-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInternet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
In a single-blind randomised trial, Emma Wilson and colleagues assess the impact of an e-STI service on testing uptake and cases diagnosed.
Published in the journal: . PLoS Med 14(12): e32767. doi:10.1371/journal.pmed.1002479
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002479Summary
In a single-blind randomised trial, Emma Wilson and colleagues assess the impact of an e-STI service on testing uptake and cases diagnosed.
Introduction
Sexually transmitted infections (STIs) continue to be a global public health concern, with an estimated 357 million new infections of curable STIs (chlamydia, gonorrhoea, syphilis, and trichomoniasis) each year [1]. In England, there were 436,928 new diagnoses of STIs and 5,684 new cases of HIV in 2015 [2,3]. The burden of infection is disproportionately high among young adults (under 25 years), men who have sex with men (MSM), and black and minority ethnic (BME) groups [4].
Left undiagnosed and untreated, curable STIs such as chlamydia, trichomoniasis, gonorrhoea, and syphilis can facilitate the transmission of HIV and can cause sub-fertility, ectopic pregnancy, chronic pelvic pain, neurological and cardiovascular disease, neonatal mortality, and infant morbidities [5]. Undiagnosed HIV and late diagnosis of HIV lead to diminished health outcomes and reduced life expectancy [6].
Increasing testing, diagnosis, and treatment of STIs and reducing time to treatment is a global priority to reduce the prevalence of STIs and their associated sequelae [7,8]. In the UK, STI testing coverage remains sub-optimal. The 3rd National Survey of Sexual Attitudes and Lifestyles (NATSAL) found that two-thirds of 16–44-year-olds who tested positive for chlamydia had not had a chlamydia test in the past 12 months [9]. Further, timely diagnosis of HIV is a challenge [10]. In 2015, 39% of adults diagnosed with HIV in the UK were diagnosed late (CD4 count < 350 cells/mm3) [3]. Interventions that increase access among high-risk and hard-to-reach groups are needed to maximise the public health benefits of STI testing.
Digital technologies are increasingly utilised to deliver sexual health interventions (e-sexual health) [11,12]. These include internet-accessed STI testing (e-STI testing). Typically this testing modality enables users to order a test kit from a virtual service (via a website or app), collect their own samples, return test samples to a laboratory, and be notified of their results by short message service (SMS) text message or telephone [13,14].
e-STI testing may bypass the inconvenience and stigma associated with face-to-face services [15,16], and overcome supply constraints where clinical services are scarce [11,17]. In doing so, it could expand access to populations who do not use face-to-face services [18,19]. Shifting tasks to patients via virtual services, particularly for non-complex testing and treatment, may prove cost-effective [20].
Public sector providers in the UK, Canada, the United States, Australia, and some European countries offer e-STI testing to high-risk groups [21–26]. Yet the international evidence base on e-STI testing is scant. To our knowledge there have been no randomised controlled trials evaluating the effect of internet-based services offering testing for chlamydia, gonorrhoea, syphilis, and HIV on testing, diagnosis, or treatment of STIs. In this trial we assessed the effects of an e-STI testing and results service (SH:24) on uptake of STI testing and STI cases diagnosed and treated, when delivered alongside usual care. The version of SH:24 evaluated offered postal self-sampling test kits for chlamydia, gonorrhoea, HIV, and syphilis; results delivered via text message or telephone; and web-based safer sex health information.
Methods
We carried out a single-blind randomised controlled trial of an e-STI testing and results service. The trial was conducted in London, UK, and participants were recruited between 24 November 2014 and 31 August 2015. Ethical approval was obtained from the National Research Ethics Service (NRES) Committee London–Camberwell St Giles (Ref 14/LO/1477). The trial protocol was accepted for publication in April 2015 and was published in January 2016 [27,28].
Young people aged 16 to 30 years of age, resident in the London boroughs of Lambeth and Southwark, sexually active (at least 1 sexual partner in the last 12 months), with stated willingness to take an STI test, and with access to the internet were eligible for inclusion. People who were unable to read English (the websites were only in English) or unable to provide consent were excluded.
We recruited in community settings to reach individuals who may not use conventional STI testing services. We utilised both face-to-face and online recruitment strategies. We promoted the trial in universities, further education colleges, market stalls, barber shops, bars, and nightclubs in South East London and via Facebook, Twitter, and Grindr (a dating application for gay and bisexual men). Advocacy and health promotion groups advertised the trial among their networks. The study was promoted in conjunction with a health promotion message, to motivate participants to join the trial and consider taking an STI test.
Research assistants assessed eligibility, provided study information, obtained written consent, and collected baseline data. Alternatively, participants read the information, entered their eligibility data, provided online consent, and entered their baseline data on the trial website. An independent computer-based randomisation programme allocated participants to the intervention or control group. Participants were sent 1 automated SMS text message with the uniform resource locator (URL) of the intervention or control STI services according to their allocation.
The randomisation system utilised a minimisation algorithm balancing for gender (male, female, transgender), age (16–19, 20–24, 25–30 years), number of sexual partners in last 12 months (1, 2+), and sexual orientation (MSM, all other groups). All factors had equal weight in determining marginal imbalance. To minimise imbalances on these selected factors, allocation was weighted towards the underrepresented group using a probability of 0.8. In the case of equal representation, participants were allocated by simple randomisation in a 1 : 1 ratio. Laboratory staff and researchers assessing outcomes were blinded to the treatment allocation.
All participants were sent 1 text message inviting them to get an STI test (see Box 1).
Box 1. Wording of control and intervention text messages
Control text message:
You have been invited to use a clinic-based sexual health service.
Please visit https://text4health.lshtm.ac.uk/trials/UI/public_htm/info/clinic.aspx to obtain your free STI test at a walk-in sexual health clinic.
If you have problems accessing this link, please text ‘HELP’
Intervention text message:
You have been invited to use an internet-based sexual health service.
Please visit https://sh24.org.uk/betatester to order your free STI test online.
Please do not share this link with anyone.
If you have problems accessing this link, please text ‘HELP’
If participants contacted the research team to say they had difficulty accessing the URL, they were resent the text message.
Participants in the intervention group were sent a text message with the URL of SH:24 (https://www.sh24.org.uk). SH:24 offers free postal self-sampling test kits for chlamydia, gonorrhoea, HIV, and syphilis. Participants who ordered a test kit from SH:24, were required to complete a short order form. Those reporting STI symptoms were advised via a pop-up message to visit their local clinic for immediate treatment. Those reporting complex needs such as depression, drug and alcohol dependency, or exploitative sexual partnerships were telephoned by a clinician and referred to relevant clinical services. All participants could continue to use the online service if they wished.
All test kits contained a lancet and collection tube to obtain a blood sample for serological testing for syphilis and HIV. For chlamydia and gonorrhoea, women were sent vaginal swabs and men were sent a container for first-catch urine samples. Test kits for MSM also contained swabs to take pharyngeal and rectal samples.
The tests kits included pictorial leaflets with guidance on how to collect the specimens. A video demonstrating blood sample collection was available on Youtube and could be accessed via the SH:24 website. Participants were kept informed of their order via text message. In the text messages, they were asked to contact the SH:24 team with any questions or concerns. After 2 weeks, non-returners were sent reminders via text and resent test kits if required, as per SH:24’s protocols.
Chlamydia, gonorrhoea, and syphilis test results were delivered by text message. Participants with reactive results for syphilis or positive results for chlamydia or gonorrhoea were signposted to local clinics for confirmatory testing and treatment as necessary. Reactive results for HIV were communicated by phone by a clinician.
Participants in the control group were sent the URL of a bespoke website with the contact details, websites, and locations (Google map images) of sexual health clinics in Lambeth and Southwark. These clinics provided usual care via walk-in services. Some clinics also offered an appointment service for those with symptoms or complex needs. Those diagnosed with an STI were asked to attend clinic for treatment. All participants were free to use any other sexual health services or interventions during the trial period. We used evidence-based methods to maximise response rates [29].
Our co-primary outcomes were self-reported diagnosis of an STI at 6 weeks, confirmed by patient health records, and self-reported completion of an STI test at 6 weeks, confirmed by patient health records. We defined completion of an STI test as samples processed by the laboratory and results delivered to SH:24 or to clinic.
Nucleic acid amplification tests (NAATs) were used to detect chlamydia and gonorrhoea in all services. In the online service, positive gonorrhoea results were confirmed using a second NAAT (Cepheid GeneXpert–Dual Target). Syphilis IgG/IgM was assessed (sensitivity > 99%; specificity > 99%), and HIV I and II/p24Ag were assessed (sensitivity 99.8–100%; specificity 99.9%). Reactive results for HIV or syphilis were counted as positive test results only when confirmed by assays in clinic. We defined STI diagnoses as those arising from laboratory testing.
Our secondary outcomes were the proportion of participants prescribed treatment for an STI, time from randomisation to completion of an STI test, and time from randomisation to treatment of an STI.
Our process outcomes were the proportion of STI tests that were positive in each group, median time from diagnosis to treatment in each group, the proportion of participants who completed an STI test in each group by service type, the proportion of participants diagnosed in each group by service type, and, in the intervention group only, the proportion who agreed that the intervention was acceptable and the proportion who adhered to an appropriate e-STI testing pathway. All pathways were considered appropriate unless participants completed a test via SH:24, received a negative result, and then retested for the same STI in a face-to-face setting within 6 weeks. In addition to our pre-specified process outcomes, we report the proportion of participants who tested positive for an STI among those who completed a test at 6 weeks, with 95% confidence intervals. All outcomes and their definitions are summarised in S1 Table.
Participants provided self-reported data by post or directly entered data on a website. Participants were sent £5 with a request to complete a follow-up questionnaire and an additional £5 on receipt of the completed questionnaire.
To obtain objective measures for our endpoints, we searched the SH:24 database, and data managers at the hospital trusts searched patient record databases, for all randomised participants using either (1) mobile phone and gender or (2) name and date of birth as identifiers. If participants reported using another service (general practitioner [GP] surgery or sexual health service outside of Lambeth and Southwark), we contacted the service to collect STI testing, diagnosis, and treatment data.
Statistical analysis
The trial steering committee approved the pre-specified statistical analysis plan prior to unblinding. Our study was powered for our co-primary outcome of the proportion of participants diagnosed with an STI in each group [27,28]. Two factors determined the number of participants needed: the estimated proportion of participants with an STI and the size of the treatment effect.
We anticipated that not all of the intervention group would order a test kit. We estimated that 30% would not complete this first step. Among the 70% who ordered a kit, we assumed that 50% would return the kit for analysis (based on return rates of an e-STI testing service in the London borough of Greenwich).
There were no available data that would give us an estimate of the likely number of individuals who would complete an STI test in the control group. We assumed that fewer people (10%) would seek a test in clinic-based settings.
We based our STI prevalence estimates on the proportion of positive chlamydia tests among 15–24-year-olds in general practice settings in Lambeth and Southwark, which was 6% in 2012 [30]. We based our estimated loss to follow-up on previous e-health studies in the UK, which achieved 90% follow-up [31].
A sample size of 3,000 participants would lead to 90% power (2-sided alpha = 5%) to detect a relative risk (RR) of 3.5 (2.1% risk of diagnosis in the intervention group versus 0.6% risk of diagnosis in the control group), allowing for 10% loss to follow-up. This equates to 10% of the control group being tested, with a 6% probability of infection as in general practice settings, and 35% of the intervention group being tested, with a 6% probability of infection as in general practice settings.
With regard to our other co-primary outcome measure, 3,000 participants would lead to 99% power (2-sided alpha = 5%) to detect an absolute difference of 25% in the proportion of participants who completed a test in the intervention group versus the proportion who completed a test in the control group (35% versus 10%).
All analyses were undertaken on an intention to treat basis with Stata version 14.2. Effect measures were RRs with 95% confidence intervals and time to outcomes. We assessed overall heterogeneity for subgroups by summing the individual chi-squared statistics and their degrees of freedom to 1 overall chi-squared test on the sum of the degrees of freedom at a 5% level of significance.
For the primary analysis we used multivariate imputation by chained equations (MICE) to correct for any potential bias caused by missing data, assuming data are missing at random (MAR). Under this assumption, the distribution of the outcome for both missing and non-missing groups is the same for individuals with the same observed data. All baseline data were complete, except for sexual orientation, for which a missing category was used.
To obtain more precise estimates and confidence intervals with the correct coverage, we accounted for baseline factors by estimating the propensity score for randomised allocation for all participants [32]. We used a logistic regression model with randomised group as the response, and gender, age (years), number of sexual partners in the last 12 months, sexual orientation, and ethnicity as covariates.
We imputed our 2 co-primary outcomes (STI testing and STI diagnosis) and the secondary outcome proportion of participants prescribed treatment using 3 conditional models. Each imputation model included randomised group as a covariate and was weighted by the inverse of the estimated propensity score (for compatibility with the model for analysis). In addition, the 2 models to impute STI testing and STI diagnosis conditioned on self-reported testing, self-reported diagnosis, and self-reported treatment. The model to impute treatment conditioned on self-reported testing and self-reported treatment only, due to collinearity with other variables, which led to non-convergence. Each imputed data set was produced with 10 cycles. We generated 100 imputed data sets for each missing outcome. Multiple imputation inference proceeded via Rubin’s rules [33].
To explore departures from MAR assumptions for our co-primary outcomes, we performed a sensitivity analysis to explore the impact of possible differences between participants with complete outcome data and participants with missing outcome data. We multiply imputed missing outcome data, using inverse probability weighting on the estimated propensity score and with allocated group and self-reported testing, diagnosis, and treatment as covariates. The odds of STI diagnosis and the odds of a completed STI test for missing participants were varied to be 1/4, 1/2, 1, 2, and then 4 times as large as the MAR analyses; this was done factorially for the 2 randomised groups, giving a total of 25 analyses (including the principal analysis assuming MAR).
We explored heterogeneity of the intervention effect on our primary outcomes. We tested for interaction at a 5% level of significance to assess whether effectiveness varied by gender (male, female), ethnicity (white, black/African/Caribbean/black British, Asian/Asian British/all other groups), sexual orientation (MSM, all other groups), age group (16–19 years, 20–24 years, 25–30 years), number of sexual partners (1, 2+), SH:24 availability (period when available to study participants only, period when available to all residents in Lambeth and Southwark), and Index of Multiple Deprivation rank (linear). The Index of Multiple Deprivation is a relative measure of deprivation that ranks every small area in England from 1 (most deprived area) to 32,844 (least deprived area) [34].
These analyses were conducted in the complete cases under a MAR assumption. They were not weighted by the inverse of the estimated propensity score, as specified in the analysis plan, due to non-convergence of the models.
We used survival analysis to estimate time from randomisation to test completion and time from randomisation to treatment. We estimated the restricted mean survival time (RMST), which is a meaningful measure even when the proportional hazards assumption is in doubt. As with other analyses, the RMST accounted for covariates by weighting on the inverse of the estimated propensity score. We set the restricted mean time t* = 6 weeks (42 days) for time to test and t* = 3 months (84 days) for time to treatment using a ‘3df/1df’ Royston–Parmar model [35].
Results
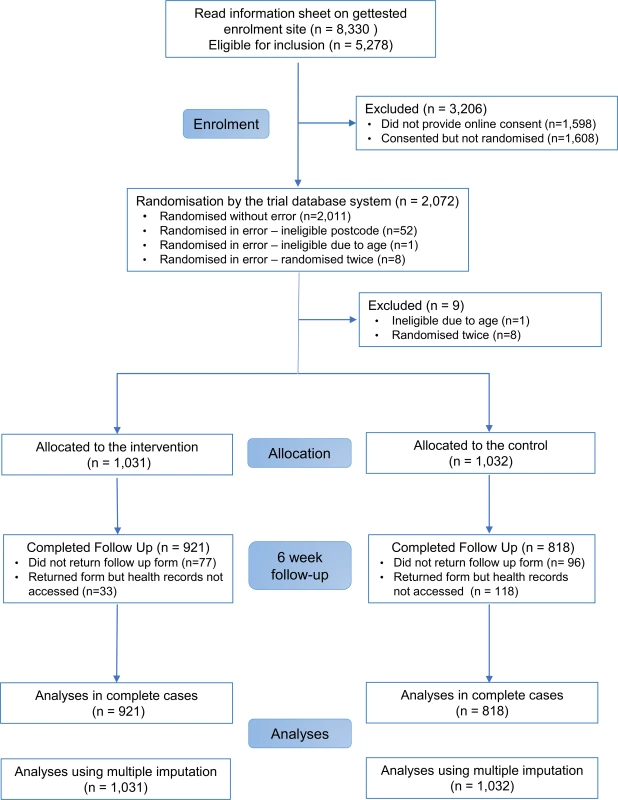
In all, 2,072 participants were randomly assigned to the SH:24 online testing and results service or to the control group (Fig 1). We excluded 8 participants who were randomised twice and 1 participant who was randomised and did not meet the age criterion (Fig 1). We were unable recruit to target, and therefore we lacked power for the co-primary outcome of STI diagnoses. Baseline characteristics are presented in Table 1.
Tab. 1. Baseline characteristics of participants. 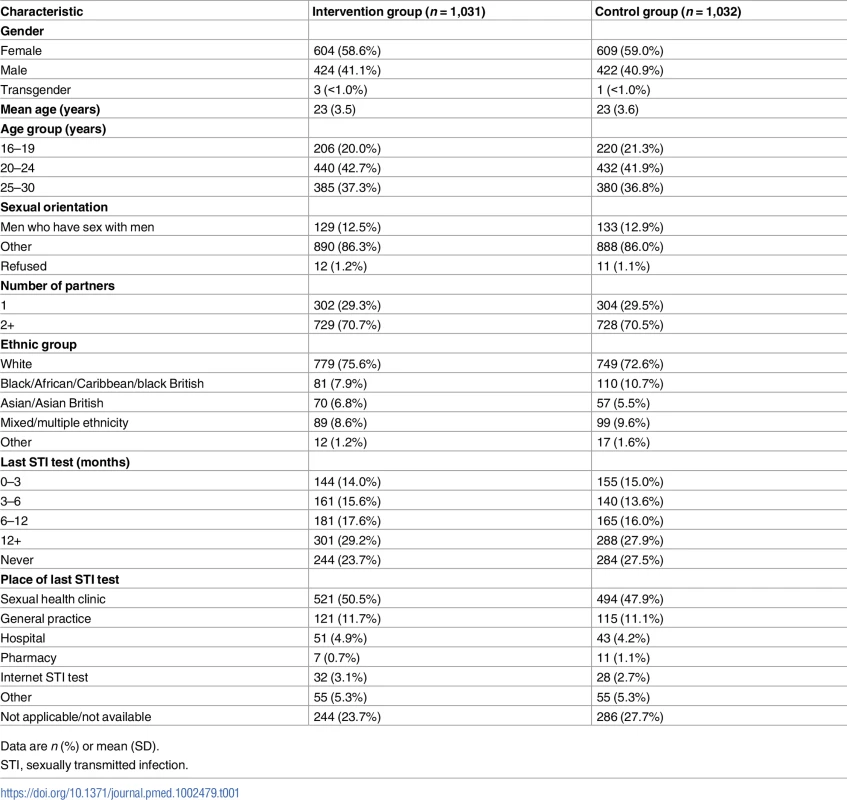
Data are n (%) or mean (SD). Primary outcome data, prior to multiple imputation, were available for 921 (89%) participants in the intervention group and 818 (79%) in the control group (Fig 1).
The proportions of participants in each group who reported completing a test at 6 weeks, and who were confirmed to have tested via patient record checks, are provided in S1 Fig. Record checks in clinics, SH:24, and GP surgeries were completed by 17 June 2016.
Our primary analyses were based on multiply imputed data sets. In all, 1,031 in the intervention group and 1,032 in the control group were included in the analyses. At 6 weeks, 50.0% of the intervention group had completed an STI test compared to 26.6% in the control group (RR 1.87, 95% CI 1.63 to 2.15, P < 0.001; Table 2); 2.8% of the intervention group versus 1.4% in the control group had been diagnosed with an STI (RR 2.10, 95% CI 0.94 to 4.70, P = 0.079; Table 2).
Tab. 2. Primary and secondary outcomes. 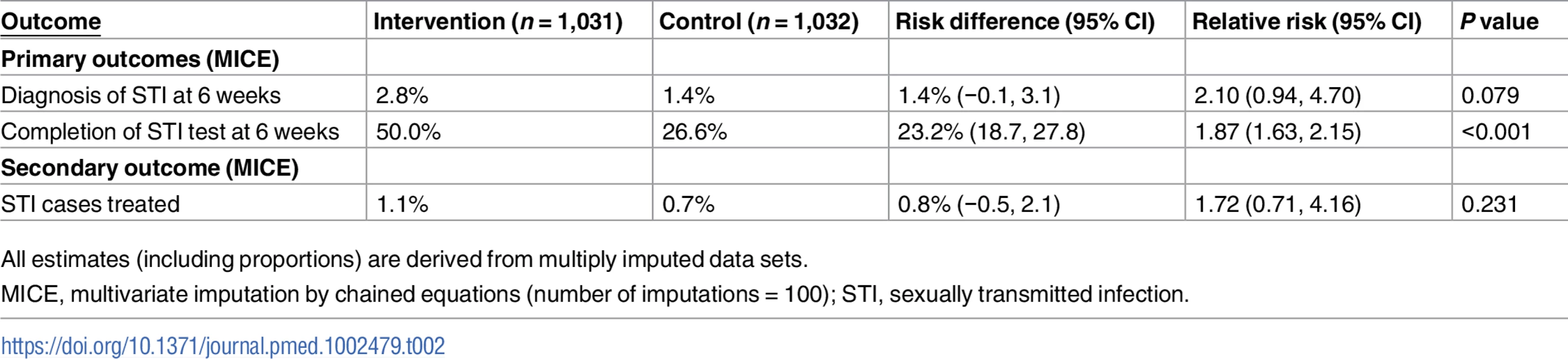
All estimates (including proportions) are derived from multiply imputed data sets. We obtained similar results for the complete case analysis (S2 Table) and for all the scenarios that we investigated under the assumption that our missing outcome data were missing not at random (S2 and S3 Figs).
The proportion of participants treated was 1.1% in the intervention group versus 0.7% in the control group (RR 1.72, 95% CI 0.71 to 4.16, P = 0.231; Table 2). In the complete cases, time to test, estimated by the RMST, was shorter in the intervention group compared to the control group (28.8 days versus 36.5 days, P < 0.001; Table 3); no differences were observed for time to treatment (83.2 days versus 83.5 days, P = 0.51; Table 3).
Tab. 3. Secondary outcomes (time to event). 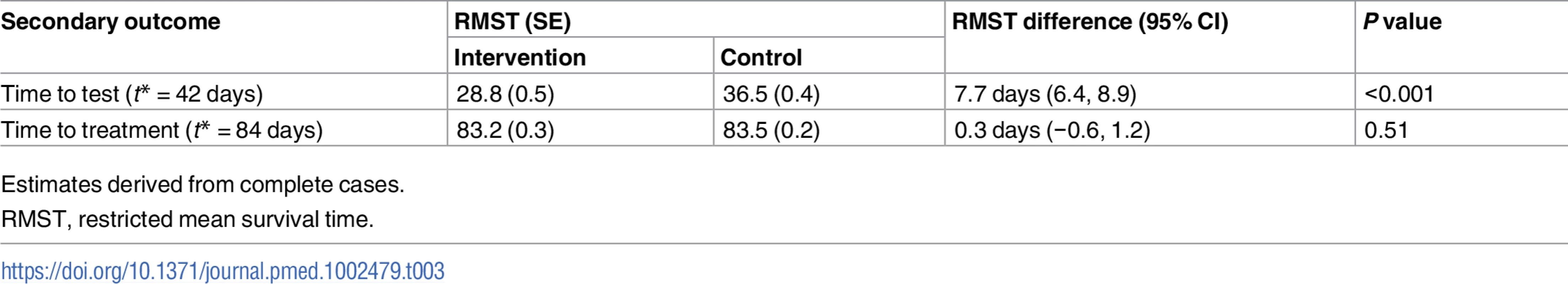
Estimates derived from complete cases. Kaplan–Meier plots for time to test and time to treatment are presented in S4 Fig and S5 Fig. We identified no evidence of heterogeneity for any of the pre-specified subgroup analyses, which were conducted in the complete cases (Figs 2 and 3). Given that we lacked power for the analyses of STI diagnoses, the subgroup analyses for this outcome are even more underpowered (Fig 3).
Fig. 2. Effect of the SH:24 intervention on STI testing by subgroup. 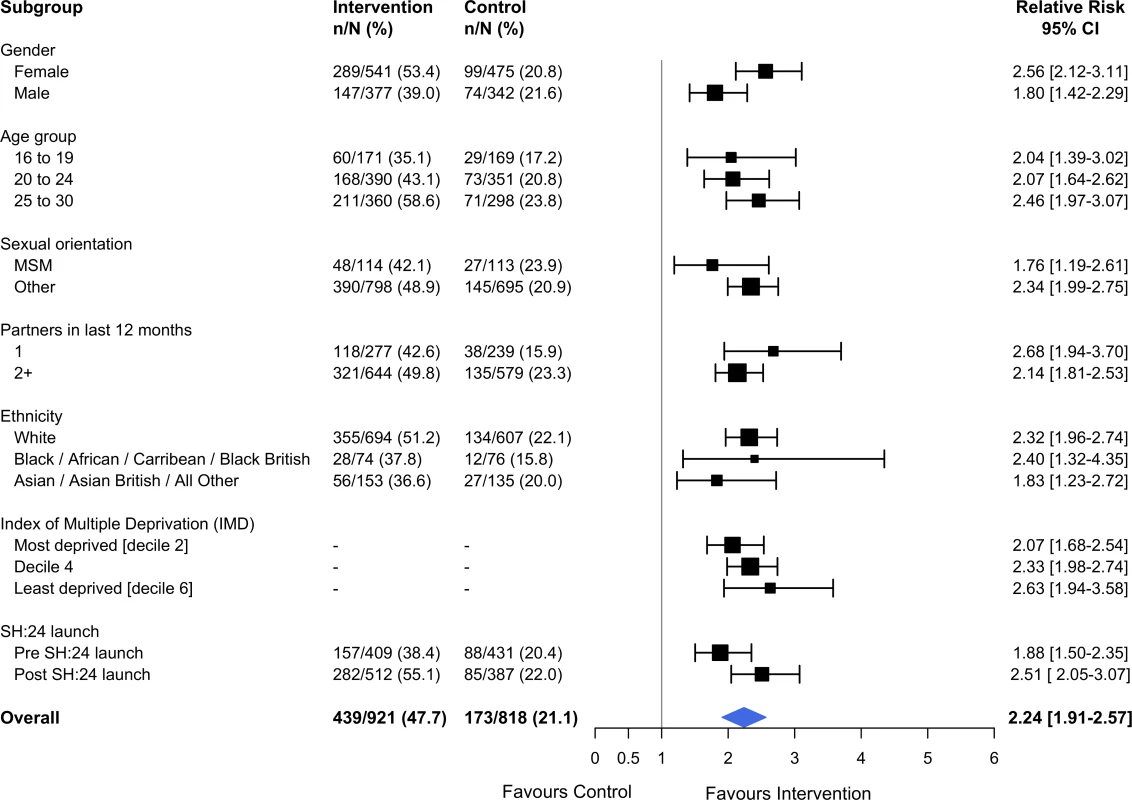
Interaction test: chi-squared = 12.36 on 9 degrees of freedom, P = 0.19. Estimates derived from the complete cases. MSM, men who have sex with men; STI, sexually transmitted infection. Fig. 3. Effect of the SH:24 intervention on STI diagnoses by subgroup. 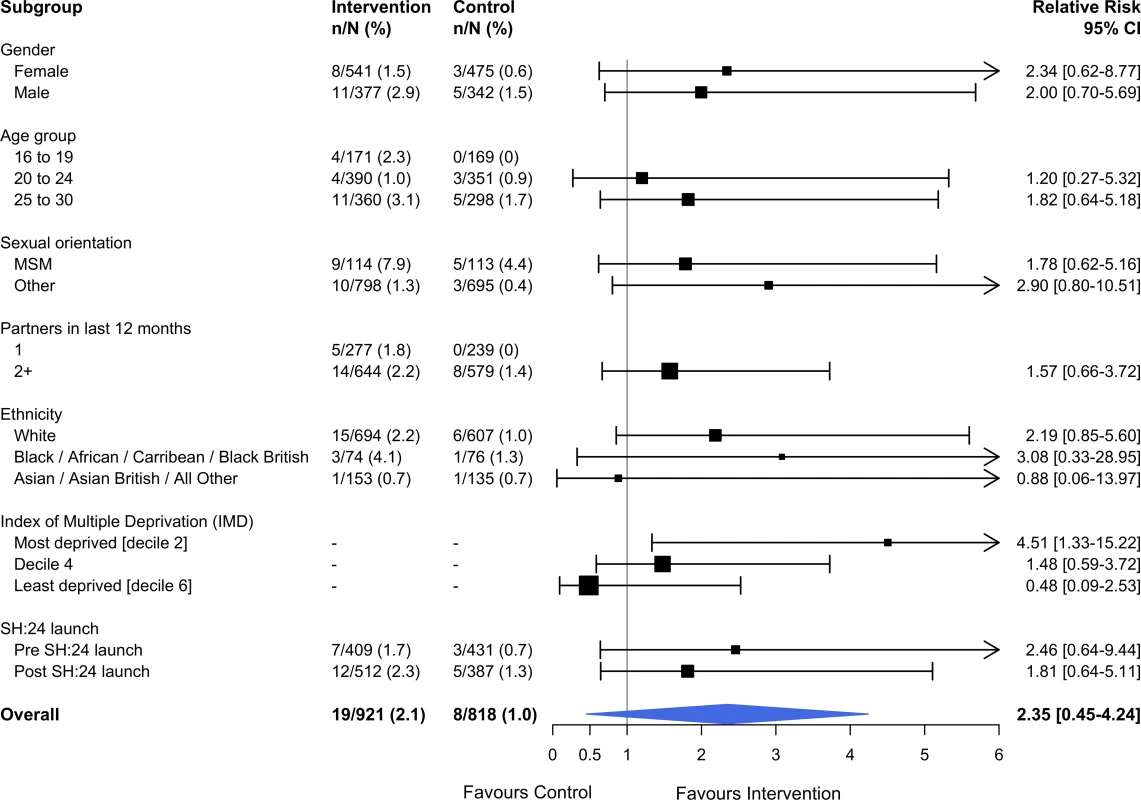
Interaction test: chi-squared = 4.57 on 5 degrees of freedom, P = 0.46. Estimates derived from the complete cases. MSM, men who have sex with men; STI, sexually transmitted infection. Process outcomes
Among participants who completed an STI test at 6 weeks, 4.3% (19/439; 95% CI 2.8 to 6.7) tested positive for an STI in the intervention group and 4.6% (8/173; 95% CI 2.3 to 9.0) tested positive for an STI in the control group. The median time from diagnosis to treatment among those with complete treatment data was 2 days in the intervention group and 4 days in the control group (S3 Table). Four of the 18 cases treated received treatment prior to a laboratory-confirmed diagnosis and were not included in these summary statistics. The excluded cases were evenly distributed between groups.
Three participants in the intervention group tested via SH:24, received a negative result, and retested in clinic for the same STI within 6 weeks (S3 Table).
Regarding intervention acceptability, 76% (294/388) of participants in the intervention group who tested via SH:24 provided acceptability data. Of these, 71% (209/294) found the intervention to be acceptable (S3 Table).
In all, 88% (388/439) of participants in the intervention group who completed an STI test at 6 weeks tested via SH:24 (Table 4). Nineteen participants in the intervention group were diagnosed with an STI. Of these, 12 were diagnosed via SH:24 and 7 were diagnosed in a sexual health clinic (Table 4).
Tab. 4. STI test completion and STI diagnoses by service type. 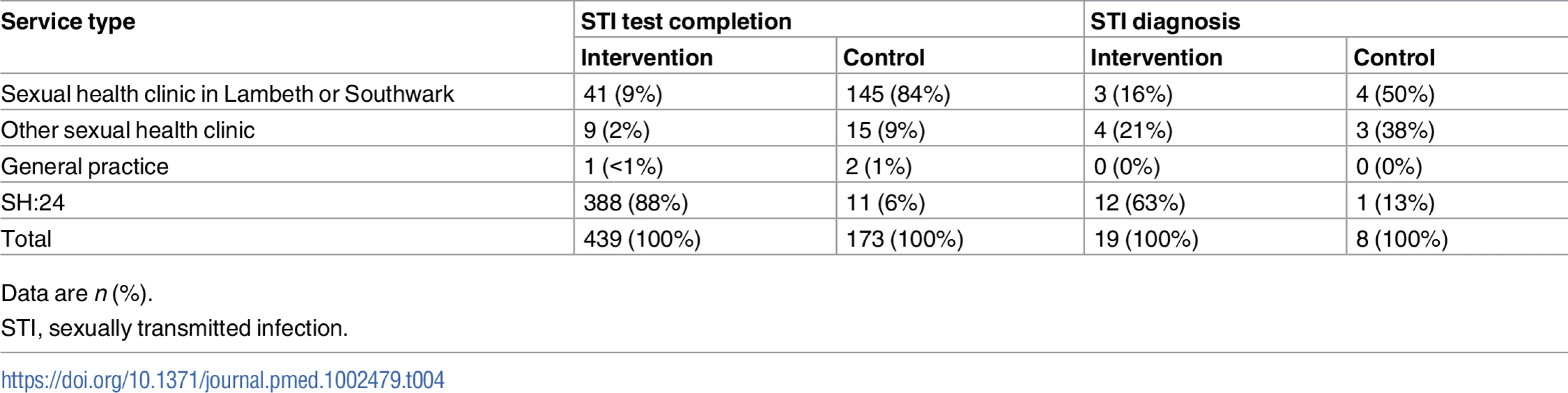
Data are n (%). In the intervention group, 2.8% (12/432) of chlamydia tests were positive, 1.4% (6/433) of gonorrhoea tests were positive, and 0.8% (3/363) of syphilis tests were positive. In the control group, 2.4% (4/169) of chlamydia tests were positive, 3% (5/169) of gonorrhoea tests were positive, and none of the 137 (0/137) syphilis tests were positive.
In all, 365 participants tested for HIV in the intervention group, and 140 in the control group. There were no confirmed HIV diagnoses. One participant tested for hepatitis B in the intervention group, and 11 in the control group. There were no hepatitis B diagnoses in either group.
Among participants who were diagnosed with an STI, most (24/27) were diagnosed with a single STI, and 3 with more than 1 STI.
Discussion
Statement of principal findings
When STI testing is promoted, offering e-STI testing alongside usual care significantly increases uptake of STI testing. The intervention increased STI testing in all groups including those at high risk for STIs. We lacked power for the analyses of STI diagnoses and STI cases treated, but our estimates are in the expected direction. The intervention reduced time to test but not time to treatment.
Strengths and weaknesses
This study has several strengths. We used an independent, remote computer-based randomisation system to ensure study staff had no prior knowledge of the treatment allocation. We collected objective outcomes even for those participants who tested outside of Lambeth and Southwark or via a different pathway (e.g., at their GP). Laboratory staff and researchers carrying out the analyses were blinded to the allocation. Baseline prognostic factors were well balanced between groups, and our co-primary outcomes were known for 84% of participants. In trials where it is not possible to blind participants, allocation to the control group could reduce motivation to carry out the desired behaviour. To mitigate potential performance bias, participants were informed at the time of recruitment that they would be invited via text message to use one type of sexual health service without stating the options. All analyses were intention to treat.
We successfully recruited high-risk groups including MSM (262/2,063; 13%), 16–24-year-olds (1,298/2,063; 63%), and individuals reporting 2 or more sexual partners in the last year (1,457/2,063; 71%). We enrolled individuals who reported limited prior contact with conventional STI testing services. A quarter of our study population had never tested for STIs prior to the trial (528/2,063) (Table 1). In all, 17% of MSM (45/262) and 21% of 16–24-year-olds (277/1,298) had not tested within the last 12 months, despite national guidelines that recommend annual STI testing among these groups [36,37].
Our trial has a number of limitations. We fell short of our recruitment target of 3,000 participants, and we were unable to extend the recruitment period due to a pre-existing plan to promote SH:24 widely across the study area. As a result, the study lacked power to detect differences in STI diagnoses and STI cases treated. As with other online enrolment systems [38], a high number of potential participants started, but did not complete, the enrolment process (Fig 1).
It is likely that those who enrolled in the study had a greater interest in STI testing than those who declined. Testing uptake in the control and intervention groups in all trials may be higher than in the general population. This could result in a smaller risk difference if the intervention were to be implemented in the general population. In all, 26% of our study population identified as BME at baseline. This is lower than the proportion of individuals in Lambeth and Southwark identifying as BME (44% and 48%, respectively [39,40]), which may limit the generalisability of our results.
While there is some reporting bias in the self-reported data (S1 Fig), the potential to bias our co-primary endpoints is limited, as these were objectively verified via participants’ health records. There was potential for contamination as the URL for SH:24 was promoted in Lambeth and Southwark when the service was launched in March 2015. However, only 11 control group participants used SH:24 in the trial, and any contamination would have biased our results towards the null. In our subgroup analyses, there was no evidence of heterogeneity as a result of SH:24’s change in availability.
At enrolment, participants were informed that the £10 incentive was for completing follow-up, but some participants later reported that they thought it was for completing an STI test. Given that all participants were told about the £10 incentive, and sent money at follow-up, the impact of this incentivisation should be non-differential and would not explain our statistically significant results.
Although the laboratory tests used by services are highly sensitive and specific, some misclassification is possible. This misclassification could have biased our STI diagnosis results towards the null. We randomised 8 people twice and excluded them from the analyses. It is possible that we randomised other people twice but only if they provided an incorrect name and date of birth. All service providers were motivated to record STI testing data in line with national surveillance requirements. If some providers were more accurate than others, this might result in differential misclassification and bias.
Although we achieved high response rates for our co-primary outcomes, these rates were differential as we achieved higher follow-up in the intervention group than in the control group. This can result in biased estimates under a complete case approach [41]. To deal with missing outcome data, our primary analyses used multivariate imputation techniques under the assumption that data were MAR. This approach is well established, and it is more valid and efficient than other approaches to deal with missing data [42]. It is reassuring that the results of all sensitivity analyses were similar to the results for the primary analyses.
Strengths and weaknesses in relation to other studies
To our knowledge, this is the first trial of an e-STI testing service that offers testing for 4 STIs (chlamydia, gonorrhoea, HIV, and syphilis). Descriptive studies from the US suggest that services that offer internet-based testing for chlamydia, gonorrhoea, and trichomoniasis can attract at-risk populations (young and BME groups) and yield high STI positivity [43,44]. One randomised controlled trial in France has evaluated self-sampling for chlamydia accessed via the internet compared to chlamydia testing in face-to-face settings. It reports an increase in testing uptake (29.2% in the intervention group versus 8.7% in the control group, RR 3.37, 95% CI 3.05 to 3.74). However, outcomes were assessed using different measures in the intervention and control groups, and there was low and differential follow-up (47% follow-up in the intervention group versus 30% follow-up in the control group) [45].
Our finding of increased STI testing uptake with e-STI testing is similar to increases in testing reported in trials of self-sampling and self-testing interventions that are not accessed via the internet [46,47].
Meaning and mechanisms
The theory of change underpinning SH:24 proposes that online diagnostic pathways will increase testing uptake as they are convenient, private, and non-judgemental and offer more choice than traditional clinic pathways [17].
The similar effect of e-STI testing on STI testing uptake across different population groups is of public health importance as it suggests a potential to increase testing among those most in need. Moreover, higher proportions of participants in the intervention group tested for all infections, including HIV and syphilis, compared to the control group.
Seven of the 19 participants diagnosed in the intervention group were diagnosed in a sexual health clinic. This highlights the continued importance of face-to-face services and is consistent with the theory of change, which proposes that e-STI testing offers patients more choice.
While our findings are in line with the proposed theory of change, they provide little evidence regarding the mechanism of action. Qualitative research is underway to explore participants’ experiences of using the intervention and their views on how it may have worked. These findings will be reported separately.
National guidelines in the UK recommend increasing testing among key population groups, and in areas of high HIV prevalence, in order to detect asymptomatic infection and normalise testing practices [18,19]. Our results for STI testing uptake suggest that e-STI testing could play a role in achieving these public health objectives. STI testing uptake and STI diagnosis are important intermediary outcomes on the pathway to increasing cases treated and cured or managed in community settings. However, our study provides limited evidence on these latter outcomes.
e-STI testing is currently being implemented in the UK as one measure to meet increasing demand for STI testing against a backdrop of severe budget cuts [48,49]. Publication of the cost-effectiveness evaluation of the intervention is pending and may provide additional insights on the contribution of e-STI testing. A larger trial is required to assess outcomes later in the cascade of STI care including STI diagnoses and cases treated and cured.
The effect size for STI cases treated was lower than for STI diagnoses, as we were unable to confirm if all those diagnosed were treated. At the time of the trial, SH:24 required those diagnosed with an infection to attend clinics in person for treatment. Attendance at clinic for treatment was confirmed for 11 of 19 participants diagnosed with an STI in the intervention group and 7 of 8 participants diagnosed in the control group. Some participants may have obtained treatment outside the study area, but as they did not state where they were treated, we were unable to verify this. The intervention did not reduce time to treatment.
It is plausible that whilst the intervention removed the barrier of having to attend a clinical service for testing, the subsequent requirement to attend clinic for treatment may have deterred some participants. Additional inputs are required so that increases in STI testing and STI diagnoses translate into similar increases in cases treated. This is likely to ensure that the potential public health benefits of e-STI testing can be fully realised.
Conclusions
We trialled SH:24 in a community setting, in 2 boroughs well served by face-to-face clinical services. Providing e-STI testing in contexts where supply is more limited, or targeting particular high-risk groups, might strengthen the contribution of e-STI testing to the control and management of STIs.
e-STI testing models could be adapted for countries with sufficient laboratory facilities. Established distribution channels for health products may be suitable for sending and receiving test kits, where postal services are limited. Future iterations of e-STI testing could include a wider range of services such as self-testing for HIV. Self-testing differs from the self-sampling modality evaluated by this study. Self-testing enables individuals to take a sample, perform a test, and interpret the results by themselves, without the need of a laboratory [14].
Delivering e-STI testing and results services to scale is technically feasible as demonstrated by SH:24, which currently delivers 42,000 tests per year in 6 regions in the UK. The long-term public health benefits of e-STI services will depend on testing, diagnosis, and treatment rates when implemented. These outcomes should be subject to ongoing monitoring and evaluation.
Supporting Information
Zdroje
1. World Health Organization. Sexually transmitted infections (STIs). Geneva: World Health Organization; 2016 [cited 2017 Nov 28]. Available from: http://www.who.int/mediacentre/factsheets/fs110/en/.
2. Public Health England. Table 1: STI diagnoses & rates in England by gender, 2007–2016. London: Public Health England; 2017 [cited 2017 Nov 22]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/626359/2016_Table_1_STI_diagnoses___rates_in_England_by_gender.pdf.
3. Public Health England. HIV: annual data tables. National HIV surveillance data tables. No. 1 : 2017. London: Public Health England; 2017 [cited 2017 Nov 28]. Available from: https://www.gov.uk/government/statistics/hiv-annual-data-tables.
4. Public Health England. Sexually transmitted infections and chlamydia screening in England, 2016. Health Protection Report. Volume 11 Number 20. London: Public Health England; 2017 Jun 9 [cited 2017 Nov 28]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/617025/Health_Protection_Report_STIs_NCSP_2017.pdf.
5. Holmes KT, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, et al., editors. Sexually transmitted diseases. 4th ed. New York: McGraw-Hill; 2008.
6. Mocroft A, Lundgren JD, Sabin ML, Monforte AdA, Brockmeyer N, Casabona J, et al. Risk factors and outcomes for late presentation for hiv-positive persons in europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med. 2013;10(9):e1001510. doi: 10.1371/journal.pmed.1001510 24137103
7. Department of Health. A framework for sexual health improvement in England. London: Department of Health; 2013 Mar [cited 2017 Nov 28]. Available from: https://www.gov.uk/government/publications/a-framework-for-sexual-health-improvement-in-england.
8. World Health Organization. Global health sector strategy on sexually transmitted infections, 2016–2021. Geneva: World Health Organization; 2016 [cited 2017 Nov 28]. Available from: http://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/.
9. Sonnenberg P, Clifton S, Beddows S, Field N, Soldan K, Tanton C, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 382(9907):1795–806. doi: 10.1016/S0140-6736(13)61947-9 24286785
10. Kirwan PD, Chau C, Gil ON, Delpech VC, Aghaizu A, et al. HIV in the UK—2016 report. London: Public Health England; 2016 [cited 2017 Nov 28]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/602942/HIV_in_the_UK_report.pdf.
11. Minichiello V, Rahman S, Dune T, Scott J, Dowsett G. E-health: potential benefits and challenges in providing and accessing sexual health services. BMC Public Health. 2013;13(1):790.
12. Burns K, Keating P, Free C. A systematic review of randomised control trials of sexual health interventions delivered by mobile technologies. BMC Public Health. 2016;16(1):778. doi: 10.1186/s12889-016-3408-z 27514851
13. Woodhall SC, Sile B, Talebi A, Nardone A, Baraitser P. Internet testing for Chlamydia trachomatis in England, 2006 to 2010. BMC Public Health. 2012;12 : 1095. doi: 10.1186/1471-2458-12-1095 23253518
14. Harding-Esch EM, Hollis E, Mohammed H, Saunders JM. Self-sampling and self-testing for STIs and HIV: the case for consistent nomenclature. Sex Transm Infect. 2017;93(2):445–448. doi: 10.1136/sextrans-2016-052841 27811311
15. Hottes TS, Farrell J, Bondyra M, Haag D, Shoveller J, Gilbert M. Internet-based HIV and sexually transmitted infection testing in British Columbia, Canada: opinions and expectations of prospective clients. J Med Internet Res. 2012;14(2):e41. doi: 10.2196/jmir.1948 22394997
16. Lorimer K, McDaid L. Young men’s views toward the barriers and facilitators of internet-based chlamydia trachomatis screening: qualitative study. J Med Internet Res. 2013;15(12):e265. doi: 10.2196/jmir.2628 24300158
17. Baraitser P, Syred J, Spencer-Hughes V, Howroyd C, Free C, Holdsworth G. How online sexual health services could work; generating theory to support development. BMC Health Serv Res. 2015;15 : 540. doi: 10.1186/s12913-015-1200-x 26637295
18. National Institute for Health and Care Excellence, Public Health England. HIV testing: increasing uptake among people who may have undiagnosed HIV. London: National Institute for Health and Care Excellence; 2016 Dec [cited 2017 Nov 28]. Available from: https://www.nice.org.uk/guidance/ng60/resources/hiv-testing-increasing-uptake-among-people-who-may-have-undiagnosed-hiv-1837567043269.
19. Public Health England. Internet-based chlamydia screening: guidance for commissioning. London: Public Health England; 2015 Mar [cited 2017 Nov 28]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/413834/Internet_based_chlamydia_screening.pdf.
20. Blake DR, Spielberg F, Levy V, Lensing S, Wolff PA, Venkatasubramanian L, et al. Could home STI specimen collection with e-prescription be a cost-effective strategy for clinical trials and clinical care? Sex Transm Dis. 2015;42(1):13–9. doi: 10.1097/OLQ.0000000000000221 25504295
21. Klamydia.se. 2017 [cited 2017 Nov 28]. Available from: https://www.klamydia.se/.
22. IWTK. About IWTK. 2017 [cited 2017 Nov 28]. Available from: https://www.iwantthekit.org/about/.
23. SHine SA. Get checked now. 2017 [cited 2017 Nov 28]. Available from: http://www.getcheckednow.com.au/get-checked.
24. Santé Publique France. Chlamydia. 2017 [cited 2017 Nov 28]. Available from: http://www.info-ist.fr/savoir/.
25. BC Centre for Disease Control. Get checked online. 2017 [cited 2017 Nov 28]. Available from: https://getcheckedonline.com/Pages/default.aspx.
26. Preventx. Freetest.me. 2017 [cited 2017 Nov 28]. Available from: https://www.freetest.me/.
27. Wilson E, Free C, Morris TP, Kenward MG, Syred J, Baraitser P. Can internet-based sexual health services increase diagnoses of sexually transmitted infections (STI)? Protocol for a randomized evaluation of an internet-based STI testing and results service. JMIR Res Protoc. 2016;5(1):e9. doi: 10.2196/resprot.4094 26772143
28. Wilson E, Free C, Morris PT, Syred J, Baraitser P. Addendum to: Can internet-based sexual health services increase diagnoses of sexually transmitted infections (STI)? Protocol for a randomized evaluation of an internet-based STI testing and results service. JMIR Res Protoc. 2016;5(3):e141. doi: 10.2196/resprot.6280 27783558
29. Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ. 2002;324(7347):1183. 12016181
30. National Chlamydia Screening Programme. Tables 1–4: chlamydia testing data for 15–24 year olds in England, January to December 2012. London: National Chlamydia Screening Programme; 2012 [cited 2015 Apr 29]. Available from: http://webarchive.nationalarchives.gov.uk/20150505151027/http://www.chlamydiascreening.nhs.uk/ps/resources/data-tables/CTAD%20Tables%20annual%202012%20republished%20for%20publication_FINAL13062014.xls.
31. Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378(9785):49–55. doi: 10.1016/S0140-6736(11)60701-0 21722952
32. Williamson EJ, Forbes A, White IR. Variance reduction in randomised trials by inverse probability weighting using the propensity score. Stat Med. 2014;33(5):721–37. doi: 10.1002/sim.5991 24114884
33. Rubin D. Multiple imputation for nonresponse in surveys. New York: Wiley and Sons; 1987.
34. Department for Communities and Local Government. The English Index of Multiple Deprivation (IMD) 2015—guidance. London: Department for Communities and Local Government; 2015 [cited 2017 Nov 28]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/464430/English_Index_of_Multiple_Deprivation_2015_-_Guidance.pdf.
35. Royston P, Parmar MKB. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011;30(19):2409–21. doi: 10.1002/sim.4274 21611958
36. British HIV Association, British Association of Sexual Health and HIV, British Infection Society. UK national guidelines for HIV testing 2008. London: British HIV Association; 2008 Sep [cited 2017 Nov 28]. Available from: https://www.bashhguidelines.org/media/1067/1838.pdf.
37. Public Health England. National chlamydia screening programme standards (seventh edition). Updated October 2016. London: Public Health England; 2016 [cited 2017 Nov 28]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/574351/NCSP_Standards_7th_edition.pdf.
38. Bailey VJ, Pavlou M, Copas A, McCarthy O, Carswell K, Rait G, et al. The Sexunzipped Trial: optimizing the design of online randomized controlled trials. J Med Internet Res. 2013;15(12):e278. doi: 10.2196/jmir.2668 24334216
39. Southwark Council. Southwark demographic factsheet. London: Southwark Council; 2015 May [cited 2017 Nov 28]. Available from: https://www.southwark.gov.uk/assets/attach/2653/Demographic%20factsheet.pdf.
40. Lambeth Council. Lambeth demographic factsheet. London: Lambeth Council; 2015 May [cited 2017 Nov 28]. Available from: https://www.lambeth.gov.uk/sites/default/files/ssh-lambeth-demography-2015.pdf.
41. Bell ML, Kenward MG, Fairclough DL, Horton NJ. Differential dropout and bias in randomised controlled trials: when it matters and when it may not. BMJ. 2013;346:e8668. doi: 10.1136/bmj.e8668 23338004
42. Carpenter J, Kenward M. Missing data in randomised controlled trials—a practical guide. Birmingham: Health Technology Assessment Methodology Programme; 2007.
43. Chai S, Aumakhan B, Barnes M, Jett-Goheen M, Quinn N, Agreda P, et al. Internet-based screening for sexually transmitted infections to reach nonclinic populations in the community: risk factors for infection in men. Sex Transm Dis. 2010;37(12):756–63. doi: 10.1097/OLQ.0b013e3181e3d771 20644498
44. Ladd J, Hsieh Y-H, Barnes M, Quinn N, Jett-Goheen M, Gaydos CA. Female users of internet-based screening for rectal STIs: descriptive statistics and correlates of positivity. Sex Trans Infect. 2014;90(6):485–90.
45. Kersaudy-Rahib D, Lydié N, Leroy C, March L, Bébéar C, Arwidson P, et al. Chlamyweb Study II: a randomised controlled trial (RCT) of an online offer of home-based Chlamydia trachomatis sampling in France. Sex Transm Infect. 2017;93(3):188–95. doi: 10.1136/sextrans-2015-052510 28377422
46. Fajardo-Bernal L, Aponte-Gonzalez J, Vigil P, Angel-Muller E, Rincon C, Gaitan HG, et al. Home-based versus clinic-based specimen collection in the management of Chlamydia trachomatis and Neisseria gonorrhoeae infections. Cochrane Database Syst Rev. 2015;9:CD011317.
47. World Health Organization. Guideline on self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. Geneva: World Health Organization; 2016 Dec [cited 2017 Nov 28]. Available from: http://apps.who.int/iris/bitstream/10665/251655/1/9789241549868-eng.pdf?ua=1.
48. Robertson R, Wenzel L, Thompson J, Charles A. Understanding NHS financial pressures. How are they affecting patient care? London: The King’s Fund; 2017 Mar.
49. Cleary M, O’Sullivan J. P145 London sexual health transformation programme. Sex Transm Infect. 2017;93(Suppl 1):A64–5.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 12- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Cell salvage and donor blood transfusion during cesarean section: A pragmatic, multicentre randomised controlled trial (SALVO)
- Re-emerging and newly recognized sexually transmitted infections: Can prior experiences shed light on future identification and control?
- Antiretroviral therapy and population mortality: Leveraging routine national data to advance policy
- Psychosocial and socioeconomic determinants of cardiovascular mortality in Eastern Europe: A multicentre prospective cohort study
- Research on HIV cure: Mapping the ethics landscape
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- Effects of women’s groups practising participatory learning and action on preventive and care-seeking behaviours to reduce neonatal mortality: A meta-analysis of cluster-randomised trials
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
- Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: A modeling study
- Sexually transmitted infections—Research priorities for new challenges
- Healthcare provider perspectives on managing sexually transmitted infections in HIV care settings in Kenya: A qualitative thematic analysis
- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Dual-strain genital herpes simplex virus type 2 (HSV-2) infection in the US, Peru, and 8 countries in sub-Saharan Africa: A nested cross-sectional viral genotyping study
- Association between infrastructure and observed quality of care in 4 healthcare services: A cross-sectional study of 4,300 facilities in 8 countries
- Bridging the quality chasm in maternal, newborn, and child healthcare in low- and middle-income countries
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání