-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from
article has not abstract
Published in the journal: . PLoS Pathog 9(8): e32767. doi:10.1371/journal.ppat.1003433
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003433Summary
article has not abstract
Introduction
Like other free-living nematodes, the one-millimeter-long nematode Caenorhabditis elegans lives in soils and composts rich in microorganisms, including human microbial pathogens. In the laboratory, C. elegans animals are typically propagated by feeding them Escherichia coli. This bacterium is effectively disrupted by the C. elegans pharyngeal grinder and essentially no intact bacterial cells can be found in the intestinal lumen of healthy, young animals. Once in the gut, however, pathogenic bacteria are capable of proliferating, invading host cells, and killing C. elegans by infectious processes. Bacterial pathogens can also adhere to the cuticle of the nematode, causing a defensive swelling response of the epidermal cells. While C. elegans lacks adaptive immunity, it responds to pathogen exposure by avoiding certain potentially pathogenic bacteria and by activating an inducible innate immune system. Thus, pathogen avoidance, grinding, swelling, peristalsis, and secretion of antimicrobial substances prevent microbial colonization of C. elegans by bacterial pathogens. Increasing evidence highlights the role of the C. elegans nervous system in the control of some of these immune responses against bacterial pathogens.
Importance of Using C. elegans to Study Neural-Immune Communications
Activation of the innate immune system upon pathogen recognition results in a rapid activation of microbial killing pathways that need to be highly controlled because deficiencies or excesses in the response have deleterious consequences. Indeed, the activation of the immune system appears to account for the major physiological, metabolic, and pathological responses to infections. Furthermore, while insufficient immune responses can lead to infection and cancer, excessive immune responses have been linked to conditions such as Crohn's disease, rheumatoid arthritis, atherosclerosis, diabetes, and Alzheimer's disease. Increasing evidence indicates that metazoans take advantage of the nervous system to receive inputs from infected sites and integrate them in a reflexive manner to coordinate appropriate immune responses.
The 1986 Nobel laureate Rita Levi-Montalcini commented during her Nobel lecture how the complexity of neural-immune networks, which at that time were already known to be closely interrelated, opens endless possibilities for interventions. However, given the complexity of the nervous and immune systems of most model organisms, the precise mechanisms by which the two systems influence each other remain largely understudied. C. elegans is emerging as a model system that may help to fill this gap and identify neural mechanisms capable of regulating immune responses.
The nervous system of most model organisms consist of billions or hundreds of billions of neurons, whose wiring at the cellular level is far from characterized. In contrast, the information about the identity, morphology, and synaptic connectivity of each of the 302 neurons in the nervous system of C. elegans is known. Thus, this information provides a unique opportunity to study the activity of specific neurons involved in neural-immune communications and to study the flow of signals between neurons in response to pathogen infection.
Neurons and Neural Signals Involved in the Control of Immune Responses in C. elegans
The study of neurally expressed G-protein coupled receptors, NPR-1 and OCTR-1, highlights the role of specific neurons in the control of immune responses [1], [2]. Animals carrying mutations in the npr-1 gene exhibit enhanced susceptibility to infections by Pseudomonas aeruginosa, Salmonella enterica, and Enterococcus faecalis, suggesting that NPR-1–expressing neurons control immune pathways important for defense against a broad range of pathogens. Interestingly, avoidance of certain bacterial pathogens, such as P. aeruginosa, is also an important defense mechanism in C. elegans that is regulated by the NPR-1 neural circuit [1], [3]. A full-genome expression analysis on animals with altered neural function due to mutation in npr-1 showed an enrichment in genes that are markers of innate immune responses, most of which are expressed in the intestine and/or regulated by a conserved PMK-1/p38 mitogen-activated protein kinase (MAPK) signaling pathway [1]. This indicates that the nervous system controls not only avoidance to certain pathogens but also the expression of immune genes in somatic tissues. The use of mosaic animals expressing NPR-1 in selected cells and neurally ablated animals link NPR-1–expressing neurons AQR, PQR, and URX to the control of immunity [1] (Figure 1).
Fig. 1. Schematic of the neural control of different pathways required for immunity in C. elegans. 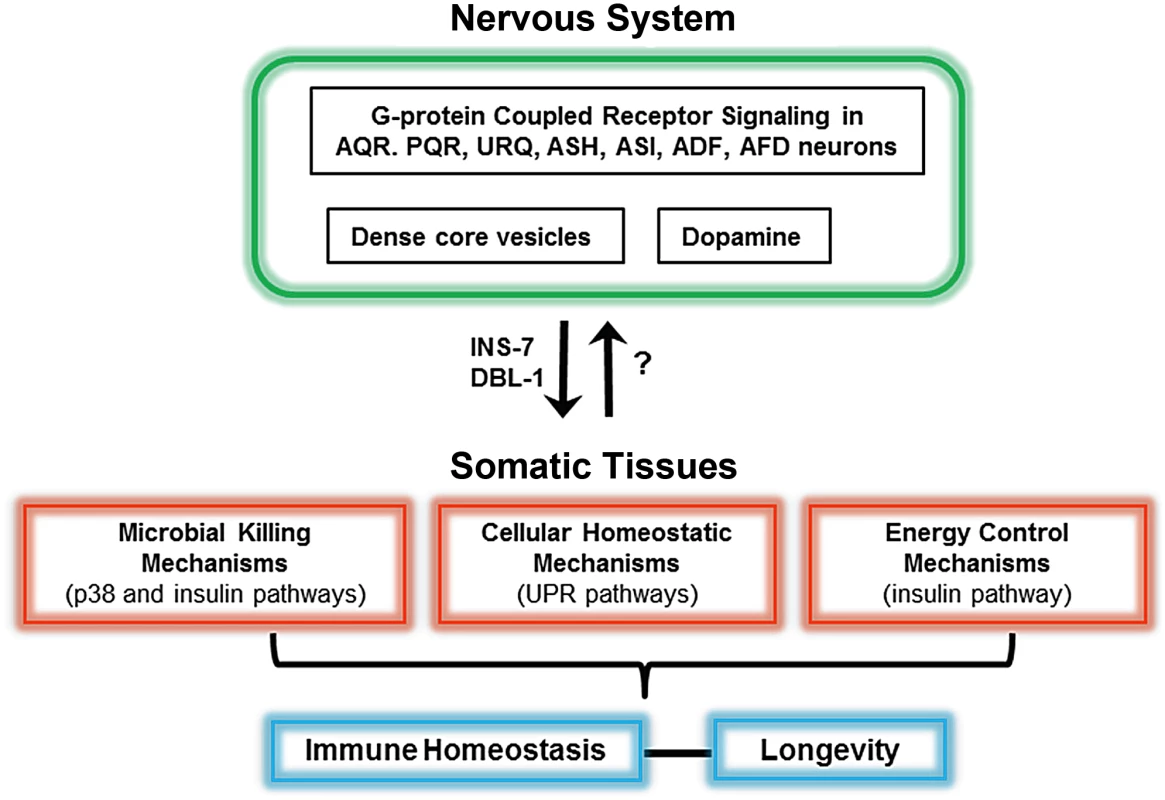
At least seven neurons in the nervous system control microbial killing pathways and cellular homeostatic pathways. In addition to AQR, PQR, URX, ASH, and ASI neurons, dopaminergic neurons also control the p38 MAPK pathway. While ASH and ASI control the unfolded protein response (UPR), ADF and AFD control both the UPR and the insulin pathway. Neurally produced INS-7 and DBL-1 target somatic tissues to control immunity. A fine-tuned control of protein homeostasis and metabolism by the nervous system seems to be important to maintain immune homeostasis. Similar studies using neuron-specific rescues and genetic ablation of neurons indicate that OCTR-1 functions in ASH and ASI neurons to suppress the p38 MAPK pathway and unfolded protein response (UPR) genes that are expressed in pharyngeal and intestinal cells [2], [4] (Figure 1). A unique component of GPCR signaling in C. elegans, the GPCR adaptor protein arrestin-1, functions downstream OCTR-1 and NPR-1 in the nervous system [5], providing further evidence for the role of AQR, PQR, URX, ASH, and ASI in the control of innate immunity. In addition, the use of mosaic animals expressing arrestin-1 only in ADF and AFD indicate that these cells also control C. elegans immunity [5] (Figure 1).
OCTR-1 is a catecholamine receptor related to vertebrate adrenergic receptors whose ligand is octopamine, an invertebrate equivalent of noradrenaline. In mammals, it is known that noradrenaline mediates responses to acute stress that result in a decreased immune response. The findings indicating that immunity can be activated by inhibiting a pathway involved in the arousal response suggest that there is a strong selective advantage to suppressing immune responses during stress, and that similar chemical cues in the nervous system of C. elegans and mammals may have similar immune consequences. In C. elegans, chemosensory neurons and serotonin signaling also control pathogen detection and avoidance [6], [7], but it is unclear to what extent those responses to pathogen exposure are found across metazoans. In addition to octopamine and serotonin, dopamine also appears to play a role in the control of immunity as dopaminergic neurons participate in a circuit that makes C. elegans more resistant to reinfections with enteropathogenic E. coli [8]. This dopamine-dependent resistance to reinfection mechanism requires both p38 MAPK and the insulin-like pathway that regulates lifespan and immunity [8] (Figure 1).
C. elegans neurons are known to express numerous secreted peptides of the transforming growth factor beta (TGF beta) family, the insulin family, and neuropeptide families. This myriad of secreted factors has the potential to act at a distance to modulate various physiological processes by regulating the function of neural and nonneural cells throughout the animal. It is known that the insulin-like neuropeptide INS-7 released from dense-core vesicles in the nervous system controls the expression of immune genes in the C. elegans intestinal cells [9], [10]. Moreover, the TGF beta receptor ligand DBL-1, which is produced by the nervous system, regulates the expression of immune genes that are expressed in the epidermis of C. elegans [11] (Figure 1).
Role of the Nervous System in the Control of Proteostasis during Immune Activation
In metazoans, the endoplasmic reticulum (ER) is an important intracellular organelle in which newly synthesized proteins are folded, assembled, and matured. When protein homeostasis, or proteostasis, is disrupted by environmental stresses or changes in physiological conditions, a series of unfolded protein response (UPR) pathways are activated. When the increased demand for protein folding is not met with an appropriate UPR, the accumulation of damaged proteins typically results in cell death. In fully immunocompetent nematodes, bacterial infections are controlled by a range of immune effectors that are highly expressed in pharyngeal and intestinal cells. In response to bacterial infection, genes involved in the UPR are also upregulated, indicating that the increased demand for protein folding in the ER must be successfully alleviated for a complete immune response to be mounted [2], [4], [5].
To maintain organismal homeostasis in response to bacterial infections, C. elegans uses both canonical and noncanonical UPR pathways. The X-box binding protein 1 (XBP-1) branch of the UPR, which is the most conserved branch of the UPR across species, is activated in response to exposure to pore-forming toxins by a mechanism that requires the p38 MAPK pathway [12]. The XBP - 1 branch is also activated by exposure to Pseudomonas aeruginosa and it is essential for larval development in infected animals [13], [14]. A noncanonical UPR pathway that is independent of XBP-1 is also activated by P. aeruginosa infection, and is required for survival when the animals are infected with P. aeruginosa or S. enterica [2], [15]. This noncanonical UPR pathway comprises a family of genes encoding prion-like glutamine[Q]/asparagine[N]-rich domain-bearing proteins that are activated in xbp-1 mutant animals when ER stress is induced by tunicamycin treatment. Functional studies demonstrate that these genes encode UPR proteins that function in parallel with the canonical UPR pathway [16].
Maintenance of protein homeostasis in response to the stress caused by P. aeruginosa infection is controlled at the cell nonautonomous level by OCTR-1. OCTR-1 signaling in ASH and ASI neurons controls the activation of noncanonical UPR genes that are upregulated in response to pathogen infection and strongly expressed in nonneuronal tissues such as those corresponding to the pharynx and the intestine, primary interfaces between host cells involved in immune responses and bacterial pathogens [2], [5]. Interestingly, OCTR-1 does not control the canonical XPB-1 branch of the UPR during development, but it does gain control of it during adulthood [4] (Figure 1). This temporal control of the canonical UPR pathway by the nervous system suggests that the high demand for protein folding that takes place during development does not need to be fine-tuned by cell nonautonomous mechanisms. In contrast, immune activation in adult animals seems to be tightly controlled by neural influences. The activation of the UPR in response to toxins and infections requires p38 MAPK. Further experimentation will be required to identify potential neural mechanisms that fine-tune this interaction during the immune response.
Concluding Remarks
Animals have evolved sophisticated mechanisms to modify specific properties in response to changes such as those that take place during response to microbial infections. The nervous system, which can sense many types of environmental stimuli, appears to integrate inflammatory signals to maintain a balanced immune response against invading microorganisms. In response to pathogen infection, the C. elegans nervous system appears to play a role in the maintenance of organismal homeostasis by controlling killing mechanisms such as those regulated by the p38 MAPK pathway, cellular stress pathways such as the UPR, and metabolic pathways such as the insulin signaling pathway (Figure 1).
The studies discussed here have linked specific neurons and neuroendocrine signals to the control of immune responses in C. elegans. However, they do not provide insights into the effects of pathogen infection on neuron activity or the flow of information required for the control of organismal homeostasis during immune activation. A unique advantage to neural-related studies in C. elegans is the unparalleled characterization of its nervous system at the cellular level. The precise and characteristic identity of each of the 302 neurons in the C. elegans nervous system, detailed anatomical reconstructions by electron microscopy, and functional data provide a map of the synaptic connectivity for the entire nervous system. This information will be invaluable to fully dissect the neural-immune pathways involved in the control of immune homeostasis in C. elegans. A detailed understanding at the molecular level of how the nervous system and the immune system cross communicate should yield potential new targets to treat not only a variety of infectious diseases but also a range of conditions that arise as a consequence of malfunctioning immune responses.
