be Not Proud—Cell Death Control in Plant Fungal Interactions
- Self and Non-self Discrimination of Intracellular Membranes by the Innate Immune System
- Innate Immune Sensing of Flaviviruses
- Bringing Culture to the Uncultured: and Lessons for Obligate Intracellular Bacterial Pathogens
- Atomic Force Microscopy: A New Look at Pathogens
- Methionine Biosynthesis in Is Tightly Controlled by a Hierarchical Network Involving an Initiator tRNA-Specific T-box Riboswitch
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Memory of Infections: An Emerging Role for Natural Killer Cells
- Emergence of the Middle East Respiratory Syndrome Coronavirus
- Emerging and Emerged Pathogenic Species: Beyond the Paradigm
- Death Be Not Proud—Cell Death Control in Plant Fungal Interactions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



