-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Physical activity levels in adults and older adults 3–4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care
In this follow-up of participants from two completed trials of pedometer-based walking interventions, Tess Harris and colleagues present evidence that increases in physical activity levels seen at 12 months are still present 3-4 years later.
Published in the journal: . PLoS Med 15(3): e32767. doi:10.1371/journal.pmed.1002526
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002526Summary
In this follow-up of participants from two completed trials of pedometer-based walking interventions, Tess Harris and colleagues present evidence that increases in physical activity levels seen at 12 months are still present 3-4 years later.
Introduction
Strong evidence exists for the health benefits of physical activity (PA) for a wide range of conditions [1,2]. Physical inactivity leads to high health service costs [1,3] and is the fourth leading risk factor for global mortality [2]. Adult and older adult guidelines advise ≥150 minutes of moderate-to-vigorous PA (MVPA) weekly, or 75 minutes of vigorous PA, or a combination, in ≥10-minute bouts [1,4], but any increase in PA for inactive people is valuable [5]. Many PA interventions, including pedometer-based interventions, increase PA levels in the short term [6–8]. However, long-term health effects require sustained PA changes [1], and evidence for maintenance is lacking. A meta-analysis of PA interventions (including pedometers) in 55–70-year-olds [8] only identified 2 trials with objective PA data beyond 12 months [9,10]. One showed a significant step-count effect 18 months post-baseline, but only 6 months post-intervention [10]; the other showed a significant increase in step count in the lifestyle group 23 months post-baseline, but only 12 months post-intervention [9]. The meta-analysis authors [8] repeated requests made by previous systematic reviews [11,12] and guidelines [13] for trials to be conducted with longer follow-up periods and objective PA measures.
We previously conducted two pedometer-based walking interventions with adults and older adults, which increased step count and MVPA in bouts at 12 months and provided longer-term follow-up opportunities [14,15]. Both trials recruited postally from primary care and delivered 12-week pedometer-based walking interventions incorporating behaviour change techniques (BCTs) through dedicated practice nurse PA consultations (3 in PACE-UP, 4 in PACE-Lift) or by post (PACE-UP only). PACE-Lift nurse consultations additionally provided feedback on accelerometry findings to participants. PACE-UP recruited 1,023 predominantly inactive 45–75-year-olds. Average baseline daily step count was 7,479 (standard deviation [SD]: 2,671) and average time in MVPA in bouts was 94 (SD: 102) minutes/week. PACE-Lift recruited 298 patients aged 60–75 years. Average baseline daily step count was 7,347 (SD: 2,839) and average time in MVPA in bouts was 92 (SD: 108) minutes/week. Despite age-group and intervention differences, both trials and all intervention groups showed increases in step counts of approximately one-tenth and time in MVPA of over one-third between baseline and 12 months [14,15].
The study aim was to follow up both trial cohorts to examine objectively measured PA levels at 3 years in PACE-UP and 4 years in PACE-Lift. Given the different but overlapping age ranges, interventions that were similar but differed in intensity, and different lengths of follow-up, we analysed the two trials separately, using identical methods, and present the results in parallel.
Methods
Study design and participants
PACE-UP 3-year follow-up
For the PACE-UP trial 3-year follow-up, London, Hampstead, Research Ethics Committee (UK) granted approval (12L/LO/0219). Written informed consent was gained from all research participants. Trial methods are published [16]; the postal and nurse interventions are summarised in Table 1 and baseline findings are summarised in S1 Table. The handbook and diary are available at www.paceup.sgul.ac.uk/materials. After a 12-month follow-up, 212/322 (66%) of controls were posted a pedometer, handbook, and PA diary and 64/322 (20%) opted for a single nurse appointment, during which they also received these materials. No further follow-up was offered at that point (compared with the trial postal-intervention group, who were telephoned to check that materials had arrived and encouraged to return completed PA diaries). Three-year follow-up collected accelerometry and patient-reported outcome measures (PROMs) by post. To minimise seasonal effects on PA levels, baseline, 12-month, and 3-year outcomes were assessed in the same month. Follow-up ran from October 2015 to November 2016. The protocol, including 3-year follow-up details, is included (S1 Protocol).
Tab. 1. Components of interventions for PACE-UP and PACE-Lift trials. 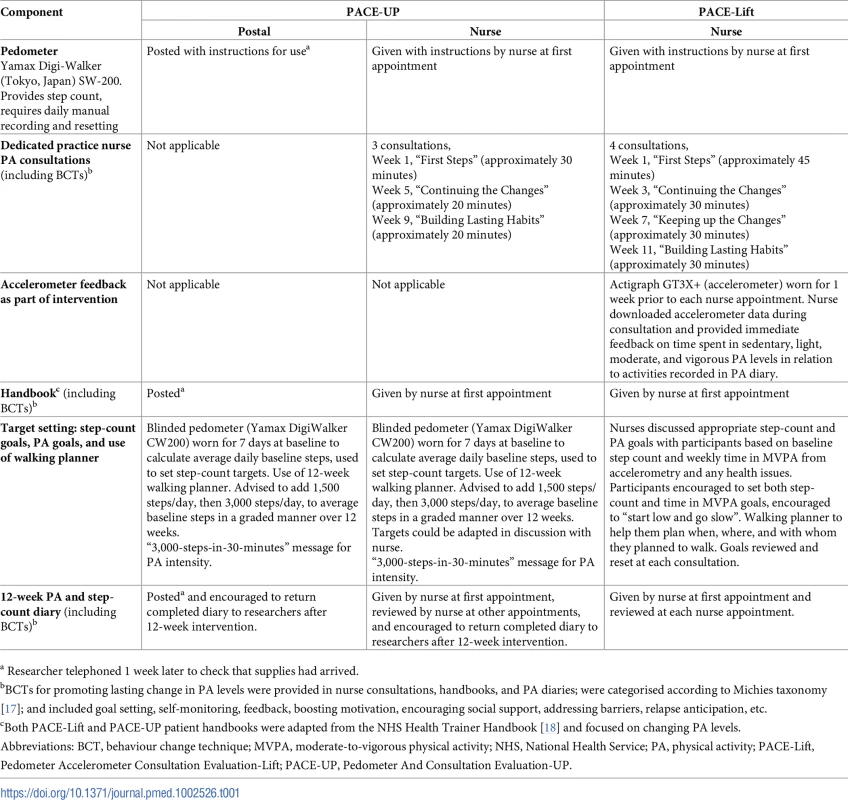
a Researcher telephoned 1 week later to check that supplies had arrived. PACE-Lift 4-year follow-up
For the PACE-Lift trial 4-year follow-up, Oxfordshire Research Ethics Committee C (UK) granted approval (11/H0606/2). Written informed consent was gained from all research participants. Trial methods are published [19]; the intervention is summarised in Table 1 and baseline findings are summarised in S1 Table. After 12-month follow-up, all control group participants were sent a pedometer and instructions; no support was offered. Four-year follow-up collected the accelerometry and PROMs by post, as in PACE-UP. Additionally, for PACE-Lift, the opportunity to meet study participants face to face after postal return of accelerometers and questionnaires was offered to measure anthropometric variables. For consistency with PACE-UP (in which face-to-face contact was not offered), only postal outcomes (accelerometry and PROMs) are reported in this paper. Baseline, 12-month, and 4-year outcomes were assessed in the same month. Follow-up ran from October 2015 to October 2016. The protocol, including 4-year follow-up, is included (S2 Protocol).
Procedures
Participants who had not withdrawn from either trial by 12 months were eligible. Practices excluded participants who had died, moved away, or developed a terminal illness or dementia. Eligible participants were sent a trial follow-up letter, participant information sheet, consent form, and freepost return envelope. Researchers telephoned participants to discuss any queries. Those interested returned signed consent forms. Participants and researchers were unmasked to intervention allocation.
Instruments, questionnaire measures, and protocols were the same as during the trial. Participants were not asked to increase their PA levels, just to continue usual activity, and thus health limitations did not preclude participation. Participants were instructed to wear the accelerometer (Actigraph GT3X+) on a belt over one hip for 7 consecutive days, from getting up until going to bed. A diary (to record activities) questionnaire and freepost envelope were provided. If accelerometry recording did not result in ≥5 days with ≥540 minutes/day, participants were asked to re-wear monitors (re-wears were required for 20 PACE-UP and 1 PACE-Lift participants). Participants were posted a £10 gift voucher.
The Actigraph GT3X+ measures vertical accelerations in magnitudes from 0.05 to 2.0 g, sampled at 30 Hz, and then summed over a 5-second epoch time period. It can record PA continuously for up to 21 days. Actigraph data were reduced using Actilife software (V6.6.0), ignoring runs of ≥60 minutes of 0 counts [14,15]. Summary variables were as used in the trials [14,15]: step counts, accelerometer wear time, time spent in total MVPA (≥1,952 counts per minute, equivalent to ≥3 metabolic equivalents), time spent in ≥10-minute bouts of MVPA, and time spent sedentary (≤100 counts per minute, equivalent to ≤1.5 metabolic equivalents). Only days with ≥540 minutes of registered time were used. To lessen attrition bias, main analyses of effect included all subjects with ≥1 satisfactory day of recording at 3 (or 4) years.
Outcomes
Outcomes focussed on changes between baseline measures and follow-up measures at 3 years (PACE-UP) or 4 years (PACE-Lift). For accelerometry, we analysed: (i) change in average daily step count, (ii) change in time spent weekly in MVPA in ≥10-minute bouts, and (iii) change in weekly sedentary time.
Questionnaire PROMs were as for 3 - and 12-month outcomes [16,19]: quality of life [20], exercise self-efficacy [21], pain [22], depression [23, 24], and anxiety [23, 25].
Statistical analysis
Analysis and reporting followed CONSORT guidelines (S1 Protocol, S2 Protocol). Primary analyses were conducted using STATA version 14.0 (StataCorp), with a two-step process to estimate change. In step 1, average daily step counts at 3 years (PACE-UP) or 4 years (PACE-Lift) were computed from a random-effects model, allowing for day of the week and day of wearing the accelerometer as fixed effects and participant as a random effect. In step 2, average daily step count at 3 years (PACE-UP) or 4 years (PACE-Lift) was regressed on estimated baseline average daily step count, with treatment group, age, gender, practice, and month of baseline accelerometry as fixed effects and household as a random effect in a multilevel model. Identical analyses were carried out for MVPA in ≥10-minute bouts, sedentary time, and wear time. Changes in PROMs were estimated using step 2 only.
Primary analyses used 681 (PACE-UP) or 225 (PACE-Lift) participants who provided accelerometry data at 3 or 4 years, respectively. Sensitivity analyses assessed the effect of missingness: (1) multiple imputation methods were used to impute outcome data for those missing at 3 or 4 years, assuming outcomes were missing at random (MAR), conditional on model variables, and using the STATA procedure mi impute, and (2) missing not at random (MNAR) analyses. The purpose of the MNAR analyses was to assess how extreme the missing data needed to be in order to explain away our positive effect estimates. To do this, we used the Stata module rctmiss (Statistical Software Components [SSC] https://ideas.repec.org/s/boc/bocode.html) [26]. Essentially, the rctmiss programme takes as its starting point MAR estimates for all subjects with missing data. It then adds or subtracts steps to the estimates before re-estimating the treatment effects. Thus, we left the control group missing values at their MAR estimates and first subtracted 500 steps/day from the MAR estimates in the treatment groups; we then took a more extreme scenario, in which we subtracted 1,000 steps/day for those in the treatment groups, again leaving the control group missing values at their MAR values.
Results
Of 1,023 PACE-UP participants, 32 withdrew by 12 months, 2 died before the 3-year follow-up, 1 was excluded, and 681 provided ≥1 day of adequate accelerometry data. The 3-year follow-up rate was 69% (681/988), or 67% (681/1,023) of initial trial participants, the mean age was 59 (SD = 7.9), and 64% (438/681) were female. Of 298 PACE-Lift participants, 15 withdrew by 12 months, 2 died before the 4-year follow-up, and 225 provided ≥1 day of adequate accelerometry data. The 4-year follow-up rate was 80% (225/281), or 76% (225/298) of original trial participants, the mean age was 67 (SD = 4.2), and 53% (120/225) were female. The CONSORT diagram (Fig 1) shows 3 - and 4-year follow-up data by randomised groups. Ninety-two percent (625/681) in PACE-UP and 93% (209/225) in PACE-Lift provided ≥5 days of accelerometry data at 3 and 4 years, respectively (S2 Table and S3 Table).
Fig. 1. CONSORT diagrams for PACE-UP and PACE-Lift studies. 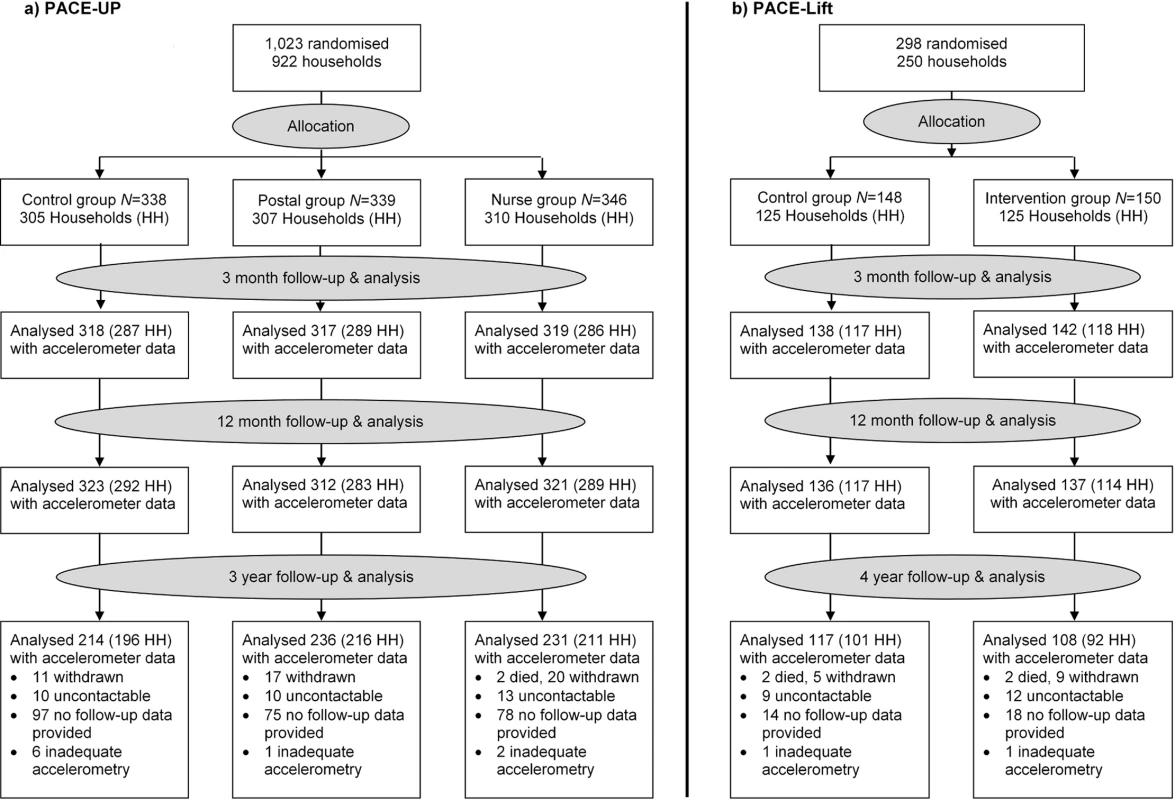
HH, household; PACE-Lift, Pedometer Accelerometer Consultation Evaluation Lift; PACE-UP, Pedometer And Consultation Evaluation-UP. Accelerometry summary measures are shown for the three PACE-UP groups (S2 Table) and two PACE-Lift groups (S3 Table) at each time point. Fig 2 displays effect estimates for different groups from both trials at all time periods for step counts and time in MVPA in bouts, respectively. Table 2 shows these estimates plus sedentary time and wear time in tabular form. At 3 years in PACE-UP, both intervention groups are doing more steps/day than controls, with no significant intervention group differences: postal +627 (95% CI: 198–1,056); nurse +670 (95% CI: 237–1,102). For PACE-Lift, at 4 years, the intervention group is doing more steps/day than the control group, although the difference is not statistically significant: +407 (95% CI: −177–992). For total weekly MVPA in ≥10-minute bouts (minutes/week), PACE-UP 3-year findings compared with control are as follows: postal +28 (95% CI: 7–49); nurse +24 (95% CI: 3–45). For PACE-Lift at 4 years, the intervention group is still doing significantly more MVPA in bouts (minutes/week) than the control group: +32 (95% CI: 5–60). Effect estimates for both steps per day and MVPA were stable when we limited analyses to subjects with at least 4 days of measurement at follow-up (S4 Table).
Fig. 2. PACE-UP and PACE-Lift studies. 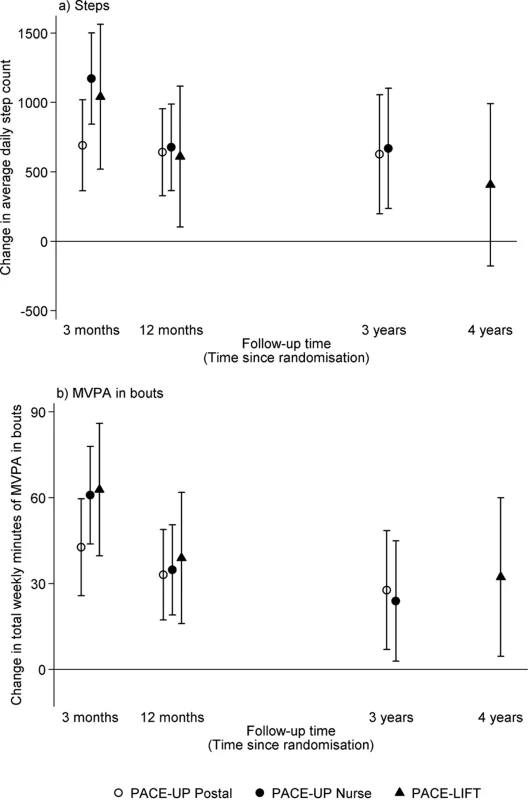
Effect estimates and 95% confidence intervals for change in (a) average daily steps and (b) total weekly minutes of MVPA in bouts at 3 months, 12 months, and 3 years (PACE-UP) and 4 years (PACE-Lift). Effect sizes, 95% confidence intervals, and p-values were obtained from multilevel linear regression models (see Methods). 3 months: p < 0.001 for all PACE-UP and PACE-Lift steps and MVPA intervention effects. 12 months: p < 0.001 for PACE-UP steps and PACE-UP MVPA; p = 0.02 for PACE-Lift steps and p < 0.001 for PACE-Lift MVPA. 3 years: p < 0.01 for PACE-UP steps and PACE-UP MVPA postal group; p = 0.03 for PACE-UP MVPA nurse group. 4 years: p = 0.17 for PACE-Lift steps and p = 0.02 for PACE-Lift MVPA. MVPA, moderate-to-vigorous physical activity; PACE-Lift, Pedometer Accelerometer Consultation Evaluation-Lift; PACE-UP, Pedometer And Consultation Evaluation-UP. Tab. 2. PACE-UP and PACE-Lift studies: Accelerometry outcomes at 3 months, 12 months, and 3 years (PACE-UP) and 4 years (PACE-Lift). 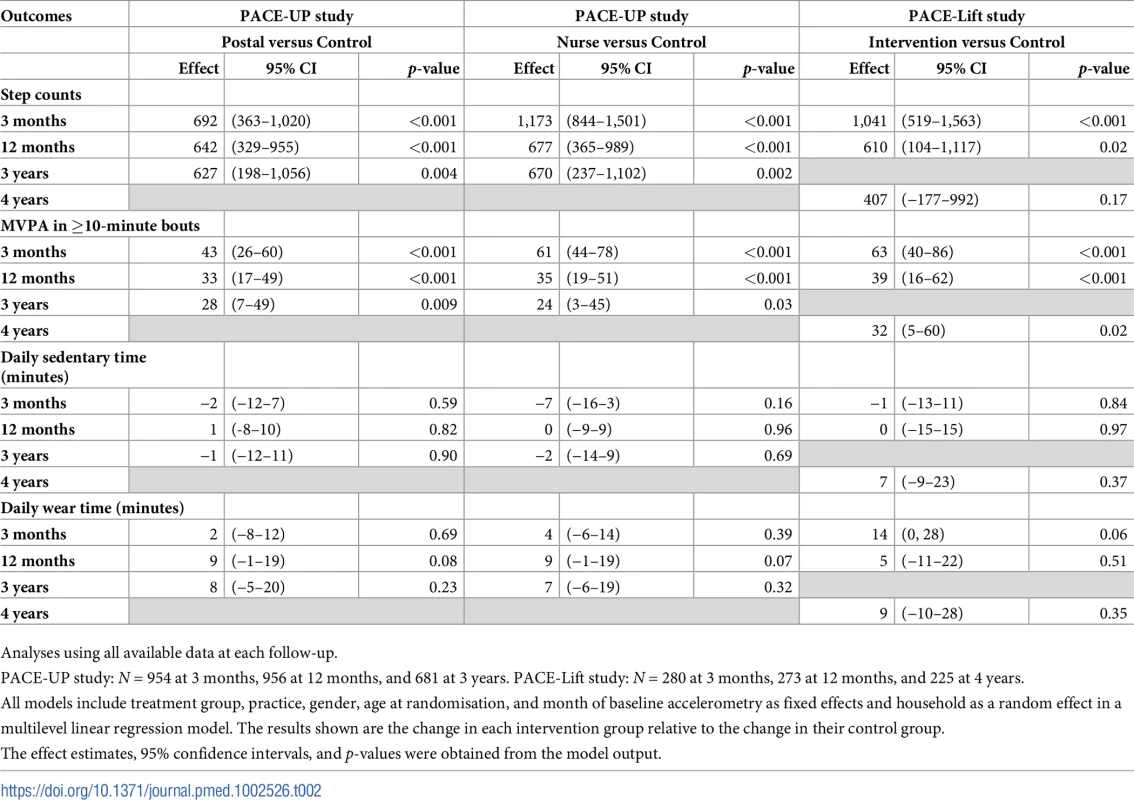
Analyses using all available data at each follow-up. In PACE-UP, the 3-year treatment effects for steps/day were 98% (postal) (627/642) and 99% (nurse) (670/677), respectively, of the 1-year estimates; in PACE-Lift, the 4-year estimate was 67% (407/610) of the 1-year estimate. For minutes of MVPA in 10-minute bouts, PACE-UP estimates were 85% (postal) (28/33) and 69% (nurse) (24/35), respectively, of the 1-year estimates, while the PACE-Lift estimate was 82% (32/39). Neither PACE-UP nor PACE-Lift showed differences between intervention and control groups at 3 and 4 years for sedentary time or daily wear time (Table 2). A PACE-UP subgroup analysis demonstrated similar effects for steps/day in 45–59 - and 60–75-year-olds (S1 Fig).
None of the interventions had significant effects on pain, depression, anxiety, or health-related quality of life at 3 or 4 years, consistent with 3 - and 12-month findings (S4 Table). In PACE-UP, there was a persistent exercise self-efficacy effect for the nurse group at 3 years (also seen at 3 and 12 months) but not in PACE-Lift at 4 years (S5 Table).
Table 3 presents sensitivity analyses assuming that missing outcome data were MAR, conditional on a variety of predictors; analyses had little impact on the primary outcome step-count effect estimates and do not change interpretation. For the MNAR analyses, we combined both intervention groups in PACE-UP to increase power and simplify presentation; separate analyses give a similar picture. The MNAR analyses (S2 Fig) make a bigger impact for both trials but only when we assume there is a strong differential departure between the non-random effects in control and treatment groups (see solid lines in S2 Fig). For example, when we assume that the missing data in the treatment groups are 1,000 steps below their MAR values while the control group values are at their MAR values, the treatment effects for PACE-UP are no longer statistically significant; but even then, the confidence interval is still largely positive.
Tab. 3. PACE-UP and PACE-Lift studies: Imputation analyses for step counts at 3 years (PACE-UP) and 4 years (PACE-Lift). 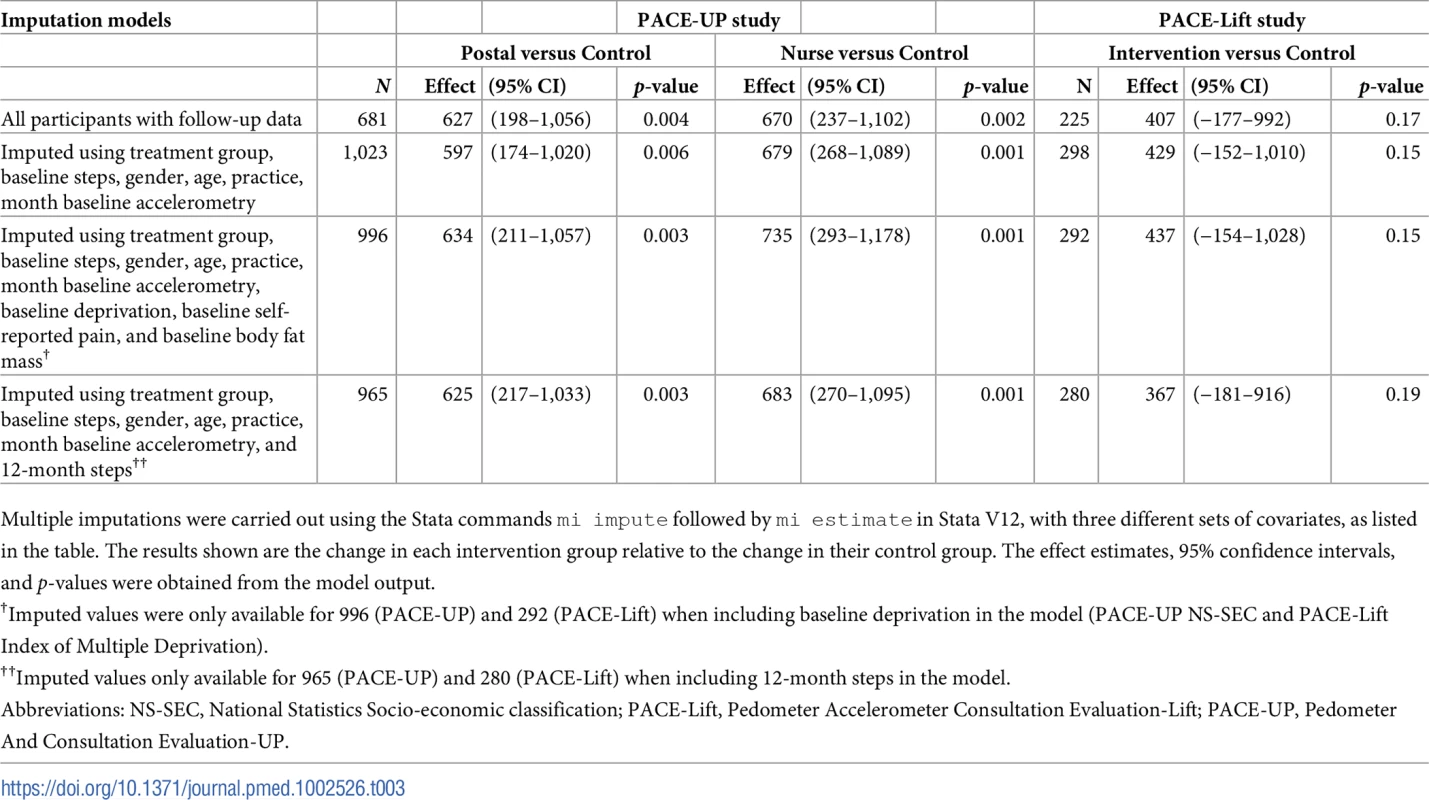
Multiple imputations were carried out using the Stata commands mi impute followed by mi estimate in Stata V12, with three different sets of covariates, as listed in the table. The results shown are the change in each intervention group relative to the change in their control group. The effect estimates, 95% confidence intervals, and p-values were obtained from the model output. Discussion
To our knowledge, these are the first population-based pedometer studies showing effects on objectively measured PA levels more than 12 months post-intervention. Compared to controls, intervention participants followed up from both PACE-UP and PACE-Lift trials showed significant increases in MVPA in bouts at 3 and 4 years of approximately an extra 30 minutes weekly, with no difference between intervention groups in PACE-UP (as was also found at 12 months). PACE-UP showed a significant step-count increase of approximately 650 steps/day; PACE-Lift showed a similar but nonsignificant step-count increase. The increases seen in PA levels were similar to those seen at 12 months. No differences were seen in sedentary or wear time.
This work’s main strength is its documentation of longer-term follow-up results beyond 12 months from trials with objective PA data relevant to guidelines. Both trials were based on population-based primary care samples and achieved good follow-up. Sensitivity analyses demonstrated that effect estimates were robust; only extreme assumptions changed interpretation. We presented findings for two trials with overlapping but different age groups and slightly different intervention intensities and follow-up periods. However, the many similarities (recruited postally from primary care; 12-week pedometer-based interventions, including nurse-support arms; accelerometer-assessed main PA outcome measures beyond 12 months) meant there was considerable value in presenting the results together. Age was not an effect modifier in PACE-UP. Despite their differences, both trials show similar consistent long-term increased time in MVPA in bouts for intervention group participants.
The study also had a number of potential limitations. Long-term follow-up data were provided by 76% of PACE-Lift and 67% of PACE-UP original trial participants. Whilst only a small proportion of participants actively withdrew from each trial, reasons for withdrawal were not systematically collected. Whilst losing between a quarter and a third of subjects at follow-up could reduce the generalizability of the findings, we have directly addressed the risk of attrition bias through sensitivity analyses using appropriate imputation methods, and this gave robust results. Participants and researchers were unmasked to group; however, PA outcomes were assessed objectively by accelerometry, and participants were blind to measurements. Participants might have tried harder with PA when monitored, but this would also have affected controls and would have been reduced by using a 7-day data collection protocol [6]. Also, the intervention groups increased their MVPA in ≥10-minute bouts, implying that participants made changes as advised. Whilst the Actigraph accelerometer provides valid estimates of time spent in different intensity levels, including MVPA [27], any waist-mounted activity monitor may underestimate upper body movement, such as weight training and carrying heavy loads [28]; it also underestimates cycling and did not measure swimming. However, crucially, accelerometers are most sensitive to ambulatory activities such as walking, which was the main intervention component of both trials. A further potential limitation is that minimal interventions were offered to both trial control groups after 12-month follow-up. However, this contamination would tend to weaken intervention effects, so the existence of differences in PA levels at 3 and 4 years is an important positive finding and helps us to understand the additional support required for a successful postal intervention.
This paper provides novel, important evidence on sustained effects of pedometer-based walking interventions on objectively measured PA levels. A recent systematic review of the effectiveness of behavioural interventions in increasing PA at 12–36 months [8] identified two studies that provided objectively measured outcomes beyond 12 months [9,10]. We identified two more recent studies using a similar search strategy [29,30]. In reviewing these studies, several issues emerge. First, interventions differed dramatically in duration, intensity, and resources needed—particularly important when considering cost-effectiveness. Second, studies reported follow-up length post-baseline, not post-intervention; maintenance of effects is defined by the latter. None of the four studies provided outcomes more than 12 months post-intervention: one was 6 months post-intervention [10], two were ongoing at the point of assessment [29, 30], and the final one was 12 months post-intervention [9]. Our two studies thus provide the first clear evidence of efficacy for pedometer-based interventions at 33 months and 45 months post-intervention, providing the type of evidence from PA interventions recently called for [8,11,13]. The simplicity of our postal intervention makes it likely to be more cost-effective than more intensive interventions, and the PACE-UP trial cost-effectiveness analyses at 12-month follow-up demonstrated this [14,31].
Our findings support guidance to promote pedometers alongside support for goal setting, self-monitoring, and feedback [32]. However, it is important to consider which factors in pedometer-based interventions are important for success. Both PACE-UP and PACE-Lift included a pedometer, step-count diary, and patient handbook, including BCTs and practice nurse PA consultations [16,19]. Despite PACE-Lift providing a more intensive nurse intervention than PACE-UP, both trials delivered similar effects on PA outcomes at 12 months [14,15] and at 3 or 4 years. Additionally, nurse and postal interventions in PACE-UP achieved similar outcomes at 12 months [14] and 3 years. These findings confirm that shorter, simpler interventions can be equally effective [8,33]. Systematic reviews suggest that individual tailoring, personalised activity goals, and using a step-count diary are important [6,8]; all interventions from both trials provided these elements. That the minimal postal interventions given to both trial control groups at 12 months were not effective at increasing PA levels suggests that the additional support given to the original PACE-UP trial postal arm (follow-up telephone call after a week and encouragement to return completed PA diary after 3 months) was important for this group’s success. The original postal group also had step-count targets set based on baseline blinded pedometer use and received the intervention when they had just been recruited to the trial, so they may have been more motivated. These factors may also have been important to the trial postal intervention’s success. PA guidelines stress the importance of increasing time in MVPA [1,4] rather than just steps. Both of our interventions addressed this: PACE-Lift by nurse feedback on PA intensity from accelerometers [19] and PACE-UP by the “3,000-steps-in 30-minutes” [34] advice given to nurse-support and postal arms [16]. Both trials were effective at increasing MVPA in bouts for all intervention groups at all outcome assessments: 3 and 12 months [14,15] and now at 3 years (PACE-UP) and 4 years (PACE-Lift).
We took the effect estimates from the simplest intervention (PACE-UP postal) to estimate long-term health benefits. Based on a systematic review that quantified the strength of association between walking and coronary heart disease [35], a 28 minutes per week increase in MVPA in bouts seen in the postal group at 3 years should reduce coronary heart disease risk by approximately 4% (95% CI: 3–5%) (see S1 Text). A cohort study that related pedometer steps to mortality [36] allowed us to estimate that a sustained increase of 627 steps/day in the postal group at 3 years should lead to a decrease in all-cause mortality of approximately 4% (95% CI: 1–5%) (see S1 Text).
Whilst environmental and policy interventions are urgently required to address the global inactivity challenge [37], individual PA behaviour change interventions are also important. The sustained effects seen on objective PA outcomes at 3 years for the lower cost postal intervention suggest that this would be an effective and cost-effective [31] intervention to roll out. Minimal support is also required to check that materials have arrived and to encourage return of completed PA diaries but need not be face to face or delivered by a healthcare professional. We are currently conducting implementation work (PACE-UP Next Steps) exploring reach, retention, and ease of adoption in primary care recruiting via postal and face-to-face routes.
The use of wearables to monitor personal PA levels has dramatically increased, through smartphones, wrist - or body-worn devices, and mobile apps, offering opportunities for increasing PA. The “3,000-steps-in-30-minutes” message captures intensity and could become an important new public health goal [34], with new, easy ways to measure steps. Small short-term studies in adults and older adults demonstrate that mobile PA apps can increase PA self-monitoring [38,39] and engagement in regular PA [38,39] and that body-worn fitness trackers can increase time spent in MVPA [39]. PACE-UP Next Steps is currently testing online resources and a mobile app to support the PACE-UP postal intervention. However, despite new PA monitoring opportunities, it is important not to ignore robust, trial-based evidence on effective and cost-effective pedometer - plus paper-based interventions.
Conclusion
We previously reported increased PA at 12 months following 12-week pedometer-based walking interventions for adults and older adults recruited through primary care, delivered either by post with minimal support or through nurse-supported PA consultations. The current paper demonstrates that these findings are still present in participants followed up at 3–4 years. The long-term success of these interventions suggests that they could help to address the public health physical inactivity challenge.
Supporting Information
Zdroje
1. Department of Health. Start Active, Stay Active: A report on physical activity for health from the four home countries' Chief Medical Officers. 2011.
2. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–29. pii: S0140-6736(12)61031-9; doi: 10.1016/S0140-6736(12)61031-9 22818936
3. Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis. 2015;57(4):315–23. doi: 10.1016/j.pcad.2014.08.002 25559060; PubMed Central PMCID: PMC4604440.
4. World Health Organisation. Global Recommendations on Physical Activity for Health. 2010 [cited 23 Feb. 2018]. Available from: http://apps.who.int/iris/bitstream/10665/44399/1/9789241599979_eng.pdf
5. Sparling PB, Howard BJ, Dunstan DW, Owen N. Recommendations for physical activity in older adults. BMJ. 2015;350:h100. doi: 10.1136/bmj.h100 25608694
6. Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–304. doi: 10.1001/jama.298.19.2296 18029834
7. Kang M, Marshall SJ, Barreira TV, Lee JO. Effect of pedometer-based physical activity interventions: a meta-analysis. Res Q Exerc Sport. 2009;80(3):648–55. doi: 10.1080/02701367.2009.10599604 19791652
8. Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013;11 : 75. pii: 1741-7015-11-75; doi: 10.1186/1741-7015-11-75 23506544
9. Opdenacker J, Boen F, Coorevits N, Delecluse C. Effectiveness of a lifestyle intervention and a structured exercise intervention in older adults. Prev Med. 2008;46(6):518–24. doi: 10.1016/j.ypmed.2008.02.017 18405960.
10. Kuller LH, Kinzel LS, Pettee KK, Kriska AM, Simkin-Silverman LR, Conroy MB, et al. Lifestyle intervention and coronary heart disease risk factor changes over 18 months in postmenopausal women: the Women On the Move through Activity and Nutrition (WOMAN study) clinical trial. J Womens Health (Larchmt). 2006;15(8):962–74. doi: 10.1089/jwh.2006.15.962 17087620.
11. Richards J, Thorogood M, Hillsdon M, Foster C. Face-to-face versus remote and web 2.0 interventions for promoting physical activity. Cochrane Database Syst Rev. 2013;9:CD010393. doi: 10.1002/14651858.CD010393.pub2 24085593
12. Vijay GC, Wilson EC, Suhrcke M, Hardeman W, Sutton S, Team VBIP. Are brief interventions to increase physical activity cost-effective? A systematic review. Br J Sports Med. 2015. doi: 10.1136/bjsports-2015-094655 26438429.
13. National Institute of Health and Care Excellence. Behaviour change: individual approaches. 2014.
14. Harris T, Kerry SM, Limb ES, Victor CR, Iliffe S, Ussher M, et al. Effect of a Primary Care Walking Intervention with and without Nurse Support on Physical Activity Levels in 45 - to 75-Year-Olds: The Pedometer And Consultation Evaluation (PACE-UP) Cluster Randomised Clinical Trial. PLoS Med. 2017;14(1):e1002210. doi: 10.1371/journal.pmed.1002210 28045890
15. Harris T, Kerry SM, Victor CR, Ekelund U, Woodcock A, Iliffe S, et al. A primary care nurse-delivered walking intervention in older adults: PACE (pedometer accelerometer consultation evaluation)-Lift cluster randomised controlled trial. PLoS Med. 2015;12(2):e1001783. doi: 10.1371/journal.pmed.1001783 25689364
16. Harris T, Kerry SM, Victor CR, Shah SM, Iliffe S, Ussher M, et al. PACE-UP (Pedometer and consultation evaluation—UP)—a pedometer-based walking intervention with and without practice nurse support in primary care patients aged 45–75 years: study protocol for a randomised controlled trial. Trials. 2013;14 : 418. pii: 1745-6215-14-418; doi: 10.1186/1745-6215-14-418 24304838
17. Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–98. pii: 938640058; doi: 10.1080/08870446.2010.540664 21678185
18. British Psychological Society. Improving Health: Changing Behaviour: NHS Health Trainer Handbook. London: Department of Health, 2008.
19. Harris T, Kerry S, Victor C, Ekelund U, Woodcock A, Iliffe S, et al. Randomised controlled trial of a complex intervention by primary care nurses to increase walking in patients aged 60–74 years: protocol of the PACE-Lift (Pedometer Accelerometer Consultation Evaluation—Lift) trial. Bmc Public Health. 2013;13. doi: 10.1186/1471-2458-13-5 23289648.
20. Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. 10158943
21. Jette AM, Rooks D, Lachman M, Lin TH, Levenson C, Heislein D, et al. Home-based resistance training: predictors of participation and adherence. Gerontologist. 1998;38(4):412–21. 9726128
22. Ware JE, Sherbourne CD. The MOS 36-item short form health survey: conceptual framework and item selection. Med Care. 1992;30 : 473–83. 1593914
23. Zigmond A, Snaith R. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67 : 361–70. 6880820
24. D'Ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: The acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract. 1994;11(3):260–6. 7843514
25. Krasucki C, Ryan P, Ertan T, Howard R, Lindesay J, Mann A. The FEAR: a rapid screening instrument for generalized anxiety in elderly primary care attenders. Int J Geriatr Psychiatry. 1999;14(1):60–8. 10029937
26. White IR, Carpenter J, Horton NJ. A mean score method for sensitivity analysis to departures from the missing at random assumption in randomised trials. Statistica Sinica. 2017; In press.
27. Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nystrom C, Mora-Gonzalez J, Lof M, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017. doi: 10.1007/s40279-017-0716-0 28303543.
28. Jorgensen T, Andersen LB, Froberg K, Maeder U, von Huth Smith L, Aadahl M. Position statement: testing physical conditions in a population—how good are the methods?. Eur J Sport Sci. 2009;9(5):257–67.
29. Sink KM, Espeland MA, Castro CM, Church T, Cohen R, Dodson JA, et al. Effect of a 24-Month Physical Activity Intervention vs Health Education on Cognitive Outcomes in Sedentary Older Adults: The LIFE Randomized Trial. JAMA. 2015;314(8):781–90. doi: 10.1001/jama.2015.9617 26305648; PubMed Central PMCID: PMC4698980.
30. Varma VR, Tan EJ, Gross AL, Harris G, Romani W, Fried LP, et al. Effect of Community Volunteering on Physical Activity: A Randomized Controlled Trial. Am J Prev Med. 2016;50(1):106–10. doi: 10.1016/j.amepre.2015.06.015 26340864; PubMed Central PMCID: PMC4691553.
31. Anokye N, Fox-Rushby J, Sanghera S, Cook DG, Kerry SM; Limb E, et al. The short-term and long-term cost-effectiveness of a pedometer-based intervention in primary care: a within trial analysis and beyond-trial modelling. Lancet. 2016. Epub 2016/11/25.
32. National Institute of Health and Care Excellence. Walking and cycling. Local measures to promote walking and cycling as forms of travel or recreation. 2012. Report No.: NICE Public health guideline 41.
33. Orrow G, Kinmonth AL, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012;344:e1389. doi: 10.1136/bmj.e1389 22451477; PubMed Central PMCID: PMC3312793.
34. Marshall SJ, Levy SS, Tudor-Locke CE, Kolkhorst FW, Wooten KM, Ji M, et al. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med. 2009;36(5):410–5. pii: S0749-3797(09)00087-7; doi: 10.1016/j.amepre.2009.01.021 19362695
35. Zheng H, Orsini N, Amin J, Wolk A, Nguyen VT, Ehrlich F. Quantifying the dose-response of walking in reducing coronary heart disease risk: meta-analysis. Eur J Epidemiol. 2009;24(4):181–92. doi: 10.1007/s10654-009-9328-9 19306107
36. Dwyer T, Pezic A, Sun C, Cochrane J, Venn A, Srikanth V, et al. Objectively Measured Daily Steps and Subsequent Long Term All-Cause Mortality: The Tasped Prospective Cohort Study. PLoS ONE. 2015;10(11):e0141274. doi: 10.1371/journal.pone.0141274 26536618; PubMed Central PMCID: PMC4633039.
37. Heath GW, Parra DC, Sarmiento OL, Andersen LB, Owen N, Goenka S, et al. Evidence-based intervention in physical activity: lessons from around the world. Lancet. 2012;380(9838):272–81. doi: 10.1016/S0140-6736(12)60816-2 22818939.
38. Turner-McGrievy GM, Beets MW, Moore JB, Kaczynski AT, Barr-Anderson DJ, Tate DF. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Inform Assoc. 2013;20(3):513–8. doi: 10.1136/amiajnl-2012-001510 23429637; PubMed Central PMCID: PMC3628067.
39. Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL. Randomized Trial of a Fitbit-Based Physical Activity Intervention for Women. Am J Prev Med. 2015;49(3):414–8. doi: 10.1016/j.amepre.2015.01.020 26071863; PubMed Central PMCID: PMC4993151.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- What is the value of multidisciplinary care for chronic kidney disease?
- 2017 Reviewer and Editorial Board Thank You
- Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study
- Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance
- Causes of death and infant mortality rates among full-term births in the United States between 2010 and 2012: An observational study
- Time for high-burden countries to lead the tuberculosis research agenda
- The importance and challenges of shared decision making in older people with multimorbidity
- Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study
- Mortality, ethnicity, and country of birth on a national scale, 2001–2013: A retrospective cohort (Scottish Health and Ethnicity Linkage Study)
- Validation of a genetic risk score for atrial fibrillation: A prospective multicenter cohort study
- Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort
- Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial
- Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study
- Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: A preclinical experimental study
- Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: A population-based cohort study
- Global child and adolescent mental health: The orphan of development assistance for health
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Primary prevention of cardiovascular disease: The past, present, and future of blood pressure- and cholesterol-lowering treatments
- Physical activity levels in adults and older adults 3–4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care
- Effect and cost-effectiveness of educating mothers about childhood DPT vaccination on immunisation uptake, knowledge, and perceptions in Uttar Pradesh, India: A randomised controlled trial
- Comorbidity health pathways in heart failure patients: A sequences-of-regressions analysis using cross-sectional data from 10,575 patients in the Swedish Heart Failure Registry
- Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry
- Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques
- Transmission of HIV-1 drug resistance mutations within partner-pairs: A cross-sectional study of a primary HIV infection cohort
- A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study)
- Integrating HIV and hypertension management in low-resource settings: Lessons from Malawi
- The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data
- Cerebral white matter disease and functional decline in older adults from the Northern Manhattan Study: A longitudinal cohort study
- HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease: The rise of the genetic risk score
- Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study
- Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: A 20-year cohort study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