Zdroje
1. StyerKL, SinghV, MacoskoE, SteeleSE, BargmannCI, et al. (2008) Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 322 : 460–464.
2. SunJ, SinghV, Kajino-SakamotoR, AballayA (2011) Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 332 : 729–732.
3. ReddyKC, AndersenEC, KruglyakL, KimDH (2009) A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science 323 : 382–384.
4. SunJ, LiuY, AballayA (2012) Organismal regulation of XBP-1-mediated unfolded protein response during development and immune activation. EMBO Rep 13 : 855–860.
5. SinghV, AballayA (2012) Endoplasmic reticulum stress pathway required for immune homeostasis is neurally controlled by arrestin-1. J Biol Chem 287 : 33191–33197.
6. PradelE, ZhangY, PujolN, MatsuyamaT, BargmannCI, et al. (2007) Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci U S A 104 : 2295–2300.
7. ZhangY, LuH, BargmannCI (2005) Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438 : 179–184.
8. AnyanfulA, EasleyKA, BenianGM, KalmanD (2009) Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 5 : 450–462.
9. KawliT, TanM-W (2008) Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol 9 : 1415–1424.
10. EvansEA, KawliT, TanM-W (2008) Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 4: e1000175 doi:10.1371/journal.ppat.1000175
11. ZugastiO, EwbankJJ (2009) Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 10 : 249–256.
12. BischofLJ, KaoC-Y, LosFC, GonzalezMR, ShenZ, et al. (2008) Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog 4: e1000176 doi:10.1371/journal.ppat.1000176
13. RichardsonCE, KooistraT, KimDH (2010) An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 463 : 1092–1095.
14. RichardsonCE, KinkelS, KimDH (2011) Physiological IRE-1-XBP-1 and PEK-1 signaling in Caenorhabditis elegans larval development and immunity. PLoS Genet 7: e1002391 doi:10.1371/journal.pgen.1002391
15. HaskinsKA, RussellJF, GaddisN, DressmanHK, AballayA (2008) Unfolded protein response genes regulated by CED-1 are required for Caenorhabditis elegans innate immunity. Dev Cell 15 : 87–97.
16. UranoF, CalfonM, YonedaT, YunC, KiralyM, et al. (2002) A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol 158 : 639–646.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits fromČlánek X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Biosecurity Implications of New Technology and Discovery in Plant Virus Research
- Pathogenic Yeasts Deploy Cell Surface Receptors to Acquire Iron in Vertebrate Hosts
- Asparagine Repeats in Proteins: Good for Nothing?
- Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from
- Intact Type I Interferon Production and IRF7 Function in Sooty Mangabeys
- Prion Replication Occurs in Endogenous Adult Neural Stem Cells and Alters Their Neuronal Fate: Involvement of Endogenous Neural Stem Cells in Prion Diseases
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
- HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women
- Crystal Structure of the Full-Length Japanese Encephalitis Virus NS5 Reveals a Conserved Methyltransferase-Polymerase Interface
- Quantitative Models of the Dose-Response and Time Course of Inhalational Anthrax in Humans
- Stabilization of Myc through Heterotypic Poly-Ubiquitination by mLANA Is Critical for γ-Herpesvirus Lymphoproliferation
- The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Biofilms
- A Novel Role for Pro-Coagulant Microvesicles in the Early Host Defense against
- Molecular Cloning and Characterization of Novel Glutamate-Gated Chloride Channel Subunits from
- CD36 Recruits αβ Integrin to Promote Cytoadherence of -Infected Erythrocytes
- X-Box Binding Protein 1 (XBP1s) Is a Critical Determinant of Homoserine Lactone-Mediated Apoptosis
- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Chikungunya Virus 3′ Untranslated Region: Adaptation to Mosquitoes and a Population Bottleneck as Major Evolutionary Forces
- Mucin Gene (PoMuc) Expression: Epigenetic Control to Shape Adaptation to a New Host
- Determinants of GPI-PLC Localisation to the Flagellum and Access to GPI-Anchored Substrates in Trypanosomes
- The Smallest Capsid Protein Mediates Binding of the Essential Tegument Protein pp150 to Stabilize DNA-Containing Capsids in Human Cytomegalovirus
- Understanding Human Variation in Infectious Disease Susceptibility through Clinical and Cellular GWAS
- Human Genetic Susceptibility to Invasive Aspergillosis
- Host Immune Response to Intestinal Amebiasis
- Fungi Infecting Plants and Animals: Killers, Non-Killers, and Cell Death
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Discovery of Anthelmintic Drug Targets and Drugs Using Chokepoints in Nematode Metabolic Pathways
- Host Immune Response to Intestinal Amebiasis
- Bed Bugs and Infectious Disease: A Case for the Arboviruses
- Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



