-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Reactive Oxygen Species Production and Survivorship in with Artificial Infection Types
Heterologous transinfection with the endosymbiotic bacterium Wolbachia has been shown previously to induce pathogen interference phenotypes in mosquito hosts. Here we examine an artificially infected strain of Aedes polynesiensis, the primary vector of Wuchereria bancrofti, which is the causative agent of Lymphatic filariasis (LF) throughout much of the South Pacific. Embryonic microinjection was used to transfer the wAlbB infection from Aedes albopictus into an aposymbiotic strain of Ae. polynesiensis. The resulting strain (designated “MTB”) experiences a stable artificial infection with high maternal inheritance. Reciprocal crosses of MTB with naturally infected wild-type Ae. polynesiensis demonstrate strong bidirectional incompatibility. Levels of reactive oxygen species (ROS) in the MTB strain differ significantly relative to that of the wild-type, indicating an impaired ability to regulate oxidative stress. Following a challenge with Brugia pahangi, the number of filarial worms achieving the infective stage is significantly reduced in MTB as compared to the naturally infected and aposymbiotic strains. Survivorship of MTB differed significantly from that of the wild-type, with an interactive effect between survivorship and blood feeding. The results demonstrate a direct correlation between decreased ROS levels and decreased survival of adult female Aedes polynesiensis. The results are discussed in relation to the interaction of Wolbachia with ROS production and antioxidant expression, iron homeostasis and the insect immune system. We discuss the potential applied use of the MTB strain for impacting Ae. polynesiensis populations and strategies for reducing LF incidence in the South Pacific.
Published in the journal: . PLoS Pathog 8(12): e32767. doi:10.1371/journal.ppat.1003075
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003075Summary
Heterologous transinfection with the endosymbiotic bacterium Wolbachia has been shown previously to induce pathogen interference phenotypes in mosquito hosts. Here we examine an artificially infected strain of Aedes polynesiensis, the primary vector of Wuchereria bancrofti, which is the causative agent of Lymphatic filariasis (LF) throughout much of the South Pacific. Embryonic microinjection was used to transfer the wAlbB infection from Aedes albopictus into an aposymbiotic strain of Ae. polynesiensis. The resulting strain (designated “MTB”) experiences a stable artificial infection with high maternal inheritance. Reciprocal crosses of MTB with naturally infected wild-type Ae. polynesiensis demonstrate strong bidirectional incompatibility. Levels of reactive oxygen species (ROS) in the MTB strain differ significantly relative to that of the wild-type, indicating an impaired ability to regulate oxidative stress. Following a challenge with Brugia pahangi, the number of filarial worms achieving the infective stage is significantly reduced in MTB as compared to the naturally infected and aposymbiotic strains. Survivorship of MTB differed significantly from that of the wild-type, with an interactive effect between survivorship and blood feeding. The results demonstrate a direct correlation between decreased ROS levels and decreased survival of adult female Aedes polynesiensis. The results are discussed in relation to the interaction of Wolbachia with ROS production and antioxidant expression, iron homeostasis and the insect immune system. We discuss the potential applied use of the MTB strain for impacting Ae. polynesiensis populations and strategies for reducing LF incidence in the South Pacific.
Introduction
Lymphatic filariasis (LF) affects 120 million people globally and has been a leading cause of morbidity in South Pacific regions [1]. An ongoing global campaign to eliminate LF relies upon mass drug administration (MDA) strategies. However, because of inherent issues associated with MDA, such as efficacy of antifilarial drugs and public compliance with drug regimens, an integrated approach that targets the vector has been suggested for the successful control of LF in some regions, such as the South Pacific.
Aedes polynesiensis is the primary vector of Wuchereria bancrofti, the filarial nematode that causes LF in the South Pacific [2]. This mosquito is naturally infected with Wolbachia, a maternally inherited endosymbiont that infects a broad range of invertebrates [3], [4]. Wolbachia infection in mosquitoes can induce cytoplasmic incompatibility (CI), a form of conditional sterility that results in early embryonic arrest when a Wolbachia infected male mates with an uninfected female or one harboring a different Wolbachia type [3]. Public health strategies under development are based upon manipulating Wolbachia induced CI in important mosquito species, either to suppress the population through releases of incompatible males or to harness CI as a gene-drive mechanism for spreading useful phenotypes, such as disease resistance, into a targeted population [5]–[7]. A recent proof of concept for this approach comes from a program in Australia that has successfully replaced an existing Ae. aegypti population with an artificially infected mosquito [8], [9].
Wolbachia infections, both natural and artificial, have been shown to interfere with pathogen development within the mosquito host. The presence of a naturally occurring Wolbachia infection in Drosophila protects flies from virus-induced mortality [10]. In mosquitoes, examples include the natural Wolbachia infection within Culex quinquefasciatus, which is associated with a significant reduction in West Nile virus dissemination and transmission rates [11]. Artificial Wolbachia infections have been observed to affect dengue, chikungunya, Plasmodium and filarial worms in Ae. aegypti [12], [13]. Formation of Plasmodium falciparum oocysts was inhibited in Anopheles gambiae that were somatically inoculated with Wolbachia [14].
Although the mechanism underlying pathogen interference is unknown, a possible explanation is the association between artificial Wolbachia infections and increased expression of key mosquito immune factors such as defensins, cecropins and Toll pathway genes [12], [13], [15]. In addition, recent studies have shown that artificial Wolbachia infections are associated with increased oxidative stress in the form of reactive oxygen species (ROS) [16], [17]. ROS are produced as the byproduct of aerobic metabolism [18] and can have detrimental effects on fecundity [19] and survival post blood meal [20]. However, a positive effect of high ROS levels is the inhibition of parasites within the mosquito host [21], [22]. Furthermore, elevated levels of ROS in Ae. aegypti are linked to the activation of the Toll immune pathway [17].
In this study, embryonic microinjection [23], [24] was used to introduce the wAlbB infection from Ae. albopictus into Ae. polynesiensis. Prior transfer of the wAlbB infection into Ae. aegypti induced strong CI in the resulting strain [25] and increased host viral resistance to dengue by increasing ROS levels and elevating expression of immune genes [15], [17]. In addition, the introduction of an artificial Wolbachia infection decreased filarial competence in Ae. aegypti [12]. We hypothesized that artificial introduction of the wAlbB infection into Ae. polynesiensis might facilitate a similar immunological response and reduce the intensity of filarial worm infection. Unlike other mosquito vector species, relatively little genomic information and molecular tools are available for Ae. polynesiensis, making examination of immune gene expression difficult. However, ROS measurement methods are a relatively robust indicator of immune system activation and have been applied to numerous species, including mosquito vectors [17], [22], [26], [27]. Here, we have compared ROS levels between the Ae. polynesiensis strains infected with different Wolbachia types. The results show an association between Wolbachia type and ROS levels. Comparisons of the Ae. polynesiensis strains show significant differences in their ability to support Brugia pahangi development. We discuss the results in relation to a possible interaction between Wolbachia infection type, ROS levels and filarial competency and the potential application to public health strategies targeting decreased LF incidence.
Results
Generation and characterization of the MTB strain
The MTB strain of Ae. polynesiensis was generated by microinjecting embryos of the aposymbiotic APMT strain with cytoplasm from naturally superinfected Ae. albopictus embryos (HOU strain). The mosquito strains used in the injection experiment are listed in Table 1, and the outcome of one injection experiment is shown in Table 2. Of 134 injected APMT embryos, 17 G0 females and 12 G0 males survived to adulthood, seven and six of which were infected with Wolbachia, respectively.
Tab. 2. Survival of microinjected <i>Ae. polynesiensis</i> embryos for each life stage and the resulting <i>Wolbachia</i> infection rates in the G<sub>0</sub> adults. 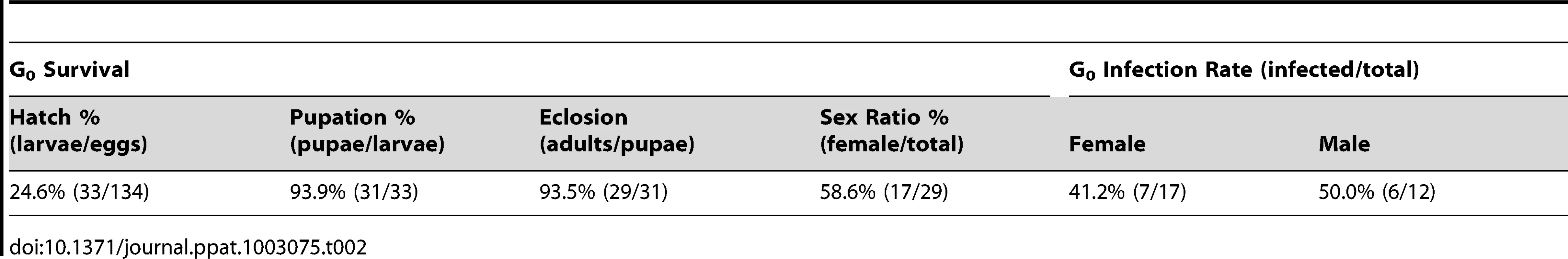
The seven PCR positive G0 females were screened for specific infection type (Table 3). A G0 female infected with both wAlbA and wAlbB was chosen for further selection. Her daughters (G1) had a 60% infection rate, with the majority of females single-infected with wAlbB only. PCR testing and selection of the subsequent generations were unable to sustain the superinfection. Thus, the resulting MTB strain is infected with wAlbB only. Using PCR-guided selection, infected females were continuously outcrossed with APMT males until G6. Beginning at G7, MTB females were mated with MTB males. Subsequent to G7, periodic testing of the MTB strain confirmed that the infection is stable and maternal inheritance rates remain at 100% (data not shown).
Tab. 3. Wolbachia infection following the generation of the MTB strain. 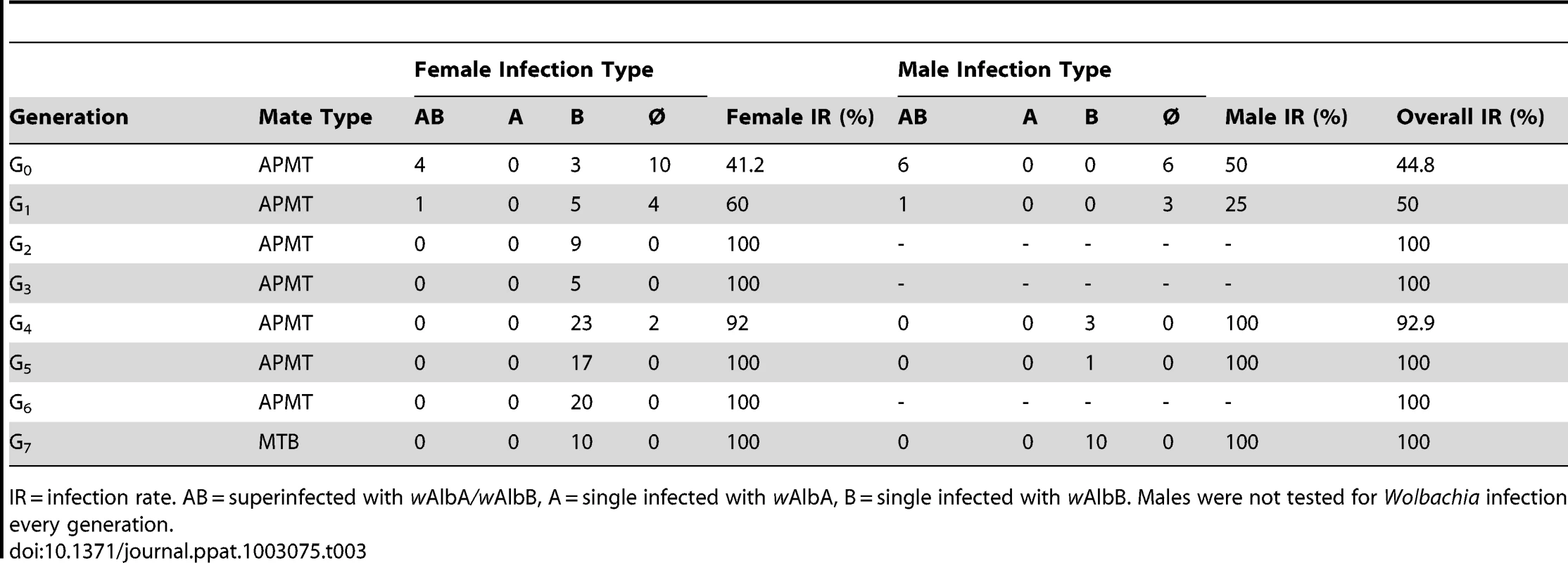
IR = infection rate. AB = superinfected with wAlbA/wAlbB, A = single infected with wAlbA, B = single infected with wAlbB. Males were not tested for Wolbachia infection every generation. Crosses were performed to examine for CI. The results demonstrate bidirectional incompatibly between APM and MTB. High egg hatch was observed in crosses between similar males and females (Table 4). In contrast, few eggs hatched in reciprocal crosses between the strain types. Incompatible crosses were significantly different from the controls (H = 12.5, df = 3, p = 0.0059).
Tab. 4. Egg hatch rates resulting from crosses. 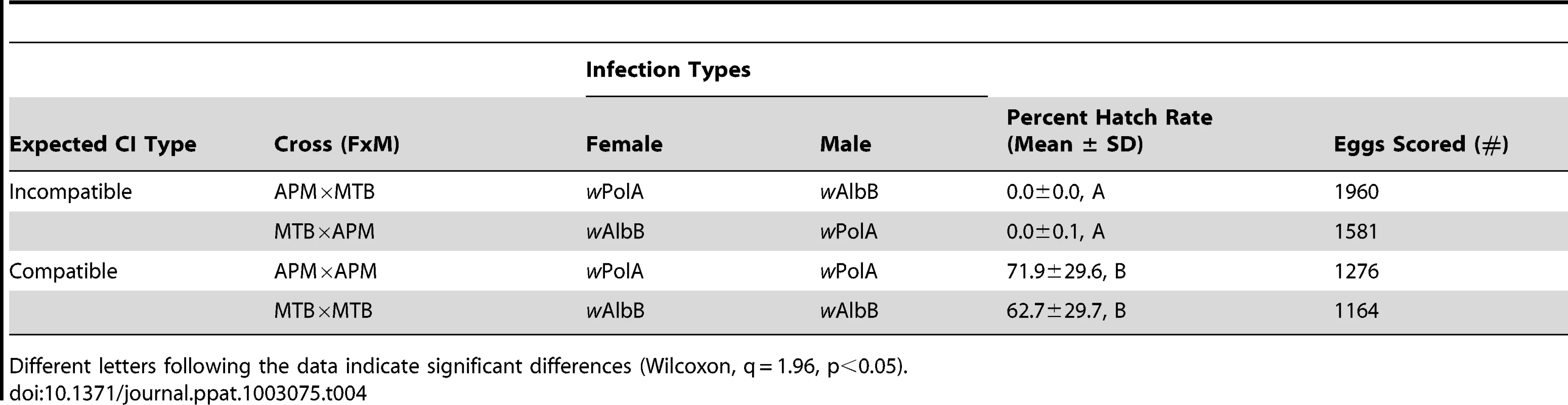
Different letters following the data indicate significant differences (Wilcoxon, q = 1.96, p<0.05). ROS levels in Ae. polynesiensis strains
ROS levels can be significantly affected in mosquitoes that are artificially infected with Wolbachia [17]. To examine for a Wolbachia-mediated effect in Ae. polynesiensis, we compared ROS levels in young adult females of MTB, APM and APMT. In addition to examining females fed sucrose only, we provided females with a blood meal to examine for an effect of blood feeding on ROS levels, which has been observed in prior studies [22], [28]. The results (Figure 1) show that blood meal status had a significant effect on ROS levels, but only for the strains in which Wolbachia had been manipulated (i.e., not in the naturally infected strain). Specifically, a model with strain and blood meal status as factors and ROS level as the variable was significant (GLM: χ2 = 38.2, df = 5, p<0.0001). Blood meal status was significant (GLM: χ2 = 27.2, df = 1, p<0.0001), while the overall strain effect was not significant (GLM: χ2 = 5.12, df = 2, p = 0.07). A significant interactive effect was observed for strain×blood meal status (GLM: χ2 = 19.3, df = 2, p<0.0001). Following a blood meal, ROS levels in the APM strain remained similar to those observed in sucrose fed females (ANOVA, F1,8 = 0.11, p = 0.75). However, significant decreases in ROS levels are observed for blood fed females of APMT (ANOVA, F1,8 = 19.09, p<0.05) and MTB (ANOVA, F1,8 = 35.08, p<0.001). Post hoc Tukey HSD tests determined that after blood feeding, APM had significantly higher levels of ROS than APMT (p<0.05) and MTB (p<0.05), the latter of which were equivalent (p = 0.9).
Fig. 1. ROS levels in Ae. polynesiensis strains. 
The concentration of H2O2 was measured in APM, APMT and MTB after feeding on sucrose (black) and 24 hours after a blood meal (white). Sucrose fed MTB had significantly higher levels of ROS than APM (ANOVA, p<0.05). Blood feeding was associated with a significantly reduced ROS level in APMT and MTB (ANOVA, p<0.05) but not APM (p = 0.75). After blood feeding, APM maintained higher ROS levels than APMT and MTB (Tukey, p<0.05), which were equivalent (p = 0.9). The data shown are the means of five replicates. Comparative filarial susceptibility and survivorship
Prior studies have shown that changes in ROS levels can be detrimental to Plasmodium development in Anopheles gambiae [21], [22], [27] and that artificial Wolbachia infection can affect filarial worm development in Ae. aegypti [12]. Therefore, the number of infective stage filarial worms were compared following a Brugia-infected blood meal (Figure 2). MTB had significantly lower worm loads relative to both APM (χ2 = 53.3, df = 1, p<0.0001) and APMT (χ2 = 44.2, df = 1, p<0.0001). Equivalent worm loads were observed with APM and APMT (χ2 = 0.52, df = 1, p = 0.47).
Fig. 2. Mean number of L3s within Ae. polynesiensis strains. 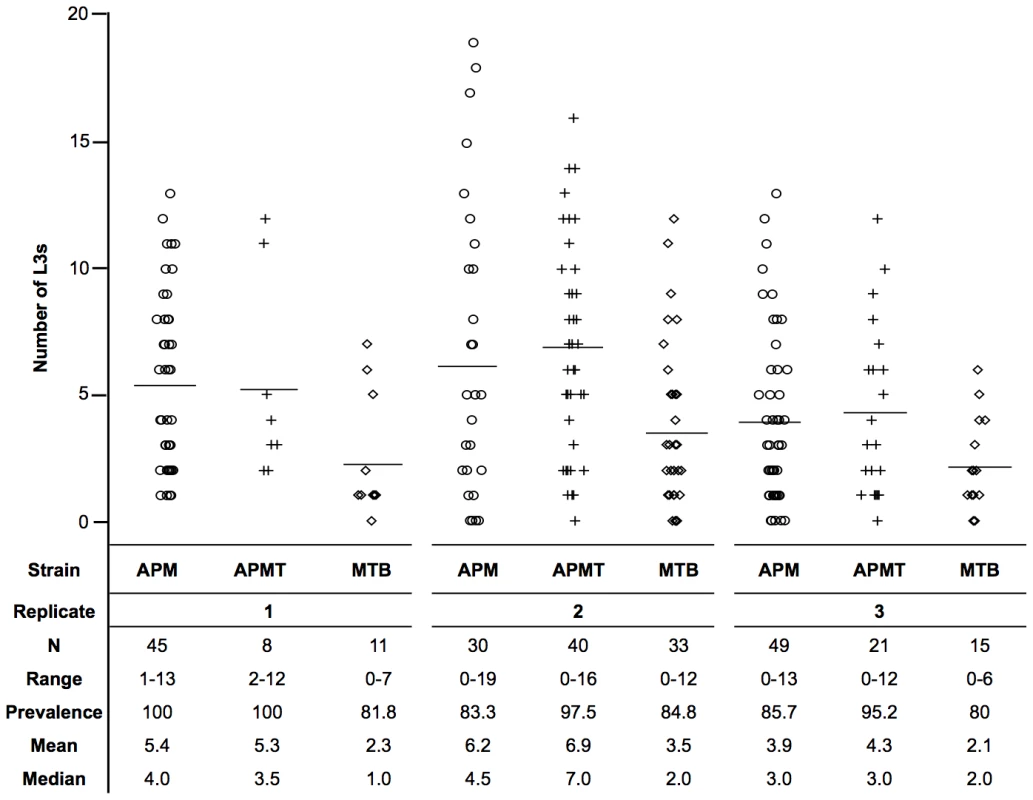
APM, APMT and MTB were given a Brugia pahangi infected blood meal. After ten days, mosquitoes were dissected and the number of infective stage L3s was counted. The MTB strain had a significantly lower worm load (p<0.0001) than APM and APMT, which were equivalent (p = 0.47). Despite significant variation between replicates (p<0.0001), the relationship between strains (APM = APMT>MTB) was consistent across the three replicates (p = 0.77). Each symbol represents a single mosquito. Lines represent mean values. Observations during competency experiments suggested a difference in strain survivorship after feeding on Brugia-infected blood. Therefore, a formal experiment was conducted to compare survivorship. Significant differences were observed in survivorship between strains fed Brugia-infected blood (GLM: χ2 = 119.6, df = 11, p<0.0001, Figure 3). There were significant differences between replications (GLM: χ2 = 28.5, df = 3, p<0.0001) and strain (GLM: χ2 = 73.79, df = 2, p<0.0001). Despite the variation between replicates, the pattern between strains remained consistent (GLM: strain×replicate, χ2 = 6.27, df = 6, p = 0.39; Figure 3C).
Fig. 3. Survivorship of Ae. polynesiensis strains after a Brugia-infected blood meal. 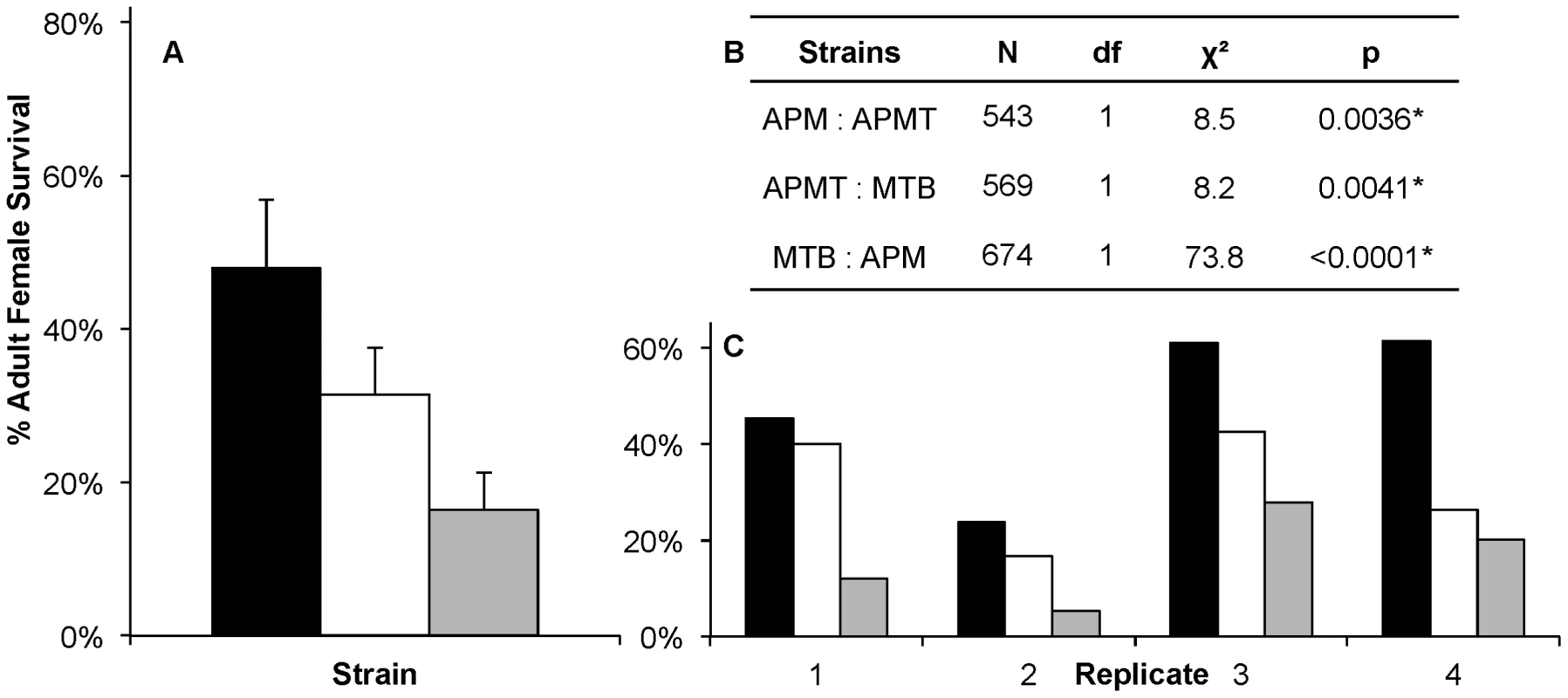
Mean survivorship of APM (black), APMT (white) and MTB (grey) ten days after feeding on a Brugia pahangi-infected blood meal. Contrast comparisons demonstrate significant differences in survivorship for each pair-wise comparison between strains. A) The mean survival of the four replicates. Error bars represent standard error. B) Contrast comparisons. C) The relationship between strains (APM>APMT>MTB) was consistent across all four replicates (GLM: χ2 = 6.27, df = 6, p = 0.39). For all tests, α = 0.05. To examine for a role of the Brugia parasite in the different survivorship, the strains were compared when fed on blood either with or without Brugia (Figure 4). The results show that, in general, each of the strains experienced lower survivorship when fed Brugia-infected blood, relative to uninfected blood (GLM: χ2 = 14.3, df = 1, p = 0.0002). Similar to the pattern observed in the preceding experiment, APM was longer lived than APMT (GLM: χ2 = 9.85, df = 1, p<0.05), which was longer lived than MTB (GLM: χ2 = 6.05, df = 1, p<0.05). There was no significant interactive effect for blood meal type×strain (GLM: χ2 = 4.5, df = 2, p = 0.1).
Fig. 4. Survivorship of Ae. polynesiensis strains after feeding on Brugia-infected blood vs. uninfected blood. 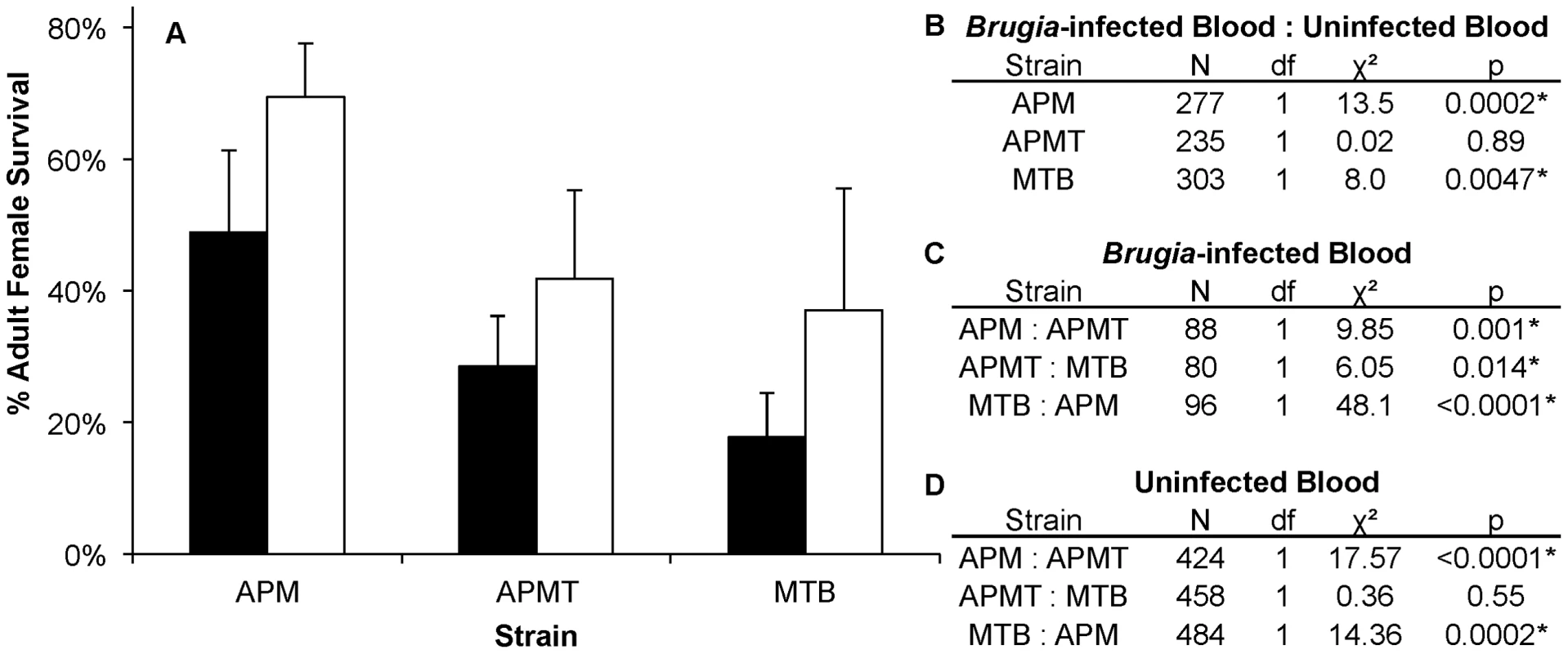
A) Mean survivorship of APM, APMT and MTB ten days after feeding on Brugia pahangi-infected blood (black) vs. uninfected blood (white). The presence of Brugia pahangi in the blood meal was associated with decreased survivorship for APM (p<0.05) and MTB (p<0.05). APMT survivorship was independent of blood meal type (p = 0.89). Error bars represent standard error for three replicates. B) Contrast comparisons for strains fed Brugia-infected and uninfected blood. C) Contrast comparisons for strains fed Brugia-infected blood. D) Contrast comparisons for strains fed uninfected blood. Comparing females fed either blood or sucrose only (Figure 5), the pattern was similar to that observed for ROS levels (Figure 1). The two artificially infected strains experienced significantly reduced survival following a blood meal. Specifically, APMT (GLM: χ2 = 6.72, df = 1, p<0.05) and MTB (GLM: χ2 = 16.98, df = 1, p<0.05) were longer lived when fed sucrose only. In contrast, APM females were longer lived following a blood meal (GLM: χ2 = 11.92, df = 1, p<0.05).
Fig. 5. Survivorship of Ae. polynesiensis strains after blood feeding vs. sucrose only. 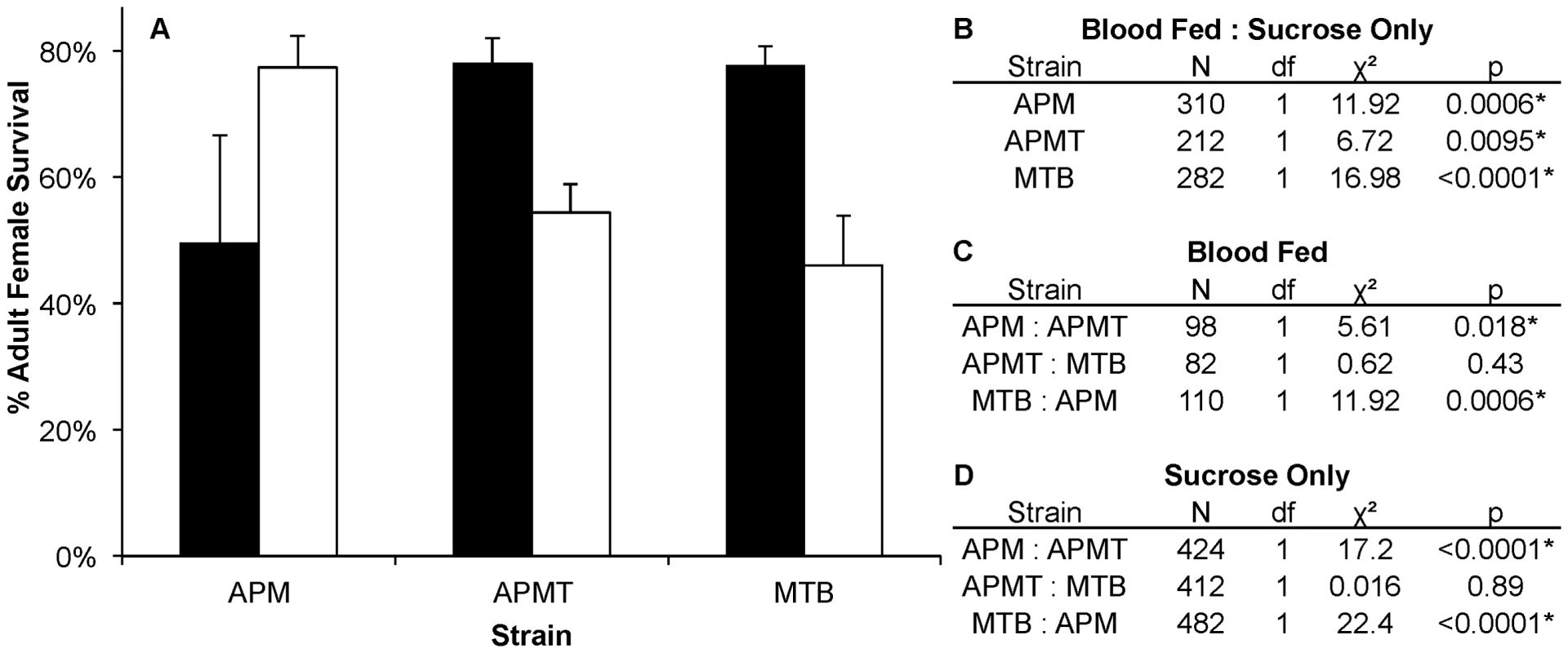
A) Mean survivorship of APM, APMT and MTB ten days after blood feeding (white) vs. sucrose only (black). Survival increased in APMT (p<0.05) and MTB (p<0.05) when fed sucrose only. However, decreased survival was observed in APM when not given a blood meal (p<0.05). Error bars represent standard error of two replicates. B) Contrast comparisons for strains fed blood or sucrose only. C) Contrast comparisons for strains fed blood. D) Contrast comparisons for strains fed sucrose only. Discussion
A novel Wolbachia infection in Aedes polynesiensis
Embryonic microinjection was used to transfer the wAlbB infection from Ae. albopictus to Ae. polynesiensis. PCR assays show that the infection is stable, with high maternal transmission. Overall mosquito survival after microinjection was high as compared to previous studies, which observed survival rates of less than 5% [24], [29]. Prior crossing experiments found that wPolA [30] and wAlbB [31] cause cytoplasmic incompatibility when crossed with different Wolbachia types. Consistent with expectations, crosses between APM and MTB were bidirectionally incompatible. Although superinfected cytoplasm was injected, only the wAlbB Wolbachia infection was established. The separation of superinfected Wolbachia types after microinjection is consistent with prior reports [31]–[34] and may result from different infection levels of wAlbB versus wAlbA within superinfected Ae. albopictus [35].
ROS levels and Wolbachia infection
The differing ROS levels observed in the artificially infected Ae. polynesiensis strains is similar to that of previous reports, which have shown differing ROS levels resulting from transinfection with wAlbB Wolbachia infection in both adult female Ae. aegypti [17] and in an Ae. albopictus cell line [16]. Multiple cellular pathways, including iron metabolism and immunity, influence ROS levels in mosquitoes [17], [36]. For example, H2O2 can be catalyzed via the Fenton reaction, along with excess labile iron [36]. Prior studies have demonstrated Wolbachia-produced bacterioferritin can scavenge labile iron, with the potential for iron competition between Wolbachia and host [16], [37]–[44]. Unfortunately, testing of specific hypotheses is limited at present, since many of the genomic and molecular tools available for better studied mosquito species (e.g., Ae. aegypti) are not yet available with Ae. polynesiensis. Our results provide additional motivation for developing such tools and methods.
Particularly intriguing is the previously reported ability of Wolbachia to upregulate dual oxidase (DUOX), which may influence the observed variation in ROS levels. A key component of innate immunity, the DUOX transmembrane protein is involved in ROS generation [26], [45]–[47] and can be increased if an artificial Wolbachia infection is recognized as foreign by its mosquito host [17]. Imbibing a blood meal can cause significant oxidative stress due to heme released from the degradation of hemoglobin, which can have pro-oxidant and cytotoxic effects when not bound to regulatory proteins [48]. An ability to maintain homeostasis despite this massive influx of iron resulting from blood feeding is important to the evolutionary success of hematophagous insects [36]. In addition, the Wolbachia genome retains genes for heme biosynthesis [49]–[51], and naturally occurring Wolbachia infections have been shown to buffer iron flux in insects, allowing iron homeostasis despite large influxes and limiting the deleterious effect of labile iron [37], [38]. In the wild type APM strain, no overall variation in ROS levels were observed following a blood meal. In contrast, a significant decrease in ROS levels was observed in the artificially infected MTB and aposymbiotic APMT strains following a blood meal. The reintroduction of wAlbB did not restore MTB to the homeostasis phenotype observed in the wild-type APM strain, indicating that not all Wolbachia types are equivalent and that the wPolA infection in Ae. polynesiensis represents an evolved symbiosis.
Whole mosquitoes were examined in this study, which may mask a tissue-specific effect. For example, mitochondrial generation of H2O2 was reduced in flight muscles after blood feeding in Ae. aegypti [52], and ROS levels were elevated in An. gambiae hemolymph following a blood meal [22]. In Ae. aegypti, blood feeding was associated with a significant decrease in ROS levels in midgut tissue through activation of a heme-mediated protein kinase C pathway [28]. While beyond the scope of our experimental design, our results encourage additional experiments designed explicitly to examine ROS in specific tissues in an effort to better understand the role of Wolbachia in influencing iron metabolism and the mosquito immune system.
Filarial worm survival
The number of infective stage filarial worms that developed within Ae. polynesiensis differed significantly between the strains. Specifically, the wild-type Wolbachia infected APM and its aposymbiotic counterpart (APMT) had similar numbers of successfully developing, infective L3 worms. This is comparable to the result of previous studies in which the removal of naturally occurring Wolbachia in Ae. pseudoscutellaris had no effect on the mean number of worms [53]. In contrast, significantly lower worm loads were observed in the artificially infected MTB compared to both the naturally infected and aposymbiotic strains. The latter is consistent with a prior experiment in which Wolbachia was artificially introduced into Ae. aegypti [12]. In the prior study, significantly lower B. pahangi numbers were observed in the artificially infected strain, relative to the naturally uninfected Ae. aegypti. Using the substantial genomic information available for Ae. aegypti, the authors speculated upon an association between an observed constitutive up-regulation of immune genes and an observed inhibition of filarial worm development. With the future development of additional genetic tools for Ae. polynesiensis, a similar approach can be used downstream to examine for an impact of artificial Wolbachia infection.
Artificial Wolbachia infections can detrimentally affect the fitness of their hosts [54]–[56]. In this study, reduced survival was observed for females with artificial Wolbachia infection types when fed on infected or uninfected blood. The decreased number of L3 filarial worms within MTB may be due, at least in part, to a reduced ability of MTB females to tolerate filarial worm infections and their premature deaths prior to dissection assays. The observed ROS variation is an additional potential explanation for the observed variation in filaria development. Recent studies show that changes in ROS levels can affect pathogen development. In An. gambiae, high levels of ROS were associated with increased melanotic encapsulation of Plasmodium parasites [22], [27]. In Ae. aegypti, increased ROS expression is associated with induction of the Toll pathway, which mediates the expression of antimicrobial peptides and antioxidants to balance oxidative stress and is associated with reduced dengue virus titer [17]. In addition, ROS generated independent of the mosquito immune system by the native microflora have also been found to negatively affect development of Plasmodium parasites [21]. It is also relevant to highlight the importance of iron to filarial worm development [57], [58]. If the artificial Wolbachia infection in Ae. polynesiensis were to affect the regulation of iron, as previously discussed, then filarial worm development and survival may be affected in the MTB strain. However, a simple direct association with overall ROS levels cannot explain the pattern of differential filarial worm development that was observed here, since the overall ROS levels were lower in MTB, relative to wild type mosquitoes. Furthermore, the lower ROS levels observed in the aposymbiotic APMT strain was not observed to be associated with reduced filarial development.
The variation observed between experimental replicates is not unexpected and is similar to prior reports [59]–[61]. Possible reasons for this variability include differences in sausage casing thickness, blood quality, and additional factors that affect mosquito feeding and microfilariae. Importantly and directly related to the study design, regardless of the variation between replications, the observed differences between strains remained the same (Figure 3C).
Survivorship and blood meal status
Filarial worm infections in mosquitoes are not benign. They can cause damage to the midgut and flight muscles [62], [63], sometimes affecting flight behavior [64]. Increasing the number of worms in an infected blood meal decreases mosquito survival rates [65]. Our results confirm the detrimental nature of filarial worms present in a blood meal, where a general reduction in survival was observed for all of the examined strains (Figure 4).
Blood contains an important nutritional component for adult mosquitoes [66]–[68] so it is not surprising that reduced survival was associated with blood deprivation of wild-type APM mosquitoes. However, this pattern was reversed in the aposymbiotic APMT and artificially infected MTB strains, where we observed a significant reduction in survivorship after blood feeding as compared to sucrose fed mosquitoes (Figure 5). As described above, this may reflect an evolved mutualism between the wPolA infection and Ae. polynesiensis, since increased survival of blood fed females is adaptive for both the anautogenous mosquito and the maternally inherited Wolbachia infection.
The observed pattern of decreased survivorship was similar to the pattern of H2O2 levels (Figure 1), suggesting an association with ROS homeostasis. Similar to our results, a recent study observed a link between decreased ROS levels and the proliferation of gut bacteria, which can be detrimental to the survival of the mosquito [27], [28]. In An. gambiae that were artificially infected with Wolbachia, no fitness effect was observed until a blood meal was taken. The authors proposed that this virulence could be due to modulated ROS levels and proliferation of gut bacteria within the mosquito following a blood meal [14].
Conclusions
In this study we were able to successfully infect Ae. polynesiensis with an artificial Wolbachia type that is stably maintained and causes bidirectional cytoplasmic incompatibility when crossed with the wild-type strain. We observed that the removal of Wolbachia and subsequent introduction of a novel Wolbachia type into Ae. polynesiensis affected host physiology and filarial worm development. We observed significant effects on ROS production both before and after blood feeding. The artificially infected mosquitoes varied also in their ability to support filarial worm development. Decreased survival was observed for blood fed mosquito strains that were cleared of their natural Wolbachia infection. The results encourage additional investigation into the specific physiological mechanisms affected by the artificial Wolbachia infection in Ae. polynesiensis. In addition, the findings presented here lend support for additional experiments with the human parasite, W. bancrofti. Although B. pahangi is a commonly used model system, it is important to determine if similar inhibitory effects can be observed against W. bancrofti. However, as there is no animal model, the latter will require transporting the transinfected mosquitoes to an endemic area or importing infected blood.
The observed experimental outcomes suggest applied strategies to impact filarial worm transmission by Ae. polynesiensis in the South Pacific. Specifically, the bidirectional incompatibility occurring in crosses of MTB and the wild-type provides a potential means to reduce the population of this important vector [2]. Another avenue of control is to purposely replace the existing population with MTB. The latter strategy would be similar to ongoing work in Australia against dengue transmission by Ae. aegypti [8], [9]. However, since MTB is bidirectionally incompatible with the wild type population, one would not expect CI to drive the spread of Wolbachia. Instead, the strategy would be suppression followed by female releases to establish the new infection type [69]. Furthermore, the decreased MTB survivorship observed in blood fed females and their reduced filarial worm development are consistent with the phenotype desired for a strategy in which the indigenous Ae. polynesiensis population is replaced with a strain less able to transmit filarial worms.
Materials and Methods
Ethics statement
This study was performed in strict accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Kentucky (Protocol number: 00905A2005).
Mosquito strains
Aedes albopictus (HOU) and an aposymbiotic Ae. polynesiensis strain (APMT) were used as Wolbachia donor and recipient, respectively. The HOU donor strain is naturally super-infected with two Wolbachia types, wAlbA and wAlbB [70]. The recipient strain, APMT, was generated by tetracycline treatment of the APM strain [2] and has been maintained for >30 generations in the laboratory. The wild-type APM strain is single infected with wPolA and exhibits a 100% infection rates in wild populations [30], [71] (Table 1). APM was used in crossing tests to characterize the newly generated strain, MTB.
Unless otherwise specified, mosquitoes were maintained using standard insectary conditions at 28±2°C, 75±10%RH, and a photoperiod of 18∶6 h (L∶D). Larvae were reared in optimal conditions, at low density in excess of 6% liver powder solution (MP Biomedicals, LLC, Solon, OH), until pupation. Adult mosquitoes were provided with a 10% sucrose solution ad libitum, and a blood meal was given once a week with anesthetized mice.
Embryonic microinjection
Collection, preparation and microinjection of embryos were based upon successful techniques used for previous mosquito transfections [23], [31]. Injection needles were prepared using quartz glass capillaries with an outer diameter (OD) of 1.00 mm, an inner diameter (ID) of 0.70 mm, and a length of 7.5 cm (QF100-70-7.5; Sutter Instrument Co., Novato, CA). Needles were beveled at a 15° angle using a micropipette beveler, model BV-10 (Sutter Instrument Co., Novato, CA). Microinjection was done using an Olympus IX70 inverted microscope (Olympus Co., Tokyo, Japan) at ×200 magnification.
Blood-fed APMT females were held in Drosophila vials (Fisher Scientific) containing wet germination paper (Anchor Paper Co., Saint Paul, MN) and allowed to oviposit. Recipient embryos (APMT) to be injected were collected, aligned on wet germination paper, briefly desiccated and covered with water-saturated halocarbon 700 oil (Sigma-Aldrich Co.). Donor HOU embryos were treated similarly, but not desiccated.
Cytoplasm was withdrawn from the posterior of donor HOU embryos and injected using an IM 300 microinjector (Narishige Scientific, Tokyo, Japan) into the posterior of the recipient APMT embryos. Recipient embryos were injected up to 90 minutes post-oviposition. After injection, the embryos were incubated under standard conditions for approximately 40 minutes. Injected embryos were removed from oil and transferred to wet germination paper, where they were allowed to develop for 5 days. The eggs were hatched (G0) and reared using standard maintenance conditions.
Rearing and selection of microinjected lines
Females of the parent generation (G0) were isolated as virgins and mated with APMT males, yielding a new strain named MTB. After oviposition, G0 females and males were assayed for both presence of Wolbachia infection and type using PCR (see below)(Table 3). Females that were negative for Wolbachia were discarded along with their progeny. Daughters (G1) from infected G0 females were isolated as virgins and outcrossed with APMT males. All G1 females that oviposited were tested for Wolbachia infection by PCR. PCR-guided selection was performed for 6 generations (G1–G6) (Table 3). At G7 the MTB strain was closed (i.e. not outcrossed with APMT males, but crossed with MTB males), and PCR was used to monitor the frequency of infection periodically through the following generations.
Infection status testing via PCR amplification
All infection types were confirmed using Wolbachia specific primers and PCR. Adults were homogenized in 100 µl of buffer containing 10 mM Tris-HCl, 1 mM EDTA and 50 mM NaCl using a Mini-beadbeater (BioSpec Products, Inc., Bartlesville, OK), boiled for 5 minutes and centrifuged at 14,000 rpm for 5 minutes. Two µl of supernatant were used for each PCR reaction. PCR reactions were amplified in 50 mM KCl, 20 mM Tris-HCl (pH 8.4), 1.5 mM MgCl2, 0.25 mM dNTPs, 0.5 mM primers and 1 U Taq DNA polymerase in a total volume of 25 µl. Wolbachia infection in all strains was confirmed using general Wolbachia primers 438F (5′CAT ACC TAT TCG AAG GGA TAG-3′) and 438R (5′AGC TTC GAG TGA AAC CAA TTC-3′) and PCR cycling conditions of 94°C 2 minutes, 39 cycles of 94°C for 30 seconds, 55°C for 45 seconds and 72°C for 1 minute 30 seconds, followed by a final extension temperature of 72°C for 10 minutes. Infection type of all strains was confirmed using A-clade (136F and 691R) or B-clade (81F and 522R) specific primers [72]. PCR cycling conditions were 94°C for 4 minutes, followed by 35 cycles of 94°C for 1 minute, 48°C (A) or 55°C (B) for 1 minute and 72°C for 1 minute and a final extension temperature of 72°C for 10 minutes.
Cytoplasmic incompatibility
Similarly aged egg papers from APM and MTB were hatched concurrently in dilute liver powder solution (∼0.6 g/L). One hundred first instar larvae were moved into a rearing container and fed optimally until pupation. Pupae were isolated in individual test tubes to ensure virginity. After eclosion, 20 virgin adults were introduced into a crossing cage at a 1∶1 sex ratio and allowed to mate. A full factorial crossing design between APM and MTB was implemented, and four replicates were performed for each cross (Table 4). An oviposition cup was available to females in each crossing cage for one week, after which the cup was removed. Eggs remained hydrated and were allowed to mature for 10 days. Egg papers were removed from the oviposition cups and hatched by submersion for two days in dilute liver powder solution. All eggs were examined by microscope to determine the total number of eggs and the proportion hatched as indicated by the position of the operculum. The normality of the hatch rate data was analyzed using a Shapiro-Wilkes test (JMP, SAS Institute, Cary, NC). A Kruskal-Wallis test was used to determine overall significance and post-hoc Wilcoxon tests were used for pairwise comparisons of hatch rates between crosses.
Determination of ROS in the whole mosquito
To determine ROS levels in mosquitoes fed sucrose only, whole bodies of seven-day-old APM, APMT and MTB were collected in 150 µl of 1× PBS containing 2 mg/ml of the catalase inhibitor 3-amino-1, 2, 4-trizole. To determine ROS levels in blood fed mosquitoes, six-day-old APM, APMT and MTB were provided with a blood meal from an anesthetized mouse. Twenty-four hours after blood feeding, the midgut was dissected from the mosquito and the blood bolus was flushed from the midgut using 1× PBS with catalase inhibitor. Mosquito carcasses and midgut tissues were collected in 1× PBS with catalase inhibitor.
For both treatments, samples were homogenized then centrifuged for 5 minutes at 10,000 g. The supernatant was filtrated through a 10 K molecular weight cutoff spin filter (Corning SpinXUF; Corning Incorporated Life Sciences). The elution was collected and tested using a Hydrogen Peroxide Assay kit (BioVision) following manufacturer's instructions. The fluorescence intensity was detected with Excitation/Emission 544/590 using a fluorescence microplate reader (Fluoroskan Ascent FL, Thermolabsystems). Five biological replicates, with three females for each strain were used for each treatment. A general linearized model with a normal distribution was used to determine if ROS levels differed between strain, feeding status or strain×feeding status. The sucrose treatment and the blood treatment were analyzed using separate ANOVAs with post hoc Tukey HSD comparisons.
Filarial susceptibility testing
Three replicates were performed to test for relative filarial susceptibility between strains. Brugia pahangi-infected dog blood was provided from the NIH/NIAD Filariasis Research Reagent Resource Center at the University of Georgia. Egg papers for APM, APMT and MTB were hatched concurrently and reared under standard maintenance conditions. Adult female mosquitoes were anesthetized using chloroform, and 75–90 mosquitoes were placed into cages. They were provided with a 10% sucrose solution and given 3 days to acclimate to the cage. Females aged 3–5 days were sucrose starved for 6 hours prior to blood feeding. They were given a Brugia-infected blood meal (10 microfilariae/µl) using sausage casing and a Hemotek membrane feeding system (Discovery Workshops, Accrington, UK) that maintained the blood at 37°C. All mosquito strains were allowed access to blood for 2 hours.
After feeding, females were allowed to rest for one hour before sorting. All mosquitoes were briefly anesthetized using chloroform and observed under a microscope for presence of a blood bolus. Blood fed and non-blood fed females were placed into separate cages. Ten days after feeding, surviving blood fed females were anesthetized on ice and dissected in sterilized Hank's balanced salt solution (Sigma-Aldrich). Individual mosquitoes were examined for L3 parasites by microscopy. The total number of filarial worms in each mosquito was recorded.
To determine whether worm load data were normal, a Shapiro-Wilkes test was used (JMP, SAS Institute, Cary, NC). We built a general linearized model with a Poisson distribution to determine if mean worm load differed across replicates or between strains. Post-hoc contrasts were used to compare worm loads between strains. To correct for multiple comparisons we used the Benjamini-Hochberg correction with an α value of 0.05 [73].
Mosquito survivorship post blood feeding
Mosquito rearing and blood feeding methods were the same as those described in “filarial susceptibility testing.” We recorded the number of mosquitoes alive and dead ten days after feeding on different blood meal types to compare differences in survival between APM, APMT and MTB. Three separate experiments were performed: 1) comparisons between strains fed on Brugia-infected blood only, 2) comparisons between mosquitoes fed uninfected and Brugia-infected blood meals, and 3) comparisons between mosquitoes that were blood fed or fed sucrose only.
For each of the above experiments, we built a general linearized model with a binomial distribution to determine if survivorship at day 10 differed between replicate, strain and blood meal type (JMP, SAS Institute, Cary, NC). Post-hoc contrasts were used to compare survivorship between strains. To correct for multiple comparisons we used the Benjamini-Hochberg correction with an α value of 0.05 [73].
Zdroje
1. BurkotT, IchimoriK (2002) The PacELF programme: will mass drug administration be enough? Trends Parasitol 18 : 109–115.
2. BrelsfoardCL, SechanY, DobsonSL (2008) Interspecific hybridization yields strategy for South Pacific Filariasis vector elimination. PLoS Neglect Trop D 2 (1)
e129.
3. WerrenJH, BaldoL, ClarkME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6 : 741–751.
4. HilgenboeckerK, HammersteinP, SchlattmannP, TelschowA, WerrenJH (2008) How many species are infected with Wolbachia? - a statistical analysis of current data. Fems Microbiol Lett 281 : 215–220.
5. TurelliM, HoffmannAA (1999) Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol Biol 8 : 243–255.
6. DobsonSL (2005) Progress toward manipulating mosquito disease vector populations via releases of Wolbachia infected mosquitoes. Am J Trop Med Hyg 73 : 348–348.
7. DobsonSL, FoxCW, JigginsFM (2002) The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc R Soc Lond B Biol Sci 269 : 437–445.
8. HoffmannAA, MontgomeryBL, PopoviciJ, Iturbe-OrmaetxeI, JohnsonPH, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 : 454–U107.
9. WalkerT, JohnsonPH, MoreiraLA, Iturbe-OrmaetxeI, FrentiuFD, et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476 : 450–U101.
10. HedgesLM, BrownlieJC, O'NeillSL, JohnsonKN (2008) Wolbachia and virus protection in insects. Science 322 : 702–702.
11. GlaserRL, MeolaMA (2010) The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 5 (8)
e11977.
12. KambrisZ, CookPE, PhucHK, SinkinsSP (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326 : 134–136.
13. MoreiraLA, Iturbe-OrmaetxeI, JefferyJA, LuGJ, PykeAT, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139 : 1268–1278.
14. HughesGL, KogaR, XueP, FukatsuT, RasgonJL (2011) Wolbachia infections are virulent and inhibit the human Malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog 7 (2)
e1001296.
15. BianG, XuY, LuP, XieY, XiZ (2010) The endosymbiotic bacterium Wolbachia induces resistance to Dengue virus in Aedes aegypti. PLoS Pathog 6 (4)
e1000833.
16. BrennanLJ, KeddieBA, BraigHR, HarrisHL (2008) The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS One 3 (5)
e2083.
17. PanX, ZhouG, WuJ, BianG, LuP, et al. (2011) Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109: E23–E31.
18. SiesH (1997) Oxidative stress: Oxidants and antioxidants. Exp Physiol 82 : 291–295.
19. DeJongRJ, MillerLM, Molina-CruzA, GuptaL, KumarS, et al. (2007) Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proc Natl Acad Sci U S A 104 : 2121–2126.
20. MagalhaesT, BrackneyDE, BeierJC, FoyBD (2008) Silencing an Anopheles gambiae catalase and sulfhydryl oxidase increases mosquito mortality after a blood meal. Arch Insect Biochem 68 : 134–143.
21. CirimotichCM, DongYM, ClaytonAM, SandifordSL, Souza-NetoJA, et al. (2011) Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332 : 855–858.
22. KumarS, ChristophidesGK, CanteraR, CharlesB, HanYS, et al. (2003) The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci U S A 100 : 14139–14144.
23. XiZY, DobsonSL (2005) Characterization of Wolbachia transfection efficiency by using microinjection of embryonic cytoplasm and embryo homogenate. Appl Environ Microb 71 : 3199–3204.
24. XiZY, KhooCCH, DobsonSL (2006) Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc R Soc Lond, B, Biol Sci 273 : 1317–1322.
25. XiZY, KhooCCH, DobsonSL (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310 : 326–328.
26. HaE-M, OhC-T, BaeYS, LeeW-J (2005) A direct role for dual oxidase in Drosophila gut immunity. Science 310 : 847–850.
27. Molina-CruzA, DejongRJ, CharlesB, GuptaL, KumarS, et al. (2008) Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chemistry 283 : 3217–3223.
28. OliveiraJHM, GoncalvesRLS, LaraFA, DiasFA, GandaraACP, et al. (2011) Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog 7 (3)
e1001320.
29. FuYQ, GavotteL, MercerDR, DobsonSL (2010) Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Appl Environ Microb 76 : 5887–5891.
30. DeanJL, DobsonSL (2004) Characterization of Wolbachia infections and interspecific crosses of Aedes (Stegomyia) polynesiensis and Ae. (Stegomyia) riversi (Diptera: Culicidae). J Med Entomol 41 : 894–900.
31. XiZY, DeanJL, KhooC, DobsonSL (2005) Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem Molec 35 : 903–910.
32. O'NeillSL, PettigrewMM, SinkinsSP, BraigHR, AndreadisTG, et al. (1997) In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol 6 : 33–39.
33. PoinsotD, MercotH (2001) Wolbachia injection from usual to naive host in Drosophila simulans (Diptera: Drosophilidae). Eur J Entomol 98 : 25–30.
34. RieglerM, CharlatS, StaufferC, MercotH (2004) Wolbachia transfer from Rhagoletis cerasi to Drosophila simulans: Investigating the outcomes of host-symbiont coevolution. Appl Environ Microb 70 : 273–279.
35. DuttonTJ, SinkinsSP (2004) Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol Biol 13 : 317–322.
36. Graca-SouzaAV, Maya-MonteiroC, Paiva-SilvaGO, BrazGRC, PaesMC, et al. (2006) Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Molec 36 : 322–335.
37. BrownlieJC, CassBN, RieglerM, WitsenburgJJ, Iturbe-OrmaetxeI, et al. (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5 (4)
e1000368.
38. KremerN, VoroninD, CharifD, MavinguiP, MollereauB, et al. (2009) Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog 5 (10)
e1000630.
39. NicholH, LawJH, WinzerlingJJ (2002) Iron metabolism in insects. Annu Rev Entomol 47 : 535–559.
40. ZhouGL, KohlheppP, GeiserD, FrasquilloMDC, Vazquez-MorenoL, et al. (2007) Fate of blood meal iron in mosquitoes. J Insect Physiol 53 : 1169–1178.
41. SchaibleME, KaufmannSHE (2004) Iron and microbial infection. Nat Rev Microbiol 2 : 946–953.
42. GeiserDL, ChavezCA, Flores-MunguiaR, WinzerlingJJ, PhamDQD (2003) Aedes aegypti ferritin - A cytotoxic protector against iron and oxidative challenge? Eur J Biochem 270 : 3667–3674.
43. PhamDQD, DouglassPL, ChavezCA, ShafferJJ (2005) Regulation of the ferritin heavy-chain homologue gene in the yellow fever mosquito, Aedes aegypti. Insect Mol Biol 14 : 223–236.
44. PhamDQD, WinzerlingJJ (2010) Insect ferritins: Typical or atypical? BBA-Gen Subjects 1800 : 824–833.
45. HaEM, LeeKA, SeoYY, KimSH, LimJH, et al. (2009) Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol 10 : 949–U919.
46. KumarS, Molina-CruzA, GuptaL, RodriguesJ, Barillas-MuryC (2010) A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327 : 1644–1648.
47. MeitzlerJL, de MontellanoPRO (2009) Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains: Insights into heme binding and catalytic activity. J Biol Chem 284 : 18634–18643.
48. JeneyV, BallaJ, YachieA, VargaZ, VercellottiGM, et al. (2002) Pro-oxidant and cytotoxic effects of circulating heme. Blood 100 : 879–887.
49. FosterJ, GanatraM, KamalI, WareJ, MakarovaK, et al. (2005) The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol 3 : 599–614.
50. GhedinE, WangSL, SpiroD, CalerE, ZhaoQ, et al. (2007) Draft genome of the filarial nematode parasite Brugia malayi. Science 317 : 1756–1760.
51. WuB, NovelliJ, FosterJ, VaisvilaR, ConwayL, et al. (2009) The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Neglect Trop D 3 (7)
e475.
52. GoncalvesRLS, MachadoACL, Paiva-SilvaGO, SorgineMHF, MomoliMM, et al. (2009) Blood-feeding induces reversible functional changes in flight muscle mitochondria of Aedes aegypti mosquito. PLoS One 4 (11)
e7854.
53. DuttonTJ, SinkinsSP (2005) Filarial susceptibility and effects of Wolbachia in Aedes pseudoscutellaris mosquitoes. Med Vet Entomol 19 : 60–65.
54. BrelsfoardCL, DobsonSL (2011) Wolbachia effects on host fitness and the influence of male aging on cytoplasmic incompatibility in Aedes polynesiensis (Diptera: Culicidae). J Med Entomol 48 : 1008–1015.
55. McMenimanCJ, LaneRV, CassBN, FongAWC, SidhuM, et al. (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323 : 141–144.
56. SuhE, MercerDR, FuYQ, DobsonSL (2009) Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl Environ Microb 75 : 7783–7788.
57. HiebWF, StokstadEL, RothsteiM (1970) Heme requirement for reproduction of a free-living nematode. Science 168 : 143–144.
58. RaoAU, CartaLK, LesuisseE, HamzaI (2005) Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci U S A 102 : 4270–4275.
59. FaillouxAB, ChanteauS, ChungueE, LonckeS, SechanY (1991) Oral Infection of Aedes polynesiensis by Wuchereria bancrofti by using Parafilm membrane feeding. J Am Mosquito Contr 7 : 660–662.
60. GublerDJ, InuiTS, BlackHR, BhattachNC (1973) Comparisons of microfilaria density in blood sampled by finger-prick, venipuncture and ingestion by mosquitoes. Am J Trop Med Hyg 22 : 174–178.
61. LowrieRC, EberhardML, LammiePJ, RaccurtCP, KatzSP, et al. (1989) Uptake and development of Wuchereria bancrofti in Culex quinquefasciatus that fed on Haitian carriers with different microfilaria densities. Am J Trop Med Hyg 41 : 429–429.
62. HockmeyerWT, SchieferBA, RedingtonBC, EldridgeBF (1975) Brugia pahangi - effects upon flight capability of Aedes aegypti. Exp Parasitol 38 : 1–5.
63. PerroneJB, SpielmanA (1986) Microfilarial perforation of the midgut of a mosquito. J Parasitol 72 : 723–727.
64. BerryWJ, RowleyWA, ChristensenBM (1986) Influence of developing Brugia pahangi on spontaneous flight activity of Aedes aegypti (Diptera, Culicidae). J Med Entomol 23 : 441–445.
65. IbrahimMS, TrpisM (1987) The effect of Brugia pahangi infection on survival of susceptible and refractory species of the Aedes scutellaris complex. Med Vet Entomol 1 : 329–337.
66. HarringtonLC, EdmanJD, ScottTW (2001) Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol 38 : 411–422.
67. ScottTW, NaksathitA, DayJF, KittayapongP, EdmanJD (1997) A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am J Trop Med Hyg 57 : 235–239.
68. StyerLM, MinnickSL, SunAK, ScottTW (2007) Mortality and reproductive dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood. Vector-Borne Zoonot 7 : 86–98.
69. HancockPA, SinkinsSP, GodfrayHCJ (2011) Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLoS Neglect Trop D 5 (4)
e1024.
70. SinkinsSP, BraigHR, OneillSL (1995) Wolbachia superinfections and the expression of cytoplasmic incompatibility. P Roy Soc B-Biol Sci 261 : 325–330.
71. PlichartC, LegrandAM (2005) Detection and characterization of Wolbachia infections in Wuchereria bancrofti (Spirurida: Onchocercidae) var. pacifica and Aedes (stegomyia) polynesiensis (Diptera: Culicidae). Am J Trop Med Hyg 73 : 354–358.
72. ZhouWG, RoussetF, O'NeillS (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. P Roy Soc B-Biol Sci 265 : 509–515.
73. BenjaminiY, HochbergY (1995) Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57 : 289–300.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Virus-Encoded microRNAs: An Overview and a Look to the Future
- Reactive Oxygen Species Production and Survivorship in with Artificial Infection Types
- Zinc Exploitation by Pathogenic Fungi
- Attenuated Typhimurium Lacking the Pathogenicity Island-2 Type 3 Secretion System Grow to High Bacterial Numbers inside Phagocytes in Mice
- The Polyfunctionality of Human Memory CD8+ T Cells Elicited by Acute and Chronic Virus Infections Is Not Influenced by Age
- How the Fly Balances Its Ability to Combat Different Pathogens
- MiniCD4 Microbicide Prevents HIV Infection of Human Mucosal Explants and Vaginal Transmission of SHIV in Cynomolgus Macaques
- Bidirectional Transfer of RNAi between Honey Bee and : Gene Silencing Reduces Population
- Global Gene Transcriptome Analysis in Vaccinated Cattle Revealed a Dominant Role of IL-22 for Protection against Bovine Tuberculosis
- Morphogenesis in Fungal Pathogenicity: Shape, Size, and Surface
- Inflammatory Responses Associated with the Induction of Cerebral Malaria: Lessons from Experimental Murine Models
- News from the Fungal Front: Wall Proteome Dynamics and Host–Pathogen Interplay
- Blood Flukes Exploit Peyer's Patch Lymphoid Tissue to Facilitate Transmission from the Mammalian Host
- Parallels in Intercellular Communication in Oomycete and Fungal Pathogens of Plants and Humans
- Influenza Human Monoclonal Antibody 1F1 Interacts with Three Major Antigenic Sites and Residues Mediating Human Receptor Specificity in H1N1 Viruses
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influenza Human Monoclonal Antibody 1F1 Interacts with Three Major Antigenic Sites and Residues Mediating Human Receptor Specificity in H1N1 Viruses
- Parallels in Intercellular Communication in Oomycete and Fungal Pathogens of Plants and Humans
- Virus-Encoded microRNAs: An Overview and a Look to the Future
- Reactive Oxygen Species Production and Survivorship in with Artificial Infection Types
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání