-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
-Mediated Detoxification of Reactive Oxygen
Species Is Required for Full Virulence in the Rice Blast Fungus
During plant-pathogen interactions, the plant may mount several types of defense
responses to either block the pathogen completely or ameliorate the amount of
disease. Such responses include release of reactive oxygen species (ROS) to
attack the pathogen, as well as formation of cell wall appositions (CWAs) to
physically block pathogen penetration. A successful pathogen will likely have
its own ROS detoxification mechanisms to cope with this inhospitable
environment. Here, we report one such candidate mechanism in the rice blast
fungus, Magnaporthe oryzae, governed by a gene we refer to as
MoHYR1. This gene (MGG_07460) encodes a glutathione
peroxidase (GSHPx) domain, and its homologue in yeast was reported to
specifically detoxify phospholipid peroxides. To characterize this gene in
M. oryzae, we generated a deletion
mutantΔhyr1 which showed growth inhibition with
increased amounts of hydrogen peroxide (H2O2). Moreover,
we observed that the fungal mutants had a decreased ability to tolerate ROS
generated by a susceptible plant, including ROS found associated with CWAs.
Ultimately, this resulted in significantly smaller lesion sizes on both barley
and rice. In order to determine how this gene interacts with other (ROS)
scavenging-related genes in M. oryzae, we compared expression
levels of ten genes in mutant versus wild type with and without
H2O2. Our results indicated that the
HYR1 gene was important for allowing the fungus to tolerate
H2O2
in vitro and in planta and that this ability
was directly related to fungal virulence.
Published in the journal: . PLoS Pathog 7(4): e32767. doi:10.1371/journal.ppat.1001335
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001335Summary
During plant-pathogen interactions, the plant may mount several types of defense
responses to either block the pathogen completely or ameliorate the amount of
disease. Such responses include release of reactive oxygen species (ROS) to
attack the pathogen, as well as formation of cell wall appositions (CWAs) to
physically block pathogen penetration. A successful pathogen will likely have
its own ROS detoxification mechanisms to cope with this inhospitable
environment. Here, we report one such candidate mechanism in the rice blast
fungus, Magnaporthe oryzae, governed by a gene we refer to as
MoHYR1. This gene (MGG_07460) encodes a glutathione
peroxidase (GSHPx) domain, and its homologue in yeast was reported to
specifically detoxify phospholipid peroxides. To characterize this gene in
M. oryzae, we generated a deletion
mutantΔhyr1 which showed growth inhibition with
increased amounts of hydrogen peroxide (H2O2). Moreover,
we observed that the fungal mutants had a decreased ability to tolerate ROS
generated by a susceptible plant, including ROS found associated with CWAs.
Ultimately, this resulted in significantly smaller lesion sizes on both barley
and rice. In order to determine how this gene interacts with other (ROS)
scavenging-related genes in M. oryzae, we compared expression
levels of ten genes in mutant versus wild type with and without
H2O2. Our results indicated that the
HYR1 gene was important for allowing the fungus to tolerate
H2O2
in vitro and in planta and that this ability
was directly related to fungal virulence.Introduction
Molecular oxygen, itself relatively nontoxic, is important to most living organisms on this planet. However, its derivatives, reactive oxygen species (ROS), can lead to oxidative destruction of cells [1]. For example, in mammals, ROS can accelerate aging by making holes in membranes, or by stealing electrons from DNA, which may result in cancer and other severe diseases [2]. However, animals, plants and fungi have all adapted to use ROS as key signaling molecules [3]. In plants, ROS play a more positive role as a defense mechanism against attacking pathogens, and are often produced as a first line of defense [4]. In the plant-pathogenic fungus, Magnaporthe oryzae, ROS regulation plays important roles in both development and virulence. ROS itself has been shown to accumulate in the developing and mature appressorium, or fungal penetration structure, while the two NADPH oxidases in M. oryzae, NOX1 and NOX2 are required for proper development of appressoria, as well as full virulence [5]. The catalase gene family member, encoded by CATB, was shown to also be involved in cell wall integrity as well as virulence, as deletion mutants were altered in hyphal, spore and appressorial morphology [6]. Organisms, therefore, must carefully balance the toxic effects of ROS and the need for ROS in cellular signaling.
There are five major types of ROS in plants: superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH), nitric oxide (NO), and singlet oxygen (1O2). In plant cells, organelles with an intense rate of electron flow or high oxidizing metabolic activity are major sources of ROS generation [7]. These organelles include mitochondria, chloroplasts and peroxisomes. ROS are also generated via enzymatic sources, such as membrane-associated NADPH oxidases, cell wall peroxidases and oxalate oxidases [8].
ROS play a crucial role during plant defense responses. Oxidative bursts have been detected when plant cells are inoculated with biotrophic pathogens [9], hemi-biotrophic pathogens [10], necrotrophic pathogens [11], and pathogen elicitors [12]. More recent studies with M. oryzae that causes rice blast disease, demonstrated that rice produces H2O2 shortly after inoculation with a virulent strain of the fungus [13], [14]. The toxic effects of ROS can directly kill pathogens, and as a result, pathogens have developed counter measures [5]. The coexistence of hosts and pathogens side-by-side determines that the increase of resistance in a host will be balanced by the change of virulence in a pathogen, and vice versa. A metabolite fingerprint study of three rice cultivars infected by M. oryzae provided evidence for suppression of plant-associated ROS generation during compatible interactions [9]. Fungal-produced catalase was secreted during infection, and appeared to play a role in breaking down the plant-produced H2O2, allowing the disease cycle to occur; in the absence of catalase, infection was largely blocked by the plant's ROS [15].
ROS production and mitigation is a multifaceted process, incorporating many genes and pathways [1]. One mechanism of sensing and ultimate detoxification of ROS in yeast is via the Hyr1 gene, formerly termed Gpx3/Orp1; this gene, upon ROS induction, activates its partner protein yAP1, which is a bZip transcription factor involved in activating cellular thiol-redox pathways, and arguably one of the most studied ROS-sensing proteins in yeast [16]. This AP1-like (activator protein) transcription factor regulates H2O2 homeostasis in Saccharomyces cerevisiae (S. cerevisiae), which in turn governs the synthesis of glutathione [17]. Hyr1p plays a key role during the oxidative response in S. cerevisiae [18]; after being directly oxidized by H2O2, it forms an intermolecular disulfide bond with yAP1 [19]. A conserved cysteine residue at position 598 in Yap1p becomes active by forming an inter-molecular disulfide bond with the Cys36 of Hyr1p. This transient inter-molecular linkage is then resolved to a Yap1p intra-molecular disulfide bond between the cysteines at positions C303-S-S-C598. During this process, the Yap1 protein is released by Hyr1p in its active form, which is then transported to the nucleus [20]. This conformational change shields its nuclear export signal from the interacting protein Crm1p, allowing it to remain in the nucleus and control a suite of antioxidant genes [21], [22]. Although YAP1 gene homologs have been analyzed in several plant pathogenic fungi such as Aspergillus fumigatus, Alternaria alternata, Cocholiobolus heterostrophus, Botrytis cinerea and Ustilago maydis [16], [20], [23], [24], [25], [26], HYR1 has yet to be studied in filamentous fungi.
In this study, we closely examined the HYR1 homolog in M. oryzae as a candidate mechanism for coping with a ROS-intensive host environment. We demonstrated that HYR1 was indeed involved in detoxifying or preventing plant basal immune responses including plant-generated ROS and callose deposits during initial stages of infection, which was correlated with its role as a virulence factor.
Results
Identification and characterization of a Glutathione peroxidase domain-containing gene in the genome of M. oryzae
As one of the key members during the oxidative stress response, the yeast Saccharomyces cerevisiae Hyr1/YIR037W (formerly termed Gpx3) was reported to be a glutathione-dependent phospholipid peroxidase (PhGpx) that specifically detoxifies phospholipid peroxides [19]. In order to identify the corresponding gene in M. oryzae, we performed a BlastP analysis against the fully sequenced genomic database of M. oryzae housed at the Broad Institute. Using an E-value of 1e-3 returned a single hit located on Supercontig 20, with an accession number of MGG_07460.6. It is 1315 bp long including two introns, with an open reading frame of 783 bp, which encodes a 172-amino acid protein. A sequence analysis was performed using Prosite on the ExPASy Proteomics Server (http://ca.expasy.org/prosite/). Hits revealed a glutathione peroxidase active site at amino acid positions 27–42, and a glutathione peroxidase signature at amino acid positions 66–73 (Figure 1A). When a BlastP search was performed against GenBank at NCBI, numerous hits were returned with high similarity scores, from many organisms including fungi and bacteria. An alignment indicates that the putative GSHPx domains of Hyr1 are highly conserved across different organisms (Figure 1B). The MoHyr1 protein shares the highest amino acid conservation with the model, non-pathogenic fungus, Neurospora crassa (93% similarity and 73% identity), but shares between 81 and 90% similarity with eight other plant pathogenic filamentous fungi examined (Table S1 and Figure 1C). Secondary structure of the HYR1 protein was determined by PSIPRED [27], and consists of eight β-sheets (or strands) and four α-helices (Figure 2). As described in Zhang et al [18], the ScHyr1p showed a typical ‘thioredoxin fold’, also consisting of four β-sheets surrounded by three α-helices [28]. We compared the crystal structure of ScHyr1p with the predicted tertiary structure of MoHyr1 protein, generated with PyMOL (http://www.pymol.org/). The MoHyr1 predicted structure appears similar to a canonical thioredoxin fold, showing four β-sheets, with β1 and β2 running parallel and β3 and β4 running anti-parallel, surrounded by three α-helices (Figure 2). We located three positionally conserved cysteines in our HYR1 protein model compared to yeast, and these are marked in Figures 1B and 2. Two important cysteines, Cys39 and Cys88, likely correspond with two active sites found in the yeast Hyr1p, Cys36 and Cys82. Together, our in silico data suggest that we have identified the structural homolog of the ScHyr1 from yeast, and that this gene is highly conserved across filamentous fungi.
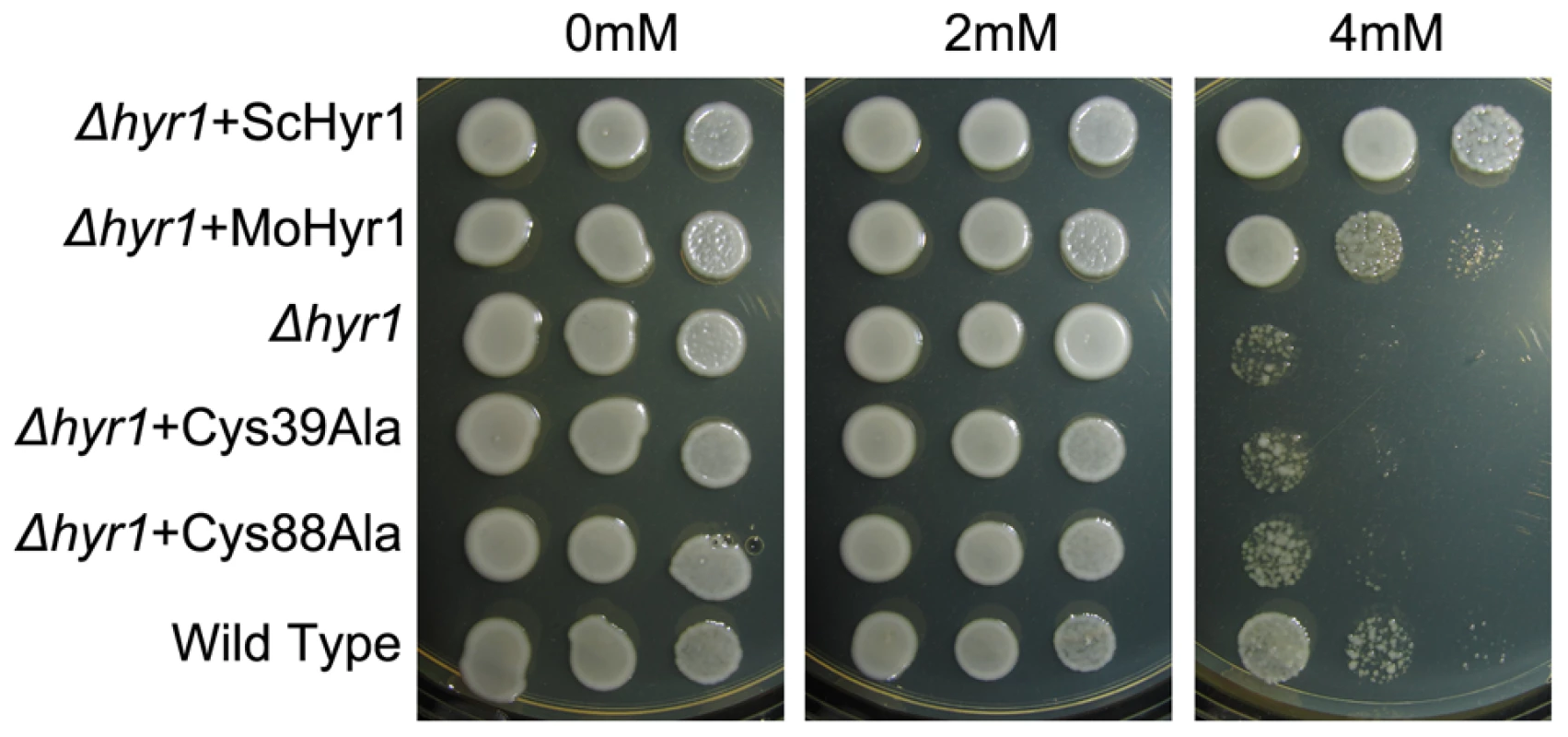
In order to functionally characterize the MoHYR1 gene, we obtained the ATCC S. cerevisiae Δhyr1 mutant and its wild type parent for complementation tests. Our hypothesis was that based on its sequence and predicted tertiary structure, the MoHYR1 gene would rescue the yeast mutant when grown on non-permissive concentrations of hydrogen peroxide. As shown in Figure 3, the yeast mutant and the wild type strain both grow well on 0 and 2 mM H2O2. However, growth of yeast Δhyr1 was significantly hindered in 4 mM H2O2. The wild type MoHYR1 gene was transformed into the yeast mutant, which restored partial growth on this higher concentration. To further support our hypothesis, we constructed mutations in the two conserved cysteine residues at positions 39 and 88. Neither of the mutations rescued the yeast phenotype on hydrogen peroxide (Figure 3).
Targeted deletion of MoHYR1
To explore the biological role of the MoHyr1 protein in the development and pathogenicity processes of M. oryzae, the deletion mutant Δhyr1 was generated through homologous recombination of the MoHYR1 open reading frame with a gene conferring hygromycin resistance (hygromycin phosphotransferase; HPH) (Figure S1A). A gene deletion fragment was generated by nested PCR amplification of the 5′ flanking region of MoHYR1, the HPH gene, and 3′ flanking region of MoHYR1, using adapters to link the three pieces together. This gene deletion fragment, which contained flanking regions homologous to the MoHYR1 gene, was introduced into protoplasts of M. oryzae via PEG-mediated fungal transformation. After PCR screening of successful knockouts and ectopics using primer pairs outside the flanking regions and inside the HPH gene, two Δhyr1 knockout mutants (B25, B33) and two ectopic mutants (B40, B60) were identified (Figure S1B) and confirmed with Southerns (Figure S1C). Real-time qRT-PCR was also employed to confirm full deletion of the MoHYR1 gene and no transcripts were detected. Deletion mutant Δhyr1 (B33) was complemented with a full-length copy of the MoHYR1 gene linked to the cerulean fluorescent protein (Figure S1D, see Materials and Methods).
MoHYR1 is required for vegetative hyphal growth in a ROS-rich environment
HYR1p in yeast was reported to not only be a sensor of ROS, but to have scavenging properties as well [19]. To investigate the role of MoHYR1 in scavenging H2O2 during vegetative hyphal growth, we inoculated the same amount of initial mycelia into complete media (CM) containing 0, 5 and 10 mM H2O2. No significant differences were detected among wild type, the Δhyr1 knockout mutants and the ectopics when growing in 0 mM H2O2. However, the mycelial growth of the Δhyr1 knockout mutants was severely and significantly affected at 10 mM H2O2 (Figure S2A and B). By contrast, the wild type and ectopics did not display much difference in mycelial growth at any concentration. The complemented mutant line grew slightly better than wild type in all concentrations of H2O2, and upon Southern analysis, we found that four copies had inserted into the genome (Figure S1E). Together, these data indicated that MoHYR1 was responsible for the H2O2 growth tolerance phenotype.
The MoHYR1 gene contributes to virulence in M.oryzae
To determine the role of MoHYR1 in virulence, we drop-inoculated detached leaves of three week-old blast-susceptible barley cultivars with conidia from two independently generated Δhyr1 mutants, B25 and B33 (Figure 4A). The mutants were still able to cause disease lesions, but there was a measurable and significant reduction in lesion size compared to those produced by wild type, ectopics, and the complemented line (Figure 4B). The complemented line, hyr1 - C, restored full virulence to the Δhyr1 mutant, B33. All pathogenicity assays were repeated on the susceptible rice cultivar Maratelli, with similar results (Figure 4C) using the spray-inoculation technique. Disease was also quantified on rice using a “lesion type” scoring assay [29] and error bars show that while lesion types 1–3 do not differ between the mutants, ectopics and wild type, lesion types 4 and 5 (severe, coalescing) did not form on mutant-inoculated plants (Figure 4D) Interestingly, no other developmental phenotype examined was compromised in the Δhyr1 mutant, including growth rate, conidia production and shape, germ tube and appressorial formation (Table 1).
Fig. 4. <i>Δhyr1</i> exhibits a virulence defect. 
Tab. 1. 
concentration equals 1×10<sup>5</sup>conidia/ml. MoHyr1 is required for breaking down ROS in planta during infection but not for internal ROS levels
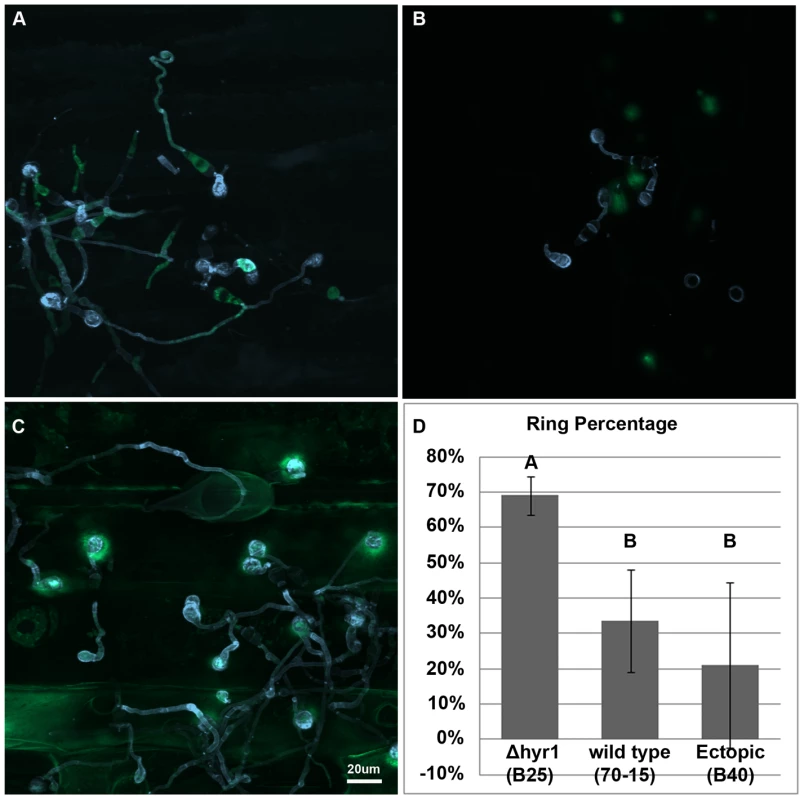
A fundamental question we wanted to assess was whether MoHYR1 was required for infection-related activities in planta. The M. oryzae's disease cycle is initiated when the conidium contacts a hydrophobic surface, inducing it to germinate. The germinated conidium forms a germ tube and appressorium that penetrates the plant surface via turgor pressure and forms a thin penetration peg into the first plant cell [30]. Thus, we first examined whether ROS was present during any of these processes, and if so whether MoHYR1 was involved in coping with it. We inoculated susceptible rice and barley cultivars with the Δhyr1 mutants, ectopics and wild type. ROS was detected using the indicator 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) [31]. Conidia of wild type, ectopics and the Δhyr1 mutant all elicited some degree of ROS when inoculated onto barley leaves (Figure 5A–C), whereas ROS was undetectable under the same imaging conditions when non-inoculated leaves were stained (data not shown). The Δhyr1 mutants showed the strongest ROS signal 24 hours post inoculation (hpi) compared to the others. The signal continued in this manner through 48 hours (data not shown). These experiments were repeated six times and the results were consistent across the two independent Δhyr1 mutant lines. ROS signals were quantified via counting the number of ‘ROS haloes’ found around appressoria and expressing this as a percentage of appressoria counted per sample; a significant difference in signals was observed between the mutants, wild type, and ectopics (Figure 5D). These results indicate that in the absence of the MoHYR1 gene, the fungus can no longer manage the ROS that is generated during initial infection events, or loses the ability to effectively cope with it.
To better understand the reason for reduced virulence in the Δhyr1 mutant, we wished to determine whether internal fungal levels of ROS were altered in the absence of the gene. The deletion mutant and wild type were grown on complete media and stained with nitroblue tetrazolium (NBT) for production of superoxide anions (Figure S3). Results showed little differences between mutant and wild type when examining the entire colony (Figure S3E and F) or aerial hyphae (Figure S3A–D).
Fungal internal ROS patterns are different from those generated in planta
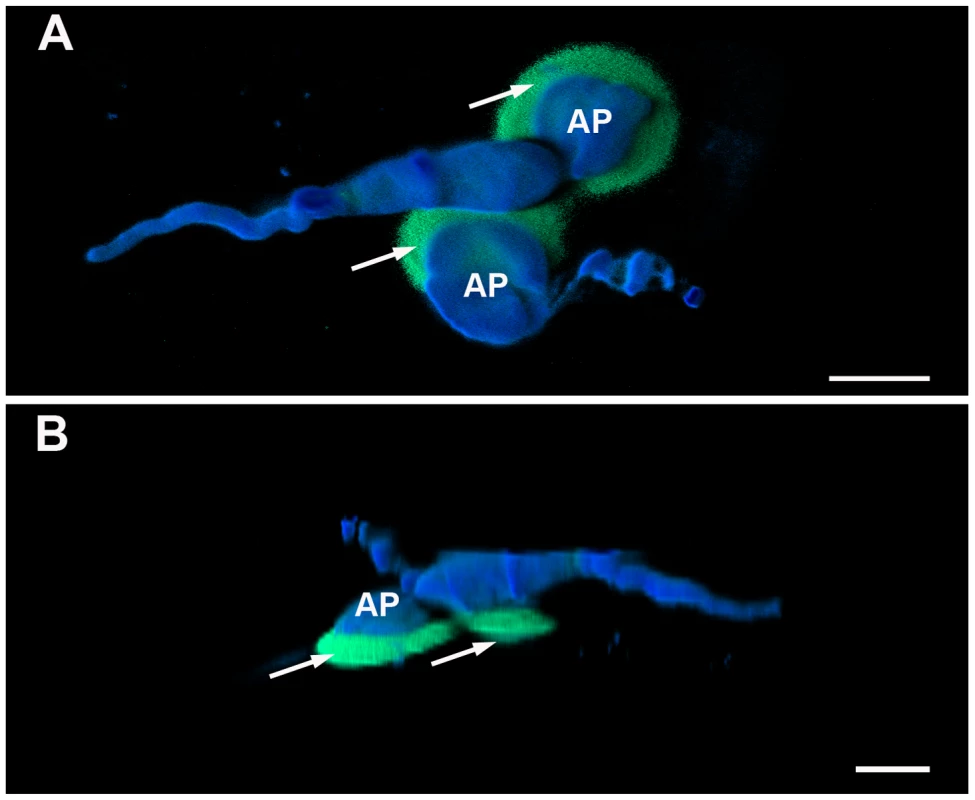
Figure 5C suggested that reactive oxygen species localized mainly around the appressoria. Upon closer inspection, we observed that the ROS “haloes” around the appressoria usually localized directly underneath the appressoria (Figure 6). Previous studies had demonstrated that the rice blast fungus also generates internal ROS during infection-related development, particularly during appressorial maturation and furthermore, that ROS can be secreted from the fungus itself [5]. In order to identify the source of the reactive oxygen species detected in our experiment, we inoculated M. oryzae onto the hydrophobic side of gel-bond, which can mimic the plant surface and induce ROS production in vitro [32]. The result shown in Figure 7 indicated that first, M. oryzae does generate ROS during germ tube and appressorial formation; second, the reactive oxygen species generated by M. oryzae were mostly intracellular and did not appear to be secreted or defused; and finally, that ROS were relatively weak in the fungal structures by 24 hpi. These observations occurred in the wild type, ectopic and mutant lines, indicating little difference in internal ROS levels regardless of the presence of HYR1. Altogether, these results were different from what we observed in planta, which was a strong ROS signal from 24–48 hpi.
Three lines of evidence suggest ROS is most likely plant-generated
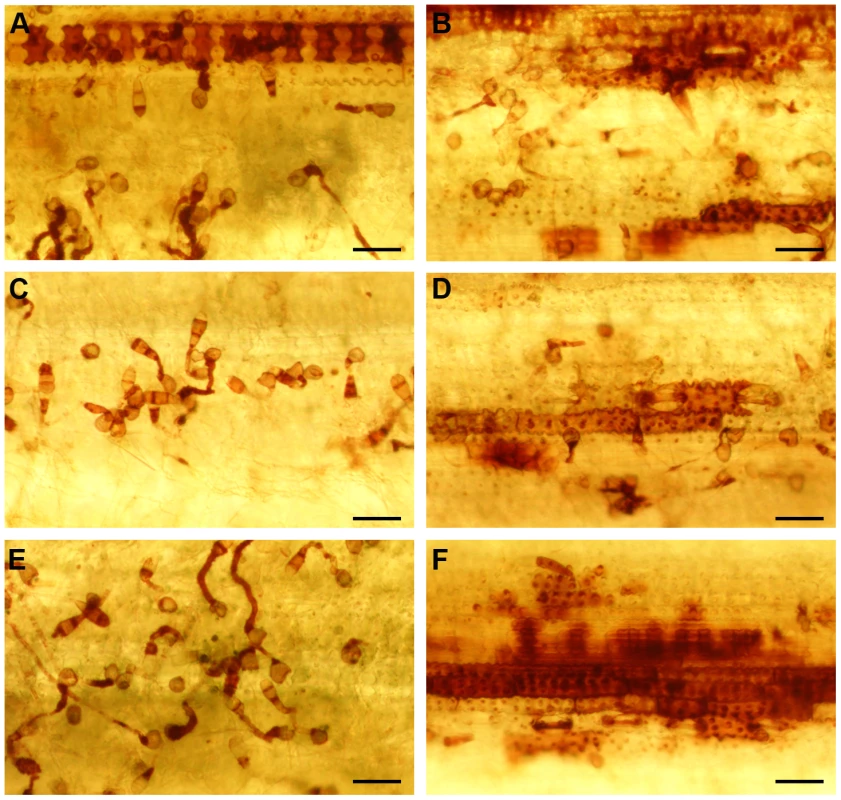
In order to identify the source of the ROS detected during susceptible interactions, we used diamino-benzidine (DAB) to study the ROS distribution pattern. Barley leaves were inoculated with Δhyr1 mutant then stained with DAB and imaged using confocal reflected light signal to visualize the DAB deposits from a top view of an interaction site (Figure 8A). The leaf samples from this same interaction site was processed further and embedded in epoxy resin to obtain a cross-section using a correlative microcopy approach. The confocal images suggested that the dark region (DAB) was localized immediately adjacent and inside the plant cell wall (Figure 8B) centered around the penetration peg (arrowhead - Figure 8B).
The second piece of evidence resulted from scavenging for ROS with ascorbic acid, an antioxidant that detoxifies hydrogen peroxide [33]. When 0.5 mM ascorbic acid was mixed with Δhyr1 mutant conidia, inoculated onto plants and stained with H2DCFDA, ROS haloes were clearly observed (Figure 9A). However, when barley leaves were pre-treated with ascorbic acid, then inoculated and stained with H2DCFDA, almost no ROS haloes were detected (Figure 9B). This experiment was repeated with another ROS-inhibitor called DPI (diphehyleneiodonium chloride), with similar results (data not shown). Ascorbic acid-treated leaves were also inoculated with mutant conidia and allowed to incubate in the growth chamber for six days, after which time we observed wild type lesions (Figure 9C). This suggested that the ROS haloes observed in this experiment are likely originated from the plant.
Futhermore, we analyzed previously characterized nox1 and nox2 mutants for ROS haloes; in M. oryzae, NOX1 and NOX2 code for NAPDH oxidases, and are largely responsible for producing internal ROS [5]. We hypothesized that if ROS was emanating from the plant, than the loss of the NOX genes should have no effect on haloes. Overall, haloes can still be produced when either of the nox mutants, or its parental strain, Guy11 was inoculated onto barley leaves (Figure S4A–F). While there was a slight significant difference among the number of haloes observed when looking at the individual mutants (nox1 made slightly more than nox2), there was no significant difference between mutants and wild type (20–30 appressoria were counted per strain, and the percentage of those with haloes, reported; Figure S4G).
MoHyr1 has an effect on later, but not immediate, plant-produced ROS
Since our data strongly suggested that Δhyr1 mutants had a lower capacity to eradicate plant-generated ROS during early stages of infection. Our next goal was to determine whether this gene played a role in fungal tolerance to ROS generated immediately following inoculation. In order to carry out this experiment, we inoculated susceptible barley leaves with either the Δhyr1 mutants or the wild type conidia, and imaged them 1 hpi. The ROS dye H2DCFDA was injected directly into the leaves, so the result only showed the redox status inside the leaves, and not inside the fungus, which might have skewed the results. Our data revealed that ROS was detected 1 hpi, which indicated that the plant detected and responded to the pathogen at an early time point (indicated by ROS fluorescence in the mesophyll cells; Figure S5A). A quantitative analysis of the signal intensities by ImageJ (available at http://rsb.info.nih.gov/ij; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) revealed no significant differences when inoculated with the Δhyr1 mutants or with the wild type conidia (Figure S5B). We thus concluded that the MoHYR1 gene does not play a role in ameliorating an early, or immediate, plant defense response.
To test whether MoHYR1 had any impact on plant-produced ROS that may occur later during infection, we inoculated Δhyr1 mutant conidia or wild type conidia onto barley leaves and stained with DAB at 24 hpi (Figure 10). Results indicated that the Δhyr1 mutant was unable to block ROS produced at 24 hpi, where the ROS was both detected in an entire plant epidermal cell, as well as in plant cells that were not in direct contact with the pathogen (Figure 10).
ROS generated during the infection process are related to cell wall appositions (CWAs)
It has been documented that the presence of reactive oxygen species around CWAs is a biochemical marker for non-penetrated cells during the interaction between barley and barley powdery mildew, Blumeria graminis [34]. To determine whether the ROS observed during a susceptible barley-M. oryzae was related to CWAs, we performed aniline blue staining on inoculated leaves. At 24 hpi, we found callose deposits specifically localized around the appressoria and penetration sites (Figure 11). Sequential correlative staining with H2DCFDA for ROS followed by analine blue for callose, showed a strong positional correlation between the two host responses when overlaid (Figure 11C).
CWAs are believed to physically block pathogen penetration [34]. To further characterize the CWAs formed during the barley - M. oryzae interaction, we examined leaves that had been inoculated with M. oryzae 24 and 40 hpi with either mutant or wild type conidia. The result showed that classical CWAs were formed within 24 hpi in both strains and no other differences in CWA morphology could be detected (Figure 12).
MoHYR1 regulates other ROS-related genes in M. oryzae
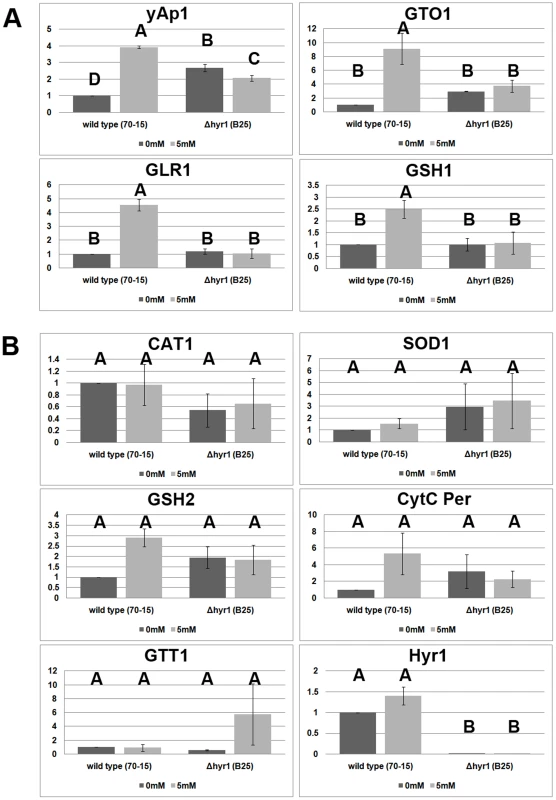
Given the fact that increased ROS accumulation occurs in the absence of MoHYR1, we next tried to determine whether the ROS scavenging system was impaired in the Δhyr1 mutants. We used real-time quantitative real time reverse transcription PCR (real-time qRT-PCR) to compare the expression of general antioxidant and redox control gene orthologs in both M. oryzae wild type and Δhyr1 strains (Figure 13). Primer pairs for the following genes were employed to examine gene expression: YAP1 (MGG_12814.6), GSH1 (γ-glutamylcysteine synthetase; MGG_07317.6), GSH2 (glutathione synthetase; MGG_06454.6), GLR1 (glutathione reductase; MGG_12749.6), GTT1 (glutathione transferase 1; MGG_05677.6), SOD1 (Cu/Zn superoxide dismutase; MGG_03350.6), CAT1 (catalase 1; MGG_10061.6), GTO1 (omega class glutathione transferase 1; MGG_05367.6), and cyt c per (cytochrome c peroxidase; MGG_10368.6). The housekeeping gene encoding Ubc (ubiquitin conjugating enzyme; MGG_04081.6) was used as an internal control. We also included the gene MoHYR1 (MGG_07460.6) in this experiment to confirm its deletion in the mutant lines. The expression patterns of these ten genes were placed into two categories. The first category (Figure 13A) is comprised of four genes that show increased expression in the wild type strain after induction with hydrogen peroxide, while expression in the mutant line is low and unchanging. GTT1, GR and GSH1 belong to this category, along with the HYR1 partner protein YAP1; YAP1 also shows slight but significant differences in expression in the Δhyr1 mutant line with and without H2O2, and has a higher expression level compared to the wild type strain without ROS. The second category contains genes whose expression does not significantly change, both in response to H2O2, as well as in the presence of the MoHYR1 gene. This category includes six genes: cyt c per, CAT I, Cu/Zn SOD, GTT I, GSHII and MoHYR1 (Figure 13B). HYR1 shows no expression at all in the mutant line, which was to be expected.
Hyr1 cellular localization
We evaluated the sub-cellular localization pattern of the MoHYR1 protein during infection, conidia of a M. oryzae deletion line (Δhyr1 B33) transformed with cerulean-MoHYR1 N-terminal fusion (the same construct that was used for complementation), was inoculated onto barley leaves and observed during the following time points: 1 hpi, 6 hpi, 12 hpi, 24 hpi and 72hpi. At 1 hpi, MoHYR1 was mainly localized in the conidial vacuoles and with low levels in the cytoplasm. When the germ tube formed, the protein was present throughout the germ tube (Figure 14A). At 6 hpi, the MoHYR1 protein showed increased cytoplasmic localization in the appressorium and conidium and at 12 hours, a concentration of HYR1 in the appressorial cytoplasm (Figures 14B and C). At the later time point, 24 hpi, the protein appeared to be localized in the vacuoles with reduced levels in the cytoplasm (Figure 14D), and a later, invasive stage time point suggests the protein was again cytoplasmically localized (Figure 14E).
Discussion
During the interaction between the pathogens and plants, plants mount defense mechanisms to protect themselves from pathogens. The cellular environment within the host can represent a major source of stress towards the invaders [16]. Pathogens, on the other hand, must possess adaptive mechanisms in order to survive. In this study, we hypothesized that the M. oryzae HYR1 protein defines one such mechanism, the glutathione synthesis pathway, involved in coping with the oxidative environment generated by plant defenses.
MoHYR1 is necessary for ROS detoxification and full virulence
In M. oryzae, MoHYR1 is the only sequence homolog of the yeast glutathione-dependent peroxidase, HYR1p, formerly termed Gpx3 [35]. In yeast, HYR1p senses H2O2 through two highly conserved cysteines that are redox sensitive. Mutations in either of these two cysteines leads to a non-functional HYR1 [18]. Indeed, we found that the wild type MoHYR1, but not the MoHYR1 cysteine mutants, was able to partially rescue the yeast HYR1p mutant on non-permissive levels of H2O2. This result is similar to Δyap1 yeast mutants complemented with homologs from two pathogenic filamentous fungi, Cochliobolus heterostrophus and Ustilago maydis, as both homologs partially complemented the yeast mutation [20], [23]. These data suggested that MoHYR1 may function similarly during redox sensing and the subsequent signaling that leads to ROS detoxification. This model was further supported by the presence of ROS haloes located underneath appressoria during infection with a much greater frequency in the Δhyr1 mutant compared to the wild type strain.
The increase in ROS haloes in Δhyr1 mutants correlated with significantly smaller lesions sizes when inoculated on susceptible rice and barley plants, suggesting that ROS scavenging regulated by MoHYR1 was required for full virulence. This was supported by a rescuing of the Δhyr1 mutant phenotype to wild type lesions by scavenging plant-derived ROS with ascorbic acid or disrupting plant-derived ROS generation with DPI. These results were similar to a gene recently reported on in the rice blast fungus called DES1 for Defense Suppressor 1 [14]. DES1 was also involved in virulence and triggers a stronger plant response upon infection, manifested by both an increase of the oxidative burst, as well as expression of two plant defense genes. Intriguingly, DES1 has no known functional domains and from sequence analysis, its function cannot be predicted, although it is well-conserved throughout fungi. It is also noteworthy that expression of MoHYR1 was tested in the Δdes1 mutant, and found to be slightly down-regulated. This could suggest that HYR1 and DES1 represent two semi-redundant, semi-dependent mechanisms evolved to cope with the plant defense response. Equally interesting is a gene recently identified in the plant and human fungal pathogens, Alternaria brassicicola and Aspergillus fumigatus, respectively, called tmpL [16]. This membrane-localized gene contains a FAD/NADP-binding domain and had not yet been studied in fungi. A deletion of tmpL resulted in a severely reduced virulence defect and hypersensitivity of exogenous oxidative stresses, however when the YAP1 gene was over-expressed in the deletion line, it rescued these and other mutant phenotypes, suggesting tmpL, YAP1 and presumably HYR1 may act in a concerted pathway to sense and trigger ROS scavenging pathways.
MoHYR1 helps the fungus negotiate a hostile host environment
A successful pathogen, which has the ability to detoxify ROS, will subsequently have fewer barriers to overcome before reaching its ultimate goal, which are the cell contents. Our results with the MoHYR1 gene suggest that while there might be no effect of MoHYR1 on ROS that's produced immediately by the plant (Figure S3), there is subsequent ROS production which MoHYR1 clearly helps the fungus overcome (Figure 10). Metabolic profiling performed by Talbot and colleagues (2008) provides support for this concept, revealing a M. oryzae-induced host metabolism re-programming that suppressed or delayed plant-produced ROS during susceptible interactions.
Although supporting evidence has shown that M. oryzae can produce ROS during infection related development [5], through scavenging experiments, the ROS observed in our studies appear to be largely plant-generated. Internal fungal ROS was unaffected by the absence of the MoHYR1 gene in vitro. Furthermore, ROS haloes were not disrupted by the ROS scavenger, ascorbic acid, when applied only to conidia, but were disrupted when ascorbic acid was specifically applied to leaves. Several pathways for plant-generated ROS include cell wall-bound peroxidases [1]. Plants defend themselves against pathogens by a battery of cell wall-associated defense reactions, including generation of ROS and cross-linking of lignin compounds [34]. During the interaction between a French bean (Phaseolus vulgaris) and a cell wall elicitor from Colletotrichum lindemuthianum, ROS appears to originate from cell wall peroxidases [36]. Apoplastic alkalization has been shown to be important in this process [34]. ROS generated from cell wall peroxidases also serve as key molecules required for lignification and cross-linking of cell walls [34]. In a study carried out between barley and the powdery mildew fungus, barley cell wall localized peroxidase HvRBOHA is responsible for generating H2O2, which was only present in non-penetrated cells [37]. Our results, particularly in Figure 8B, suggest ROS localized up against the plant cell wall. Further investigations into M. oryzae-host interactions will include analyzing plant defense-related genes, including the barley cell wall peroxidase.
Callose and ROS are two plant defensive compounds known to be involved in cell wall appositions, which are deposited during both compatible and incompatible interactions [34]. H2O2 played an important role in this process and enzymatic removal of H2O2 by catalase significantly reduces the frequency of phenolic deposition [34]. Several components were reported to be essential for this oxidative burst: peroxidases, a calcium influx and K+ Cl− efflux, extracellular alkalization, and post-Golgi vesicles [38]. ROS around the CWA areas might function as signal compounds to gather the vesicles and components needed for mature CWAs. We observed that ROS and callose deposits were positionally related during attempted penetration by both wild type and Δhyr1 mutants, immediately below the appressorium. From this result, we hypothesize that ROS generated by plant defenses activates CWA formation in a susceptible host and experiments to determine the timing of deposition of ROS versus callose are currently underway.
A hypothesis that follows from these data is that when the MoHYR1 gene is deleted, the plant responds as though it's being challenged with an avirulent pathogen. As early as 12 hours post inoculation, we observed that barley leaves inoculated with Δhyr1 mutants showed higher ROS signals compared with leaves inoculated with wild type. These data were consistent using two staining methods, H2DCFDA and DAB. In leaves inoculated with wild type, ROS was detected around appressoria but was mostly observed inside fungal structures. However, ROS was seen both around appressoria and adjacent cells when inoculated with the Δhyr1 mutants. Whole cells filled with ROS were also observed when inoculated with Δhyr1 mutants, which was related with HR-type cell death. All these data indicated that HYR1 might function to suppress later plant-generated ROS, either by detoxifying it directly, or manipulating plant ROS secretion-related gene expression.
MoHYR1 regulates several genes involved in ROS-scavenging
While our data showed that HYR1 likely played an important role in ROS-detoxification processes, our experiments did not preclude other ROS tolerance mechanisms in the fungus, particularly since mutants were reduced in virulence, but not completely non-pathogenic. Such mechanisms might involve the aforementioned DES1 and tmpL genes. Currently, we are characterizing the MoYAP1 homolog in M. oryzae; our initial Δyap1 mutant data suggested this gene was dispensable for pathogenicity, much like what has been found in Botrytis cinerea, Aspergillus fumigatus and Cochliobolus heterostrophus [23], [25], [26]. Intriguingly, YAP1 did appear to be essential for virulence in Ustilago maydis and Alternaria alternata [20], [25], suggesting that fungal lifestyle (i.e. necrotrophic vs. biotroph) had little to do with this particular oxidative stress pathway, and further supporting redundant pathways. Our real-time qRT-PCR data showed that YAP1 increases in expression when wild type was challenged with H2O2 and we also noted a decrease in YAP1 gene expression in the Δhyr1 mutant background. One interpretation of this result was that the fungal cell might be compensating for the absence of HYR1, by boosting expression of its partner gene.
The glutathione pathway-related genes GLR1, GTO1 and GSH1, all increased during H2O2 challenge in the wild type however had extremely decreased expression in the mutant line, regardless of ROS. This suggested that these genes were reliant upon HYR1, which was not unexpected, since the glutathione pathway was shown to be regulated YAP1, which occurs after interacting with HYR1 [17]. Our results were also in keeping with the C. heterostropus Yap1 homolog mutant Δchap1, which showed extremely low levels of both GLR1 and GSH1 [23]. Interestingly, we did not observe any of the other genes increasing in expression in the mutant background; this suggested that at least for the genes that we chose such as CAT1 and SOD1, they did not provide compensatory mechanisms for a loss of HYR1. While this is one hypothesis, it is also possible that these genes are regulated at the protein level, as was found in the A. fumigatus mutant, ΔAfyap1; both CAT1 and SOD1 were among the proteins down-regulated in the mutant [39], and this could also hold true for the Δhyr1 mutant. Likewise, catalase, SOD and peroxidase activities were measured in the A. alternata mutant ΔAaAp1 [25]. A transcriptomic study on the Δhyr1 deletion mutant would answer many of these questions; further, such a study would uncover redundant pathways of ROS detoxification masked by the presence of MoHYR1.
Localization of the MoHYR1 protein
While numerous studies have examined localization of the Yap1p, we were unable to find any studies on the localization of HYR1 either in yeast or filamentous fungi. Our data revealed that the HYR1 protein mostly localized either to the cytosol or to vacuoles, during early stage infection events on barley (germ tube, early appressorial formation, appressorial maturation and penetration). At one hpi, MoHYR1 was mainly moving through the germ tube, although it was difficult to definitively ascertain which organelle it might be associated with. At twelve hpi, the MoHYR1 protein shows cytoplasmic localization, mainly expressed in the cytosol of the appressorium. We suspect that by twenty-four hours, the fungus had penetrated and gained ingress to the first epidermal cell; indeed cell biology studies on events following initial penetration suggested that M. oryzae bulbous hyphae fill an entire rice leaf sheath cell and were in the process of moving onto the next one by twenty-seven hours post-inoculation [40]. Its vacuolar localization at this time-point could reflect that fact that it was no longer needed by the fungus, which had circumvented the plant's oxidative burst and at that point growing in the first epidermal cell. We examined a later time-point at 72 hpi and found the HYR1 gene to be once again cytoplasmically localized, perhaps indicating a requirement for this pathway at the invasive growth stage.
Conclusions and future directions
In conclusion, we identified and characterized the MoHYR1 gene, a functional homolog of the yeast Hyr1 (or Gpx3) gene. Although MoHYR1 does not cause dramatic effects in the disease phenotype, it nevertheless played an important role in virulence. This effect appeared to be related to the deletion mutant's inability to tolerate plant-generated ROS, or at least to do so in a timely and effective manner to cause wild type levels of disease. Together, our results help to define a mechanism that, while well-studied in yeast, has not yet been examined in filamentous fungi; furthermore, our studies pose additional questions to be answered regarding the role of the glutathione pathway in scavenging ROS in filamentous fungi, how this aids in pathogenicity and what other underlying redundant scavenging pathways exist.
Materials and Methods
M. oryzae strains and growth conditions
Rice-infecting M. oryzae, strain 70–15 (Fungal Genetics Stock Center 8958) was used as the wild type strain throughout this project, and the strain from which mutants and transgenics were derived. All strains were maintained at 25°C under constant fluorescent light on complete medium (CM 1 liter: 10 g sucrose, 6 g yeast extract, 6 g casamino acid, 1 ml trace element). Oatmeal agar medium (OAM 1 liter: 50 g oatmeal and 15 g agar) was used for sporulation. Conidia were harvested 10–12 days after plating.
Yeast strains and complementation assays
Yeast strains BY4741 (wild type) and BY4741 YIR037W (Δhyr1 mutant) were ordered from the American Type Culture Collection, grown out and maintained on YPD medium. Constructs for transformation were built using standard PCR reaction conditions and programs; briefly, pJS371 used overlapping primers to make an intron-free version of the MoHYR1 gene in pJS318. Using the intron-free plasmid, overlapping primers were used to make Cys39Ala and Cys88Ala mutant versions of the coding sequence. These were cloned into pCRScript (pJS372 & pJS373, respectively). The yeast HYR1 gene (ScHYR1) was then amplified from Sc46 and cloned into pRS423, the His3 episomal plasmid, pJS374. These plasmids then form the basis of the genes to be tested: MoHYR1 wild type, the 2 cysteine mutants of MoHYR1 and the ScHYR1 gene. These four genes are under the same promoter and terminator. Therefore ScHYR1 was engineered to have an NcoI site at the ATG and a BamHI site at the beginning of the terminator (pJS375). Since the Magnaporthe gene has a natural NcoI site at the ATG, the 3 genes of the MoHYR1 are cloned into pJS379 as NcoI/BamHI fragments (pJS381, pJS382, pJS383).
For the complementation assays, five-microliter drops from serial dilutions from cultures with anOD600 of 0.5 were spotted on plates with and without 0, 2 and 4 mM H2O2 and grown for 2 days at 30°C. This experiment was repeated 10 times. In total, the following plasmids were used in this part of the study:
pSM387 ( = pRS423) HIS3 yeast episomal plasmid; pJS374 pSM387 + ScHYR1;
pJS381 ScHYR1-Pro::MoHYR1::ScHYR1Term;
pJS382 ScHYR1-Pro::MoHYR1_Cys36Ala::ScHYR1Term;
pJS383 ScHYR1-Pro::MoHYR1_Cys82Ala::ScHYR1Term.
Plants cultivars and growth conditions
Rice cultivar Maratelli (a gift from the Dean Lab; Raleigh, NC) and barley cultivar Lacey (Johnny's Selected Seeds; Winslow, ME) were used throughout this study, as both are susceptible to M. oryzae strain 70–15. Rice was grown in a growth chamber at 80% humidity, and 12 h:12 h day:night cycles, at 28°C. Barley was grown in a growth chamber at 60% humidity, and 12 h:12 h day:night cycles, at 24°C (day) and 22°C (night).
Targeted deletion of Hyr1
The targeted gene deletion was accomplished using the homologous recombination method. We amplified 5′ and 3′ flanking regions of Hyr1 using primer pairs #1 and 2 (Table S2). Flanking regions were then linked via adaptor-mediated PCR to a 1.3 kb HPH coding sequence, providing resistance to the antibiotic hygromycin (Alexis Biochemicals, San Diego, CA). The entire length of the deletion fragment was 3.7 kb. Fungal protoplasts of the wild type 70-15 were directly transformed with the nested PCR product (primers used were forward primer of primer pair #1 and reverse primer of primer pair #2). Protoplast generation and subsequent transformation were conducted by following established procedures [41]. To confirm the knockout mutant, the genomic DNA of candidate strains was extracted and amplified with primer pairs #3, 4 and 5 (Table S2).
In vitro H2O2 growth assessment of Δhyr1 mutants
Equal-sized pieces of mycelia were cut with #3 cork-borer tool (0.7 cm in diameter), and immersed in 10 ml of liquid CM at 25°C in darkness. Colonies were grown in CM containing H2O2 at concentrations of 0 mM, 5 mM and 10 mM. Colonies were removed from each well, vacuum filtered to dryness, and measured on a scale one week post-immersion.
Pathogenicity assays
For point or drop inoculations, conidia were harvested from 12-day-old cultures grown on OMA in 20 µl of a 0.2% gelatin (Acros organics, New Jersey) suspension, for a final concentration of 1–5×105 conidia/ml. Point two percent gelatin was used as a non-inoculated control for pathogenicity assays. For drop inoculations, three week old leaves of Maratelli or Lacey were detached and laid flat in a humid chamber (90 mm Petri dish with moist filter paper). Twenty microliters of conidial suspensions, or gelatin alone, were dropped onto each leaf and kept in darkness overnight at ∼25°C. The next day, remaining water drops were wicked off and moved to a growth chamber under constant fluorescent light. For spray inoculations, conidial suspensions (10 ml; concentration as above) in 0.2% gelatin were sprayed onto three week old Maratelli or Lacey seedlings. Inoculated plants were placed in a dew chamber at 25°C for 24 hours in the dark, and then transferred into the growth chamber with a photoperiod of 16 h:8 h light:dark cycles. Disease severity was assessed seven days after inoculation.
Quantitative real-time RT-PCR of ROS-related genes and data processing
Quantitative real time reverse transcription PCR (real-time qRT-PCR) was carried out using primer pairs for the following genes: YAP1 (MGG_12814.6), GSH1 (MGG_07317.6), GSH2 (MGG_06454.6), GLR1 (MGG_12749.6), GTT (MGG_06747.6), GTO1 (MGG_05677.6), GTT1 (MGG_09138.6), SOD1 (MGG_03350.6), CAT1 (MGG_10061.6) and cytochrome c peroxidase (MGG_10368.6). The housekeeping gene encoding ubiquitin conjugating enzyme (MGG_00604.6) was used as an internal control. We also included the gene MoHYR1 (MGG_07460.6) to confirm its deletion in the mutant lines. Primer pairs are listed in Table S3. Seventy-five nanograms of cDNA generated from mycelium grown as per the H2O2 experiments described above (generated from the 0 mM and 5 mM H2O2 samples), was used as templates for each reaction. The mycelia were fragmented in a blender as per the protocol by Mosquera et al [42], before being inoculated into liquid complete medium. After 2–3 days, the mycelia were blended again to ensure the largest amount of actively growing fungal tips. The H2O2 experiment was performed 24 hours after the 2nd blending, and RNA was extracted. PCR reaction conditions were as follows for a 25 µl reaction: 13 µl H2O, 10 µl 5 Prime SYBR Green Master Mix (Fisher Scientific, Waltham, MA), 0.5 µl Forward Primer (for a final concentration of 2 µM; Integrated DNA Technologies, Coralville, IA), 0.5 µl Reverse Primer (for a final concentration of 2 µM) and 1 µl template DNA. Conditions for real-time quantitative RT-PCR conditions were as follows: 95°C for 2 min; 95°C for 15 sec, 58°C for 15 sec, 68°C for 20 sec (cycle 40 times); 95°C for 15 sec; 60°C for 15 sec (melting curve); 60°C –95°C for 20 min; 95°C for 15 sec; lid temperature constant at 105°C. The 2−ΔΔCt method was used for generating the data. ΔΔCt is defined as ΔCt treatment - ΔCt calibrator. cDNA from the strain 70-15 in 0 mM H2O2 was used as the calibrator for comparison of gene expression in 5 mM H2O2 in both the Δhyr1 deletion lines as well as the wild type For both the ΔCt treatment and ΔCt calibrator, ΔCt is defined as Ct gene - Ct housekeeping-gene. For the calibrator, which is 0 µM H2O2, this value would be 2−0 or 1. These experiments were repeated twice with similar results.
Cloning of MoHYR1 and generation of fusion protein
A HYR1 N-terminal cerulean fusion construct was generated by fusion PCR. Briefly, using M. oryzae genomic DNA as a template, a 1 kb promoter region of HYR1 was amplified with primers 6 and 7 (Table S2). Another set of primers, 8 and 9, were used to amplify the 2.4 kb HYR1 open reading frame. Three resulting fragments, the 1 kb promoter fragment, the 1328 bp ORF (including 709 bp of terminator sequence) and 740 bp cerulean fluorescent protein coding sequence [43], were mixed and subjected to a second fusion PCR with primers 7 and 8. The resulting 3.1 kb PCR product was generated with BamHI and NotI restriction enzymes (New England Biolabs, Beverly, MA) and cloned into pBlueScript II SK+. The construct was fully sequenced and found to be correct, hence was co-transformed into the M. oryzae Δhyr1 knockout mutant protoplasts to make Cerulean-HYR1 fusion transformants. Transformants with expected genetic integration events were identified by PCR using primers pairs 6 and 10 (Table S2). Properly transformed Δhyr1 mutants were also used as the complemented lines, in Figures 3 and 4, designated as “hyr1-C”.
Detection of ROS
Ten-fourteen day old rice and eight day old barley plants were used and collected 24 hours after being inoculated with 10–12 day old conidia (methods as described above). All staining procedures were performed with both rice and barley, however barley was best-suited for microscopy, hence all micrographs shown in this study are of barley. For experiments with 29,79-dichlorofluorescin diacetate (H2DCFDA) (Invitrogen, Carlsbad, CA), inoculated tissue were collected and incubated for 60 min at room temperature in 5–20 mM H2DCFDA dissolved in DMSO (less than 0.005% final concentration), then washed with 0.1 mM KCl, 0.1 mM CaCl2 (pH 6.0) and left for 60 min at 22°C before experimentation. Dye excitation was at 488 nm; emitted light was detected with a 500–550 band pass emission filter. DAB staining was carried out using the protocol developed by Thordal Christensen et al [44]. Briefly, leaves were cut at the base with a razor blade and placed in a 1 mg/mL solution of DAB for 8 h under darkness at room temperature. Leaves were decolorized by immersion in ethanol (96%) for 4 h followed by 2 hours in PBS buffer before imaging. A third method of ROS detection was employed for examining ROS internal to, or secreted from, the fungus. Nitroblue tetrazolium (Sigma-Aldrich, St. Louis) was used at 4 mg/mL (in deionized water) and the staining performed for 5 min∼30 min at room temperature prior to observation.
ROS scavenging treatments
In order to eliminate the ROS generated by fungus, conidia of Δhyr1 (B25) and wild type (70-15) were mixed with 0.5 mM ascorbic acid (AsA) and inoculated onto the leaf surface. Leaves were stained for ROS at 24 hpi. In order to eliminate ROS generated from the plant, leaves were first treated with 0.5 mM ascorbic acid for 1 hour. To remove excess AsA, leaves were then washed with 0.1 mM KCl, 0.1 mM CaCl2 (pH 6.0) buffer three times for 5 minutes each. Finally, leaves were inoculated with conidia 1hpi and stained for ROS 24 hpi. Additionally, barley leaves were injected with 5 µM DPI (diphenyleneiodonium; Sigma, St Louis), then washed and inoculated, as above.
Detection of fungal cell wall
Calcofluor White M2R (Fluorescent brightener 28, F-6258, Sigma, St Louis) was used for detection of the fungal cell wall. We made 10,000-fold dilutions from a saturated Calcofluor White stock solution. For experiments involving conidia on gel-bond (VWR, Arlington Heights, IL), Calcofluor White was applied 1, 4, 8, 12, and 24 hours post inoculation, incubated for 15 minutes, then gently rinsed off with 1X PBS buffer. For experiments involving inoculated plants, inoculated or non-inoculated (control) leaf tissue was collected and immersed in working solution for 15 minutes, then gently rinsed with 0.1 mM KCl, 0.1 mM CaCl2 (pH 6.0).
Detection of Cell Wall Appositions (CWAs)
For CWAs staining, we cleared inoculated or non-inoculated (control) leaves in ethanol:acetic acid (6 : 1 v/v) overnight and washed them with water. Subsequently, cleared leaves were incubated in 0.05% aniline blue (w/v) in 0.067 M K2HP04 buffer at pH 9.2 overnight and rinsed gently in sterilized deionized water for microscopy.
Localization of DAB
Inoculated barley leaves were stained using DAB and rinsed several times in PBS. Thereafter, samples were fixed in 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and 2% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) in sodium cacodylate (Electron Microscopy Sciences, Hatfield, PA) buffer for 1 hour overnight. Samples were then rinsed three times, 15 min each, in sodium cacodylate and post-fixed with 2% OsO4 in sodium cacodylate for 3–5 hours on a rotator. Again, samples were then rinsed three times for 15 min each, with water on a rotator. Samples then underwent an ethanol dehydration series (25%, 50%, 80% ETOH; 20 min each) on a rotator. Samples were primed with 1% gamma-glycidoxylpropyl trimethoxysilane in 80% ETOH overnight at room temperature and then washed three times for 15 min each in 100% ETOH on a rotator. Samples then underwent a series of infiltrations on a rotator as follows: 100% ETOH/n-BGE (Electron Microscopy Sciences, Hatfield, PA) (1 : 1) for 30 min, 100% n-BGE for 30 min, n-BGE/Quetol-651 (Electron Microscopy Sciences, Hatfield, PA) (1 : 3) for 1 hour, n-BGE/Quetol-651 (1 : 1) for 1 hour, n-BGE/Quetol-651 (3 : 1) for 1 hour, 100% Quetol-651 for 1 hour, 100% Quetol-651 for 1 hour, 100% Quetol-651 overnight and 100% Quetol-651 for 1 hour. Finally, samples were embedded and polymerized in an oven at 60°C for about 24 hours.
Bioinformatic and statistical analyses
BlastP analysis was done against the fully sequenced genomic database of M. oryzae housed at the Broad Institute, using an e-value of 1e-3. ClustalW (X2) was used to perform the full alignment and generate the phylogenetic tree. The final tree image was generated with Tree Viewer. The HYR1 protein secondary structure was predicted using the PSIPRED protein structure prediction server. The structural image of the HYR1 protein was created using the PyMOL molecular viewer. All student t-tests were performed using JMP8 (SAS Institute Inc. 2007. <Title>. Cary, NC: SAS Institute Inc.).
Confocal microscopy
Confocal images were taken with Zeiss LSM510 or Zeiss LSM5 DUO using a C-Apochromat 40X (NA = 1.2) water immersion objective lens. H2DCFDA ester was excited at 488 nm and fluorescence was detected using a 505–550 nm band pass filter. Calcofluor white was excited at 405 nm and detected using 420–470 nm band pass filter. Cerulean was excited at 458 nm and detected using a 475 long pass filter. We also used transmitted light and reflected light for some confocal experiments.
Supporting Information
Zdroje
1. Mittler
R
Vanderauwera
S
Gollery
M
Van Breusegem
F
2004
Reactive oxygen gene network of plants.
Trends Plant Sci
9
490
498
2. Rogers
SA
2002
Detoxify or Die.
Sarasota, , FL
Sand Key Company, Inc
409
3. Thannickal
VJ
Fanburg
BL
2000
Reactive oxygen species in cell signaling.
Am J Physiol Lung Cell Mol Physiol
279
L1005
L1028
4. Jones
JDG
Dangl
JL
2006
The plant immune system.
Nature
444
323
329
5. Egan
MJ
Wang
ZY
Jones
MA
Smirnoff
N
Talbot
NJ
2007
Generation of reactive oxygen species by fungal NADPH oxidases is
required for rice blast disease.
Proc Natl Acad Sci U S A
104
11772
11777
6. Skamnioti
P
Henderson
C
Zhang
ZG
Robinson
Z
Gurr
SJ
2007
A novel role for catalase B in the maintenance of fungal
cell-wall integrity during host invasion in the rice blast fungus
Magnaporthe grisea.
Mol Plant-Microbe Interact
20
568
580
7. Neill
S
Desikan
R
Hancock
J
2002
Hydrogen peroxide signalling.
Curr Opin Plant Biol
5
388
395
8. Sagi
M
Davydov
O
Orazova
S
Yesbergenova
Z
Ophir
R
2004
Plant respiratory burst oxidase homologs impinge on wound
responsiveness and development in Lycopersicon esculentum.
Plant Cell
16
616
628
9. Parker
D
Beckmann
M
Zubair
H
Enot
DP
Caracuel-Rios
Z
2009
Metabolomic analysis reveals a common pattern of metabolic
re-programming during invasion of three host plant species by Magnaporthe
grisea.
Plant J
59
723
737
10. Shetty
NP
Mehrabi
R
Lutken
H
Haldrup
A
Kema
GHJ
2007
Role of hydrogen peroxide during the interaction between the
hemibiotrophic fungal pathogen Septoria tritici and wheat.
New Phytol
174
637
647
11. Able
AJ
2003
Role of reactive oxygen species in the response of barley to
necrotrophic pathogens.
Protoplasma
221
137
143
12. Galletti
R
Denoux
C
Gambetta
S
Dewdney
J
Ausubel
FM
2008
The AtrbohD-Mediated Oxidative Burst Elicited by
Oligogalacturonides in Arabidopsis Is Dispensable for the Activation of
Defense Responses Effective against Botrytis cinerea.
Plant Physiol
148
1695
1706
13. Kato
T
Tanabe
S
Nishimura
M
Ohtake
Y
Nishizawa
Y
2009
Differential responses of rice to inoculation with wild-type and
non-pathogenic mutants of Magnaporthe oryzae.
Plant Mol Biol
70
617
625
14. Chi
MH
Park
SY
Kim
S
Lee
YH
2009
A Novel Pathogenicity Gene Is Required in the Rice Blast Fungus
to Suppress the Basal Defenses of the Host.
PLoS Path
5
e1000401
15. Tanabe
S
Nishizawa
Y
Minami
E
2009
Effects of catalase on the accumulation of H2O2 in rice cells
inoculated with rice blast fungus, Magnaporthe oryzae.
Physiol Plant
137
148
154
16. Kim
KH
Willger
SD
Park
SW
Puttikamonkul
S
Grahl
N
2009
TmpL, a Transmembrane Protein Required for Intracellular Redox
Homeostasis and Virulence in a Plant and an Animal Fungal
Pathogen.
PLoS Path
5
e1000653
17. Inoue
Y
Matsuda
T
Sugiyama
K
Izawa
S
Kimura
A
1999
Genetic analysis of glutathione peroxidase in oxidative stress
response of Saccharomyces cerevisiae.
J Biol Chem
274
27002
27009
18. Zhang
WJ
He
YX
Yang
Z
Yu
J
Chen
Y
2008
Crystal structure of glutathione-dependent phospholipid
peroxidase Hyr1 from the yeast Saccharomyces cerevisiae.
Proteins
73
1058
1062
19. Delaunay
A
Pflieger
D
Barrault
MB
Vinh
J
Toledano
MB
2002
A thiol peroxidase is an H2O2 receptor and redox-transducer in
gene activation.
Cell
111
471
481
20. Molina
L
Kahmann
R
2007
An Ustilago maydis gene involved in H2O2 detoxification is
required for virulence.
Plant Cell
19
2293
2309
21. Delaunay
A
Isnard
AD
Toledano
MB
2000
H2O2 sensing through oxidation of the Yap1 transcription
factor.
EMBO J
19
5157
5166
22. Lee
J
Godon
C
Lagniel
G
Spector
D
Garin
J
1999
Yap1 and Skn7 control two specialized oxidative stress response
regulons in yeast.
J Biol Chem
274
16040
16046
23. Lev
S
Hadar
R
Amedeo
P
Baker
SE
Yoder
OC
2005
Activation of an AP1-like transcription factor of the maize
pathogen Cochliobolus heterostrophus in response to oxidative stress and
plant signals.
Eukaryot Cell
4
443
454
24. Levine
A
Tenhaken
R
Dixon
R
Lamb
C
1994
H2O2 from the oxidative burst orchestrates the plant
hypersensitive disease resistance response.
Cell
79
583
593
25. Lin
CH
Yang
SL
Chung
KR
2009
The YAP1 Homolog-Mediated Oxidative Stress Tolerance Is Crucial
for Pathogenicity of the Necrotrophic Fungus Alternaria alternata in
Citrus.
Mol Plant-Microbe Interact
22
942
952
26. Temme
N
Tudzynski
P
2009
Does Botrytis cinerea Ignore H2O2-Induced Oxidative Stress During
Infection? Characterization of Botrytis Activator Protein 1.
Mol Plant-Microbe Interact
22
987
998
27. McGuffin
LJ
Bryson
K
Jones
DT
2000
The PSIPRED protein structure prediction server.
Bioinformatics
16
404
28. Martin
JL
1995
Thioredoxin - a Fold for All Reasons.
Structure
3
245
250
29. Valent
B
Farrall
L
Chumley
FG
1991
Magnaporthe-grisea Genes for Pathogenicity and Virulence
Identified through a Series of Backcrosses.
Genetics
127
87
101
30. Howard
RJ
Valent
B
1996
Breaking and entering: Host penetration by the fungal rice blast
pathogen Magnaporthe grisea.
Annu Rev Microbiol
50
491
512
31. Myhre
O
Andersen
JM
Aarnes
H
Fonnum
F
2003
Evaluation of the probes 2 ′,7 ′-dichlorofluorescin
diacetate, luminol, and lucigenin as indicators of reactive species
formation.
Biochem Pharmacol
65
1575
1582
32. Kang
SH
Khang
CH
Lee
YH
1999
Regulation of cAMP-dependent protein kinase during appressorium
formation in Magnaporthe grisea.
FEMS Microbiol Lett
170
419
423
33. Smirnoff
N
2000
Ascorbic acid: metabolism and functions of a multi-facetted
molecule.
Curr Opin Plant Biol
3
229
235
34. Huckelhoven
R
2007
Cell wall - Associated mechanisms of disease resistance and
susceptibility.
Annu Rev Phytopathol
45
101
127
35. Dai
SH
Wei
XP
Alfonso
AA
Pei
LP
Duque
UG
2008
Transgenic rice plants that overexpress transcription factors
RF2a and RF2b are tolerant to rice tungro virus replication and
disease.
Proc Natl Acad Sci U S A
105
21012
21016
36. Bolwell
GP
Bindschedler
LV
Blee
KA
Butt
VS
Davies
DR
2002
The apoplastic oxidative burst in response to biotic stress in
plants: a three-component system.
J Exp Bot
53
1367
1376
37. Trujillo
M
Altschmied
L
Schweizer
P
Kogel
KH
Huckelhoven
R
2006
Respiratory Burst Oxidase Homologue A of barley contributes to
penetration by the powdery mildew fungus Blumeria graminis f. sp
hordei.
J Exp Bot
57
3781
3791
38. Davies
DR
Bindschedler
LV
Strickland
TS
Bolwell
GP
2006
Production of reactive oxygen species in Arabidopsis thaliana
cell suspension cultures in response to an elicitor from Fusarium oxysporum:
implications for basal resistance.
J Exp Bot
57
1817
1827
39. Lessing
F
Kniemeyer
O
Wozniok
W
Loeffler
J
Kurzai
O
2007
The Aspergillus fumigatus transcriptional regulator AfYap1
represents the major regulator for defense against reactive oxygen
intermediates but is dispensable for pathogenicity in an intranasal mouse
infection model.
Eukaryot Cell
6
2290
2302
40. Khang
CH
Berruyer
R
Giraldo
MC
Kankanala
P
Park
SY
2010
Translocation of Magnaporthe oryzae Effectors into Rice Cells and
Their Subsequent Cell-to-Cell Movement.
Plant Cell
22
1388
1403
41. Sweigard
JA
Chumley
FG
Valent
B
1992
Disruption of a Magnaporthe-Grisea Cutinase Gene.
Mol Gen Genet
232
183
190
42. Mosquera
G
Giraldo
MC
Khang
CH
Coughlan
S
Valent
B
2009
Interaction Transcriptome Analysis Identifies Magnaporthe oryzae
BAS1-4 as Biotrophy-Associated Secreted Proteins in Rice Blast
Disease.
Plant Cell
21
1273
1290
43. Patterson
GH
Piston
DW
Barisas
BG
2000
Forster distances between green fluorescent protein
pairs.
Anal Biochem
284
438
440
44. ThordalChristensen
H
Zhang
ZG
Wei
YD
Collinge
DB
1997
Subcellular localization of H2O2 in plants. H2O2 accumulation in
papillae and hypersensitive response during the barley-powdery mildew
interaction.
Plant J
11
1187
1194
45. Fink
JL
Hamilton
N
2007
DomainDraw: A macromolecular feature drawing
program.
In Silico Biol
7
145
150
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Innate Immune Sensing of DNA
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Low Diversity Variety Multilocus Sequence Types from Thailand Are Consistent with an Ancestral African Origin
- Genetic Assignment Methods for Gaining Insight into the Management of Infectious Disease by Understanding Pathogen, Vector, and Host Movement
- Innate Immune Sensing of DNA
- Engineered Resistance to Development in Transgenic
- -Mediated Detoxification of Reactive Oxygen Species Is Required for Full Virulence in the Rice Blast Fungus
- SLO-1-Channels of Parasitic Nematodes Reconstitute Locomotor Behaviour and Emodepside Sensitivity in Loss of Function Mutants
- Structure-Function Analysis of the LRIM1/APL1C Complex and its Interaction with Complement C3-Like Protein TEP1
- A Effector with Enhanced Inhibitory Activity on the NF-κB Pathway Activates the NLRP3/ASC/Caspase-1 Inflammasome in Macrophages
- The MARCH Family E3 Ubiquitin Ligase K5 Alters Monocyte Metabolism and Proliferation through Receptor Tyrosine Kinase Modulation
- is an Unstable Pathogen Showing Evidence of Significant Genomic Flux
- The Cell Wall Protein CwpV is Antigenically Variable between Strains, but Exhibits Conserved Aggregation-Promoting Function
- Engineering HIV-Resistant Human CD4+ T Cells with CXCR4-Specific Zinc-Finger Nucleases
- SUMO-Interacting Motifs of Human TRIM5α are Important for Antiviral Activity
- Completion of Hepatitis C Virus Replication Cycle in Heterokaryons Excludes Dominant Restrictions in Human Non-liver and Mouse Liver Cell Lines
- : Reservoir Hosts and Tracking the Emergence in Humans and Macaques
- On Being the Right Size: The Impact of Population Size and Stochastic Effects on the Evolution of Drug Resistance in Hospitals and the Community
- Bacterial and Host Determinants of MAL Activation upon EPEC Infection: The Roles of Tir, ABRA, and FLRT3
- Respiratory Syncytial Virus Interferon Antagonist NS1 Protein Suppresses and Skews the Human T Lymphocyte Response
- NF-κB Hyper-Activation by HTLV-1 Tax Induces Cellular Senescence, but Can Be Alleviated by the Viral Anti-Sense Protein HBZ
- Human Cytomegalovirus IE1 Protein Elicits a Type II Interferon-Like Host Cell Response That Depends on Activated STAT1 but Not Interferon-γ
- A New Model to Produce Infectious Hepatitis C Virus without the Replication Requirement
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- NF-κB Hyper-Activation by HTLV-1 Tax Induces Cellular Senescence, but Can Be Alleviated by the Viral Anti-Sense Protein HBZ
- Bacterial and Host Determinants of MAL Activation upon EPEC Infection: The Roles of Tir, ABRA, and FLRT3
- : Reservoir Hosts and Tracking the Emergence in Humans and Macaques
- On Being the Right Size: The Impact of Population Size and Stochastic Effects on the Evolution of Drug Resistance in Hospitals and the Community
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání