-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study
Fumiaki Imamura and colleagues reveal profiles of plasma phospholipid fatty acids that are associated with risk of type 2 diabetes using data from the EPIC cohort.
Published in the journal: . PLoS Med 14(10): e32767. doi:10.1371/journal.pmed.1002409
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002409Summary
Fumiaki Imamura and colleagues reveal profiles of plasma phospholipid fatty acids that are associated with risk of type 2 diabetes using data from the EPIC cohort.
Introduction
Fatty acids play vital roles in metabolic homeostasis, serving as precursors of signalling molecules, energy sources, and constituents of membranes and functional lipids [1,2]. Reflecting their diverse roles, fatty acids have been evaluated as markers of physiological homeostasis, metabolic disorders, and dietary exposure in biological, clinical, and population-based research [3–5]. For example, blood or tissue levels of omega-3 polyunsaturated fatty acids (PUFAs) have been studied as a cardio-protective factor in biochemical and clinical research and as a biomarker of dietary consumption of omega-3 PUFAs in epidemiological research [1,2,4–6]. However, research to date has largely evaluated individual fatty acids or single subgroups of fatty acids, rather than combinations of fatty acids, in terms of mechanism or as potential biomarkers.
Combinations of fatty acids may have aetiological and clinical implications for metabolic diseases including type 2 diabetes (T2D). Insulin resistance and pancreatic lipotoxicity have been found to be influenced by multiple fatty acids. For example, palmitic acid (16 : 0) induces lipotoxicity, and unsaturated fatty acids may prevent it [7–9]. Pharmacological and nutritional research also warrant considering multiple fatty acids together. Interventions of lipid-lowering drugs or dietary carbohydrates or fats, for example, alter blood concentrations of individual PUFAs and saturated fatty acids (SFAs) jointly [3,9–11]. These findings support the notion that combinations of fatty acids are important to study in relation to the aetiology of T2D and to predict T2D risk.
A few epidemiological studies have identified combinations of circulating or tissue fatty acids associated with adiposity, hypertension, and risks of metabolic syndrome and cardiovascular diseases using a statistical pattern-recognition approach [12–15]. These studies have indicated potential biological and clinical importance of combinations over and above that of individual fatty acids. However, a combination of fatty acids has never been evaluated as a potential risk factor for incident T2D. Thus, we first aimed to identify 1 or more combinations of phospholipid fatty acids that explained variability in multiple fatty acid concentrations, using epidemiological data from the European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct study. Then, focussing on the single combination of fatty acids that explained the greatest variability, we tested the hypothesis that the combination is associated with the incidence of T2D. To provide mechanistic insights, we further examined the association of this combination of fatty acids with metabolic risk factors, genetic predisposition to obesity and insulin resistance, and dietary intakes in EPIC-InterAct. For metabolic and dietary factors, external validation was performed by evaluating data of the US National Health and Nutrition Examination Survey (NHANES).
Methods
Study population
We conducted this work as a substudy of the fatty acid project in EPIC-InterAct to explore a combination of fatty acids to add to our previous work on individual fatty acids and subclasses (S1 Protocol) [16,17]. EPIC-InterAct is a prospective study nested within 8 European countries of the EPIC study (Denmark, France, German, Italy, Netherlands, Spain, Sweden, and UK) [16,18]. In EPIC-InterAct, the case-cohort design was adopted to combine the advantages of a prospective design with the efficiency of a case-control design [19]. From the 340,234 adults with 3.99 million person-years of follow-up of the EPIC study, EPIC-InterAct (1) randomly selected 16,835 adults (‘sub-cohort’) and (2) identified 12,403 incident cases of T2D occurring by 31 December 2007; the identified cases included 778 cases in the sub-cohort by design (S1 Fig) [16,18]. All participants gave written informed consent. The study was approved by local ethics committees and the institutional review board of the International Agency for Research on Cancer [18].
The current study included 15,919 adults from the sub-cohort—after excluding 916 meeting 1 or more exclusion criteria: prevalent diabetes (n = 548), missing information on fatty acids (n = 156), missing information on incident T2D (n = 129), and post-censoring T2D (n = 4)—and included 12,132 incident T2D cases, after excluding 271 adults missing information on fatty acids (S1 Fig). In summary, we evaluated 27,296 adults in this study (12,132 cases, including 755 cases from the sub-cohort; and 15,919 adults from the sub-cohort).
Ascertainment of type 2 diabetes
Prevalent diabetes cases (excluded from the study) were identified by baseline self-report of a diagnosis, physician’s diagnosis, anti-diabetic drug use, or other evidence of T2D before the baseline date in EPIC-InterAct [18]. Incident T2D was ascertained from multiple information sources reviewed by each participating centre [18]: self-report, linkage to primary-care registers, secondary-care registers, medication use (drug registers), hospital admissions, and mortality data. Information from any follow-up visit or external evidence with a date later than the baseline visit was used. In Denmark and Sweden, incident cases were identified via local and national diabetes and pharmaceutical registers, and hence all ascertained cases were considered to be verified. Follow-up was to the date of diagnosis, 31 December 2007, or the date of death, whichever occurred earliest.
Assessment of fatty acids and other variables
We evaluated relative concentrations of 27 individual fatty acids expressed as mole percentage of total plasma phospholipid fatty acids (Table 1), as previously described (S1 Text) [16,20]. These measurements were masked to case status. Thirty-seven fatty acids of plasma phospholipids were quantified by gas chromatography [20]. In the current analysis, 10 fatty acids were excluded because their relative concentrations were <0.05% on average. Coefficients of variation of the 27 fatty acids ranged from 1.9% to 4.6% [20].
Tab. 1. Relative concentrations of plasma phospholipid fatty acids and their correlations with the identified fatty acid pattern score: EPIC-InterAct sub-cohort (n = 15,919). 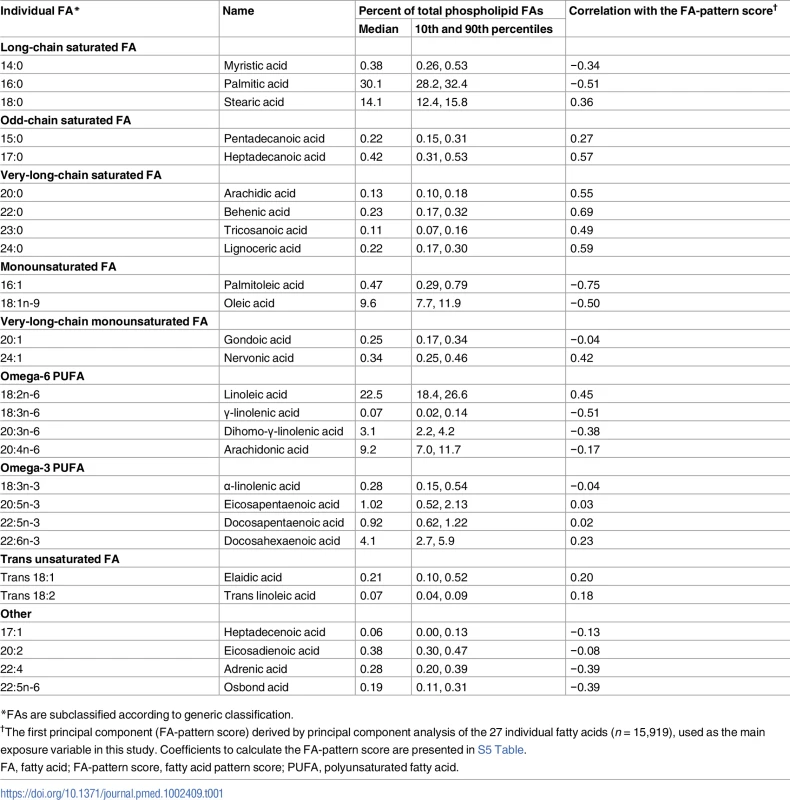
*FAs are subclassified according to generic classification. At baseline, weight, height, and waist circumference were measured directly in every centre. Waist circumference was not measured in Umea, Sweden (n = 1,845) [18]. Sociodemographic factors, smoking status, and medical history were assessed by a questionnaire for general health. Physical activity was assessed by a questionnaire validated previously [21]. Dietary variables were derived centrally based on food frequency questionnaires or diet histories standardised in each cohort [22,23]. Using blood samples stored at −196°C (or −150°C in Denmark), biochemical assays were performed at Stichting Ingenhousz Laboratory, Etten-Leur, Netherlands, for glucose, triglycerides, high-density lipoprotein cholesterol (HDL-C), triglycerides, high-sensitivity C-reactive protein (hsCRP), and proteins related to hepatic function—alanine transaminase (ALT), γ-glutamyl transferase (GGT), and aspartate transaminase (AST)—as the liver is the major organ metabolising fatty acids.
Genetic information became available in 22,179 adults with fatty acid data, assayed with Illumina Human660W-Quad BeadChip (Illumina, Little Chesterford, UK; n = 9,166) and MetaboChip (Illumina; n = 13,013) [24]. Using these data, we conducted post hoc analyses to examine whether genetic predisposition to metabolic risk was associated with the FA-pattern score. We calculated weighted genetic risk scores for body mass index (BMI) (n loci = 97) [25] and for insulin resistance (n loci = 10) [24] using published measures of genome-wide associations (S1 Text).
Derivation of fatty acid pattern score
Principal component analysis (PCA) was performed in the sub-cohort (n = 15,919) to combine multiple fatty acids (Table 1) together in a way to explain as much variation of those fatty acids as possible. Sampling weights were applied so that each of the 8 countries equally contributed to the PCA. Eigenvalues divided by 27 were assessed as percent of variance explained.
Principal components were inferred as representing fatty acid patterns. The pattern matrix from PCA was then used to calculate the scores, referred to as FA-pattern scores, among the rest of the study population (incident T2D cases not in the sub-cohort, Fig 1) and was also applied to quality control samples (n = 860) to assess the contribution of any batch effects [20]. We chose to focus on the first principal component for further aetiological analyses to provide potential biological implications of this single combination of fatty acids. This enabled us to conduct and report a detailed investigation into the associations of this combination with T2D incidence and metabolic, dietary, and genetic variables, and their biological implications; it also removed the need for subjective decisions about how many components to derive, which matrix rotation method to use, and how to account for multiple testing [26].
Fig. 1. Principal components and clusters of 27 fatty acids in the EPIC-InterAct sub-cohort (n = 15,919). 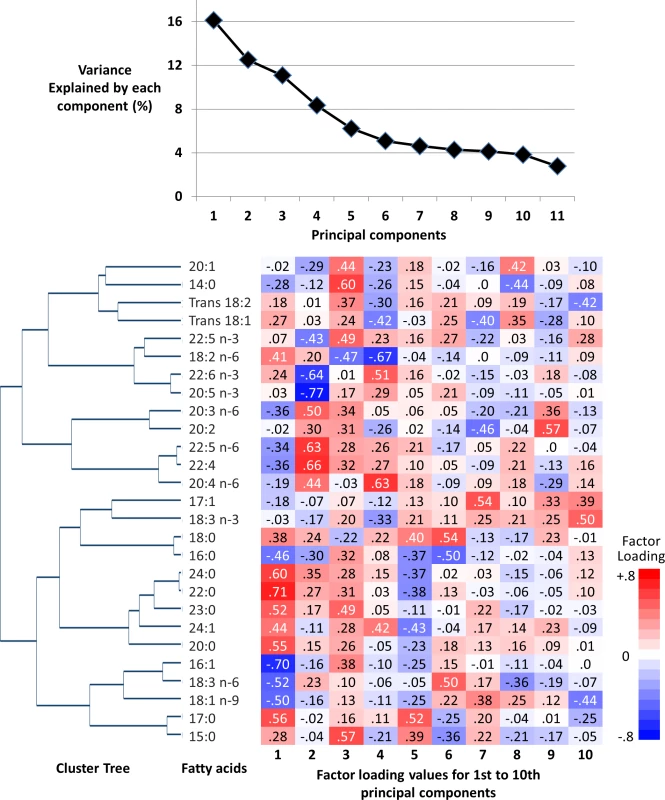
Top: The proportion of total variance of 27 fatty acids explained by each principal component. Bottom: Hierarchical cluster tree on the left and factor loadings (measures of contributions of fatty acids to principal components) on the right. Statistical analysis
All analyses were performed using Stata (StataCorp, College Station, Texas, US), with αtwo-sided = 0.05. For descriptive purposes, a hierarchical cluster tree was generated to visually assess correlation between fatty acids [27]. Pearson correlation coefficients between fatty acids were also calculated. The strength of association of the FA-pattern score with incident T2D was evaluated by estimating hazard ratios (HRs) and 95% CIs from Prentice-weighted Cox regression, with age as the underlying timescale [19]. The estimates were obtained in each country and pooled by random-effects meta-analysis [28] for quintiles specific to the sub-cohort, for a continuous term per interdecile range (the difference between the 90th and 10th percentiles of the distribution), and for cubic-spline terms to test non-linear associations [29]. We additionally computed a 95% predicted interval for the primary results by combining random-effects variation (tau2) and variation of the main estimate [28].
The models included potential confounders, including demographics, prevalent heart disease and stroke, medication use, smoking status, physical activity, and dietary factors (consumption of alcohol, soft drinks, dietary fibre, fruits, vegetables, and processed meats), that are associated with cardiometabolic health in general. We also adjusted for BMI, waist circumference, glucose, lipids, hsCRP, and liver enzymes to examine their influence on the associations of interest. Potential confounding by genetic predisposition for greater BMI and insulin resistance was also assessed.
In pre-specified analyses, we examined whether observed associations varied by baseline age, sex, and BMI, testing an interaction term for each factor and the FA-pattern score in regression analysis. Effect modification by blood triglycerides, use of lipid-lowering drugs (yes or no), and alcohol consumption (consumer or non-consumer) was also tested post hoc because of the association of triglycerides with the FA-pattern score (r = −0.29) and possible effects of lipid-lowering drugs and alcohol on de novo lipogenesis. Missing covariates were imputed by country, using multiple imputation by chained equations with variables for the FA-pattern score, covariates, survival time, and case status [30]. We report results from single imputation, because between-imputation variability was <0.2% of total variability in multiple imputation (20 datasets); we performed sensitivity analysis using multiple imputation and complete-case analysis.
As a sensitivity analysis to assess whether HR varied over the follow-up time by reverse causation, stratified analysis was performed by splitting follow-up time at 7 years after baseline and by censoring any events occurring within the first 2 years as non-cases. We additionally evaluated the stability of our findings: examining the consistency of a main finding for the single principal component when PCA was performed after Box–Cox transformation, improving normality of distribution of all fatty acid variables. We also examined whether or not the main result was driven by single fatty acids or fatty acid subclasses through 2 approaches: adjusting models for single fatty acids and subclasses separately, and repeating the analysis after PCA of fatty acids excluding each of the 27 fatty acids or subclasses one at a time. We performed internal cross-validation [31]: First, we re-derived the FA-pattern score in a subset selected by country, age, sex, and BMI (test set); second, we applied the scoring matrix to another subset (validation set) to derive the FA-pattern score, and then we examined the associations of the independently derived score with incident T2D.
Analysis of metabolic, genetic, and dietary factors
To investigate potential mechanisms for the association of the FA-pattern score with incident T2D, we estimated cross-sectional associations of the FA-pattern score with each of selected metabolic risk factors (BMI, waist circumference, lipids, glucose, hsCRP, and liver enzymes) using linear regression. Additionally, modified Poisson regression [32] was used to examine the cross-sectional association of the FA-pattern score with prevalence of hepatic steatosis defined as ALT greater than cut-points previously validated against ultrasound (30 U/l for men, 19 U/l for women) [33]. We further fitted linear regression to assess whether genetic risk scores for BMI and for insulin resistance (independent variables) could explain variability in the FA-pattern score (dependent variable). These regression models statistically adjusted for age and the other covariates used for longitudinal analyses.
We also evaluated dietary factors as potential lifestyle determinants of the FA-pattern score. Multivariable-adjusted linear models included dietary determinants as independent variables and the FA-pattern score (scaled to 1 standard deviation) as a dependent variable. This analysis evaluated major macronutrient and fibre intakes (nutrient-based analysis) and 18 selected foods or beverages (food-based analysis).
Assessment of external validity
Recognising the risk of false-positive findings based on our data-driven approach, we conducted post hoc assessment of the external validity of the FA-pattern score derived in EPIC-InterAct, using cross-sectional data from NHANES 2003–2004 (n = 1,566) on total plasma fatty acids, metabolic factors, dietary factors, and potential confounders. Using the scoring matrix derived from EPIC-InterAct, we calculated the FA-pattern score in NHANES (S2 Text) [15]. Using linear regression adjusting for potential confounders, replication analyses were performed (S3 Text). In dietary analyses, 18 dietary items were first assessed in EPIC-InterAct with backward variable selection (p = 0.2 as a cutoff, additionally for the purpose of adjustment [34]) to identify which dietary variables predicted the FA-pattern score together. Then we tested selected dietary factors in NHANES for external validation (S3 Text) in linear regression adjusting for potential confounders and including the same dietary variables. The 18 food groups first tested in EPIC-InterAct were selected by possible biology of diets, fatty acid profiles, and T2D, and evaluated both individually and simultaneously.
Results
The first component derived by PCA explained 16.1% of the variation of 27 fatty acids, and 6 to 10 components explained more variation than 1 fatty acid could explain (>3.7% of total; ‘eigenvalue’ > 1.0) (Fig 1). The first 4 components had loading values (e.g., >0.6 or <−0.6) in multiple fatty acid classes. Selected to gain insight into the biological importance of a combination of fatty acids, the first component reflected relationships between fatty acids varying in chain length and degree of unsaturation, including fatty acids that can be synthesised endogenously and those derived from dietary consumption (Table 1; Fig 1). A similar pattern was identified in cluster analysis, as fatty acids adjacent in the tree had similar loading values (Fig 1). Major contributors (correlation coefficients r > 0.5 or r < −0.5) were palmitic acid (16 : 0, r = −0.51), palmitoleic acid (16 : 1, r = −0.75), and γ-linolenic acid (18 : 3n-6, r = −0.51). Heptadecanoic acid (17 : 0) and very-long-chain SFAs (VLSFAs) with 20 or more carbons had positive contributions (r = 0.5–0.7), but their relative concentrations were low (<1% of total). While linoleic acid (18 : 2n-6) had a positive contribution (r = 0.45), the other PUFAs, and trans unsaturated fatty acids had lower contributions (−0.25 < r < 0.25) (Fig 1). The coefficient of variation of the FA-pattern score was 6.0% based on the quality control samples.
Adults with higher FA-pattern score were more likely to be women, non-smokers, non-users of lipid-lowering drugs, and those with generally healthier profiles of metabolic risk factors, while there was no significant relationship with age or education (S1 Table). Covariates had missing values in <5% of adults, except 49.9% for family history of diabetes, which was not assessed in 12 of the 26 study centres (S2 Table). Where it was assessed, 24.5% of participants had missing information.
Association of the fatty acid pattern score with incidence of type 2 diabetes
In the longitudinal analysis of 12,132 cases per 190,148.9 person-years (11.9 y of follow-up per person on average), the FA-pattern score was strongly associated with incident T2D. Adjusted for sociodemographic variables, dietary factors, and medical history, the HR (95% CI) of T2D comparing the top to the bottom fifth of the FA-pattern score was 0.23 (0.19–0.29) (p trend < 0.001) (Table 2). The association persisted after adjustment for BMI (HR 0.32; 95% CI 0.25–0.40) and for triglycerides and HDL-C (0.37; 95% CI 0.27–0.50). Results changed little when additionally adjusted for concentrations of random glucose, hsCRP, hepatic enzymes, other dietary factors, family history of T2D, and genetic risk scores for obesity and insulin resistance (S3 Table). The association varied across the 8 countries (Fig 2; I2 = 88%); this variation was partly explained by country-specific mean ages and percentage of men (p < 0.05 each), although an inverse association was observed in all countries.
Fig. 2. Prospective associations of the fatty acid pattern score with incident diabetes in 8 countries: EPIC-InterAct (n = 27,296). 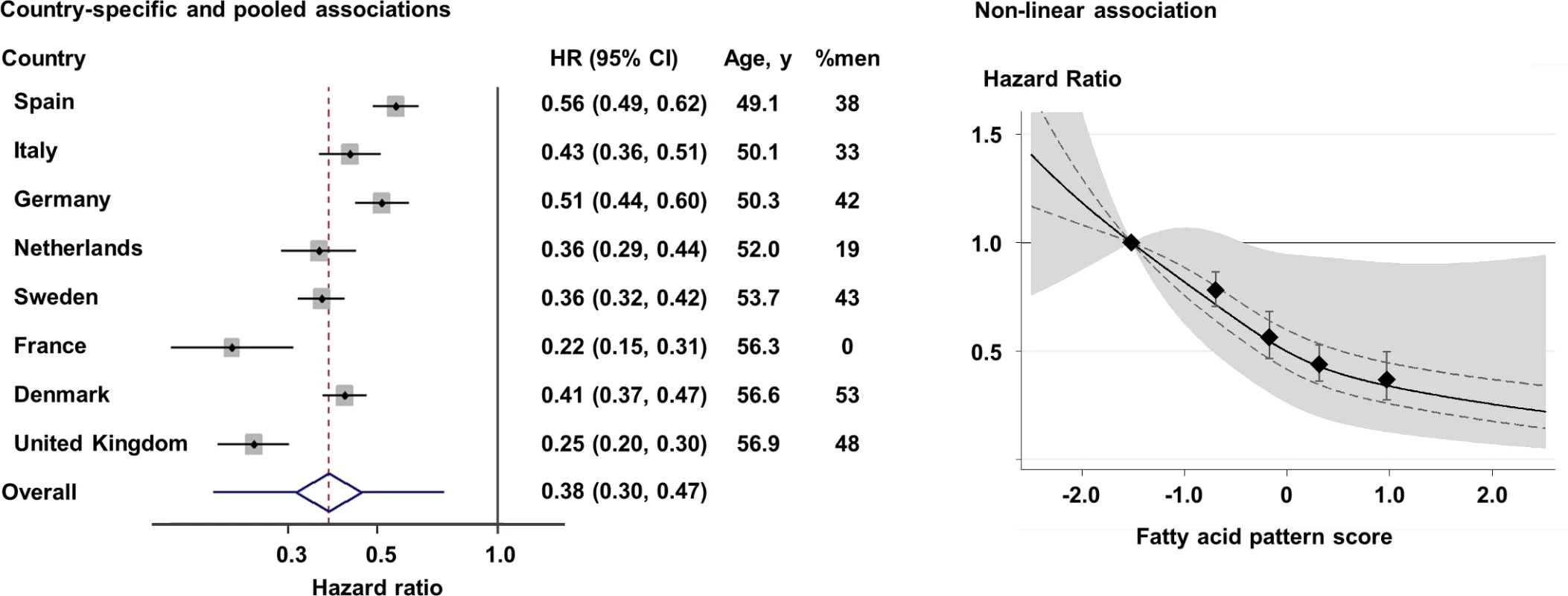
Left: Hazard ratios (HRs) per country-specific range of 10th to 90th percentiles (p for heterogeneity by age and sex = 0.005 and 0.02, respectively), and pooled by random-effects meta-analysis. The diamond and error bars of the pooled estimate represent the 95% confidence interval and predicted interval (0.20 to 0.74), respectively. Right: HR based on quintiles and restricted cubic spline (p non-linearity = 0.001) (solid line). Error bars in both panels, and dotted lines in the right panel, indicate the 95% confidence intervals of HRs, and the shaded area in the right panel is the predicted interval. All analyses adjusted for covariates as in the most adjusted model in Table 2. Tab. 2. Association of the fatty acid pattern score with incidence of type 2 diabetes: EPIC -InterAct (n = 27,296). 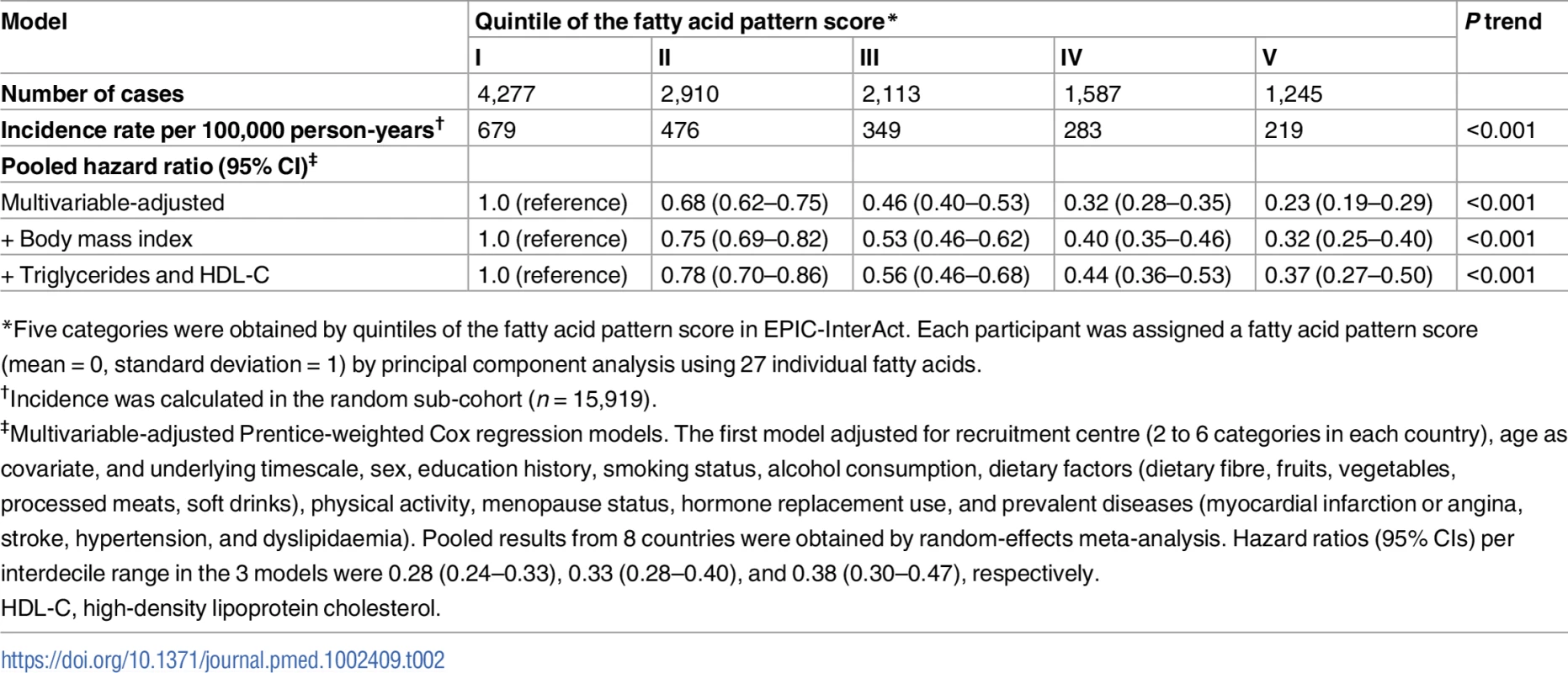
*Five categories were obtained by quintiles of the fatty acid pattern score in EPIC-InterAct. Each participant was assigned a fatty acid pattern score (mean = 0, standard deviation = 1) by principal component analysis using 27 individual fatty acids. There was no evidence of effect modification by baseline age, sex, BMI, triglycerides, lipid-lowering drug use, or alcohol consumption (p interaction > 0.1 each). The main result was stable in sensitivity analyses that explored the influence of imputation, duration of follow-up, and normality of distribution (S3 Table). In analyses adjusting for individual fatty acid variables (S2 Fig), adjustment for 17 : 0 attenuated the estimates to the greatest extent among the fatty acid variables we evaluated, shifting the HR (95% CI) of 0.38 (0.30–0.47) to 0.53 (0.42–0.67), but with the association remaining significant. Cross-validation analysis confirmed the stability of the findings (S4 Table). For instance, when the FA-pattern score was derived in 7 countries, not 8, and the scoring algorithm was applied to adults in the 1 country excluded, the summary HR (95% CI) in the most adjusted model was 0.40 (0.34–0.50).
Association of the fatty acid pattern score with metabolic, genetic, and dietary factors
In both EPIC-InterAct and NHANES (see S5 Table for scoring coefficients), the FA-pattern score was associated with metabolic risk factors in the direction consistent with the above findings for incident T2D. Inverse associations were seen with BMI, triglycerides, glucose, hsCRP, ALT, AST, GGT, and the likelihood of having hepatic steatosis (p < 0.001 each) (Table 3). A significant positive association with HDL-C was observed in NHANES (p < 0.001), but not in EPIC-InterAct (p = 0.7).
Tab. 3. Associations of the fatty acid pattern score with metabolic factors in EPIC-InterAct and with metabolic factors in the US NHANES 2003–2004. 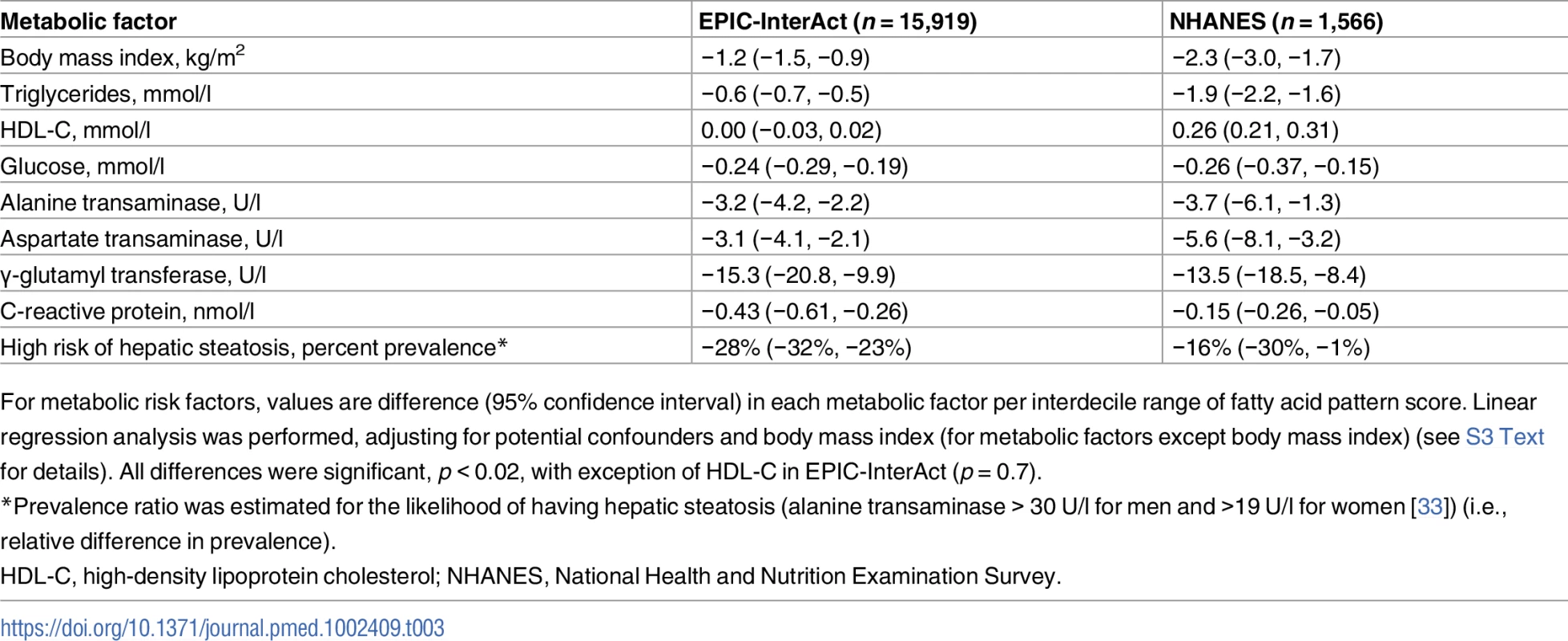
For metabolic risk factors, values are difference (95% confidence interval) in each metabolic factor per interdecile range of fatty acid pattern score. Linear regression analysis was performed, adjusting for potential confounders and body mass index (for metabolic factors except body mass index) (see S3 Text for details). All differences were significant, p < 0.02, with exception of HDL-C in EPIC-InterAct (p = 0.7). In genetic analyses (EPIC-InterAct only), a gene score related to higher BMI was not significantly associated with the FA-pattern score: +0.1% of SD of the FA-pattern score (95% CI −0.6% to +1.7%; p = 0.3) per interdecile range of the genetic score. A gene score related to higher insulin resistance was significantly associated with lower FA-pattern score: −1.9% of SD (95% CI −3.4% to −0.4%; p = 0.02).
In dietary analyses in EPIC-InterAct and NHANES, higher intakes of PUFAs and fibre were associated with higher FA-pattern score in both cohorts (Fig 3). For example, replacing carbohydrates with PUFAs in the diet by an amount equivalent to 5% of total energy was positively associated with the FA-pattern score (0.43 SD of the score, 95% CI 0.30–0.57) in EPIC-InterAct and 0.21 (95% CI 0.11–0.32) in NHANES. In food-based analysis of EPIC-InterAct, the FA-pattern score was significantly related to higher intakes of fish, margarine, and coffee and lower intakes of soft drinks and alcoholic beverages (p < 0.05 each) when assessed individually (S3 Fig) and simultaneously (Fig 3). In NHANES, findings from EPIC-InterAct for soft drinks, coffee, and alcohol were replicated (p < 0.04) (Fig 3).
Fig. 3. Associations of dietary factors with the fatty acid pattern score in EPIC-InterAct (1991–1998, n = 15,566) and the US National Health and Nutrition Examination Survey (2003–2004, n = 1,500). 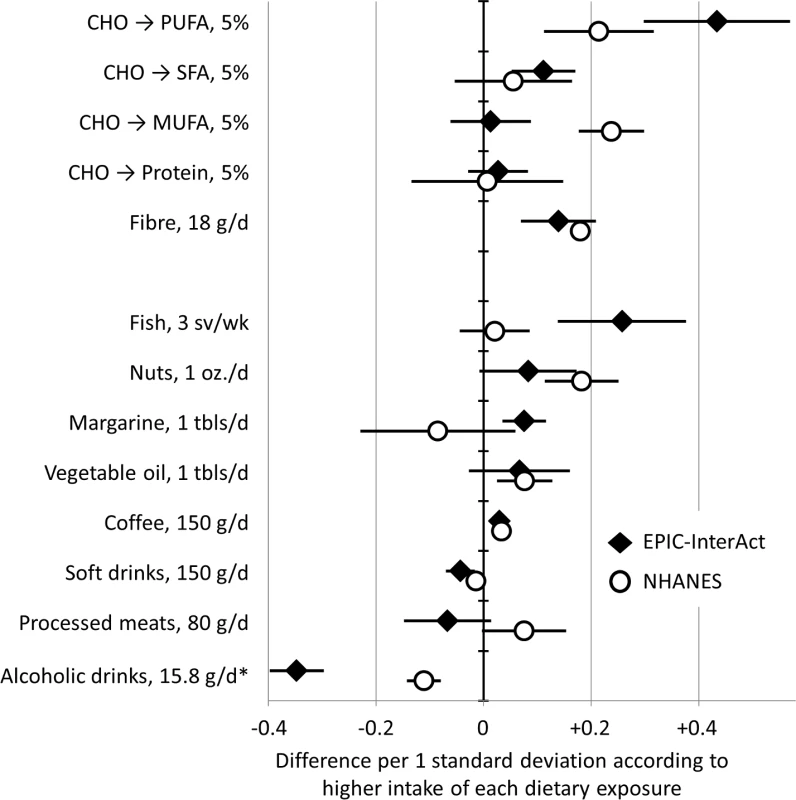
Error bars are 95% confidence intervals. In analysis for macronutrients, analysis estimated potential effects of replacing carbohydrate (CHO) intakes with intakes of polyunsaturated fatty acid (PUFA), monounsaturated fatty acid (MUFA), saturated fatty acid (SFA), and protein by the amount of 5% of total caloric intake. Dietary factors were scaled for interpretability: sv, serving; oz., ounce (28.8 g); tbls, tablespoon. *Alcoholic drinks were examined as grams of ethanol. Discussion
We evaluated fatty acid profiles among adults in 8 European countries and derived a FA-pattern score that represents a combination of both essential and non-essential fatty acids and that is characterised by high relative concentrations of linoleic acid (18 : 2n-6), stearic acid (18 : 0), odd-chain SFAs, and VLSFAs (≥20 carbons), and by low relative concentrations of γ-linolenic acid (18 : 3n-6), monounsaturated fatty acids (MUFAs), and long-chain SFAs (14 : 0 and 16 : 0). The unique combination was associated with dietary, metabolic, and genetic factors, and prospectively associated with a lower incidence of T2D. Comparing the top fifth to the bottom fifth of the FA-pattern score, T2D incidence was lower by approximately 60%. This robust association with incident T2D was independent of established risk factors and also any single fatty acids or fatty acid subclasses. These findings support the hypothesis that a combination of multiple fatty acids is an important marker for the development of T2D above and beyond the roles of single types of fatty acids. The combination of essential and non-essential fatty acids is of strong interest for further clinical or population-based investigations to predict T2D risk, identify interventional agents for T2D prevention, and better understand the aetiology of T2D.
The combination of fatty acids contributing to the identified FA-pattern score fits with known mechanisms involving the de novo lipogenesis (DNL) pathway. In DNL, fatty acids including 14 : 0, 16 : 0, 16 : 1n-7, and 18 : 1n-9 are synthesised endogenously, where stearoyl-CoA desaturase (SCD) converts 16 : 0 to 16 : 1n-7 as a rate-limiting step of fatty acid synthesis. The inverse correlation of 18 : 2n-6 with these fatty acids may reflect its role as a ligand of peroxisome proliferator-activated receptor α (PPARα) [2,5]. PPARα down-regulates SCD and ELOVL (elongation of very-long-chain fatty acid) enzymes, explaining the observed inverse correlation of 18 : 2n-6 with MUFAs, 16 : 0, 18 : 3n-6, and other n-6 PUFAs [2]. An exception of PPARα’s action is activation of ELOVL3, which leads to synthesis of VLSFAs in the adipose tissue [35] and supports the observed associations between 18 : 2n-6, 18 : 0, and VLSFAs. While our findings are in line with the benefit of dietary PUFAs (predominantly 18 : 2n-6), other major PUFAs (e.g., omega-3 PUFA) contributed little to the primary fatty acid combination. This could reflect their diverse roles in eicosanoid pathways and pro - and anti-inflammatory pathways, and their associations with dietary intakes (e.g., fish) independent of DNL-driving dietary factors [5].
These suggested mechanisms are linked to the development of T2D. Activation of PPARα suppresses hepatic DNL and pro-inflammatory pathways that lead to insulin resistance, dyslipidaemia, and fatty liver [2,5,36]. In an experimental setting, for example, PPARα knock-out mice developed fatty liver exhibiting overt hepatic lipogenesis [37]. Main products of DNL include 16 : 0 and diacylglycerols that cause a pro-inflammatory response, endoplasmic reticulum stress, and insulin resistance [1,38]. Thus, our analysis yielded a combination of multiple fatty acids that may represent biological pathways related to insulin resistance, inflammatory responses, and T2D risk. This was confirmed with the observed associations of FA-pattern score with metabolic risk factors in an expected direction and the association of FA-pattern score with genetic predisposition to insulin resistance. Associations of gene variants with fatty acids and with incident T2D cannot be confounded by long-term lifestyle characteristics. Therefore, the specific gene variants, the identified combination of fatty acids, and the risk of T2D are likely to be on the same causal pathway, warranting future research to elucidate how insulin resistance specifically alters fatty acid profiles or vice versa.
Our analysis and prior studies derived a similar combination of fatty acids [12–15], but no previous studies to our knowledge evaluated T2D incidence as an outcome. Past studies used different methods and examined varied numbers of fatty acids (10 to 42) of phospholipids [15], cholesteryl esters [12,15], plasma [13], or adipose tissue [14]. Despite the differences, all of the studies reported a combination of fatty acids partly driven by higher levels of 18 : 2n6 with lower 16 : 0, which could reflect activity of DNL [39]. These combinations were found to be associated with lower blood pressure, greater endothelial function, less weight gain over time, or lower risk of metabolic syndrome [12–14]. In contrast, an association with ischaemic heart disease or stroke was not significant [15]. The inconsistency depending on outcome is predictable as DNL could promote insulin resistance, but suppress atherosclerosis [36]. Statins and other lipid-lowering drugs also alter fatty acid profiles [10] and have divergent effects on heart disease and T2D [40]. Thus, a fatty acid pattern can be a future focus of investigations of cardiometabolic diseases and related interventions.
The association of the FA-pattern score with incident T2D was not fully explained by any single fatty acid, but was partly attenuated by adjustment for odd-chain SFAs and VLSFAs. These SFAs are associated with lower risk of cardiometabolic diseases [16,41,42], while their biological roles remains understudied. High phospholipid VLSFAs may reflect high activity of PPARα, which leads to VLSFA synthesis and less insulin resistance, apoptotic cell death, and pancreatic dysfunction [41,43]. Blood odd-chain SFAs may partly reflect dairy consumption [3,44], gut microbiota [45], or endogenous synthesis through α-oxidation [46], and thus any correlates to those factors could explain our findings. Evidence for these mechanisms and relationships with other fatty acids remains scarce and deserves further investigation.
Dietary correlates with the combination of fatty acids deserve discussion, as they were replicated in EPIC-InterAct and NHANES: alcohol and soft drinks as negative correlates, and coffee, fibre, and PUFAs as positive correlates. The finding for alcohol consumption is likely to reflect its lipogenic effect, a risk factor for liver cirrhosis and T2D [47]. Regarding coffee consumption, polyphenols may deactivate DNL [48] and lower triglyceride levels and T2D risk [49–51]. Increased PUFA intake (predominantly n-6 PUFAs) could increase insulin sensitivity as well as lower DNL [2,5,52]. Our findings for soft drinks and fibre may also reflect their glycaemic and anti-glycaemic effects, respectively, as a high glycaemic effect leads to insulin secretion and DNL [2,53].
Strengths of this work include the standardised assay of fatty acid profiles in an EPIC-InterAct population with geographic diversity; the large study size (to our knowledge by far the largest among studies of fatty acid biomarkers), allowing various sensitivity analyses; and the generalisability of our findings, strengthened by our findings across 8 European countries and the US NHANES. By focussing on a single combination of fatty acids, we were able to report details of its association with incident T2D and metabolic, genetic, and dietary factors. However, this also limited our investigation of other fatty acid patterns, in particular in relation to omega-3 fatty acids. Other limitations include possible residual confounding by factors unmeasured or measured imprecisely, although we adjusted for many covariates including major risk factors for T2D. Whether or not the combination of fatty acids itself caused the T2D onset remains unestablished. Possible exposure misclassification due to single fatty acid measurements and possible outcome misclassification were limitations, but likely to be independent of T2D case status and fatty acid profiles, respectively. We found no strong reason to think that these limitations would alter the overall conclusions. Lastly, the generalisability of our findings might be limited to high-income Western populations, and fatty acid patterns in other populations with diverse genetic backgrounds and dietary patterns are of future interest.
In conclusion, we identified a combination of plasma phospholipid fatty acids characterised by high relative concentrations of 18 : 2n-6, VLSFAs, and odd-chain SFAs and low relative concentrations of long-chain SFAs and MUFAs, some of which are synthesised endogenously. This particular profile was associated with a 3-fold lower relative risk of incident T2D in European populations after adjustment for confounding. While both genes and diet were linked to the FA-pattern score, association of the FA-pattern score with T2D was independent of established risk factors for T2D and not driven by individual fatty acids. These findings highlight that multiple fatty acids are jointly related to the development of T2D. The combination of fatty acids warrants further investigation of its determinants and potential application as a marker of metabolic characteristics.
Supporting Information
Zdroje
1. Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20(5):379–85. doi: 10.1097/MOL.0b013e32832fa5c4 19625959
2. Jump DB, Tripathy S, Depner CM. fatty acid–regulated transcription factors in the liver. Annu Rev Nutr. 2013;33(1):249–69. doi: 10.1146/annurev-nutr-071812-161139 23528177
3. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47(5):348–80. doi: 10.1016/j.plipres.2008.03.003 18435934
4. Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006;17(1):22–7. 16407712
5. Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23(7):351–63. doi: 10.1016/j.tem.2012.05.001 22704720
6. Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–67. doi: 10.1016/j.jacc.2011.06.063 22051327
7. Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell L V. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993;328(4):238–44. doi: 10.1056/NEJM199301283280404 8418404
8. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr Rev. 2008;29(3):351–66. doi: 10.1210/er.2007-0023 18048763
9. Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121(4):1402–11. doi: 10.1172/JCI44442 21403394
10. Vessby B, Lithell H. Interruption of long-term lipid-lowering treatment with bezafibrate in hypertriglyceridaemic patients. Effects on lipoprotein composition, lipase activities and the plasma lipid fatty acid spectrum. Atherosclerosis. 1990;82(1–2):137–43. doi: 10.1016/0021-9150(90)90152-9 2360915
11. King IB, Lemaitre RN, Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83(2):227–36. 16469979
12. Warensjö E, Sundström J, Lind L, Vessby B, Warensjo E, Sundstrom J. Factor analysis of fatty acids in serum lipids as a measure of dietary fat quality in relation to the metabolic syndrome in men. Am J Clin Nutr. 2006;84(2):442–8. 16895896
13. Anderson SG, Sanders TAB, Cruickshank JK. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension. 2009;53(5):839–45. doi: 10.1161/HYPERTENSIONAHA.108.123885 19307467
14. Dahm CC, Gorst-Rasmussen A, Jakobsen MU, Schmidt EB, Tjønneland A, Sørensen TIA, et al. Adipose tissue fatty acid patterns and changes in anthropometry: a cohort study. PLoS ONE. 2011;6(7):e22587. doi: 10.1371/journal.pone.0022587 21811635
15. Imamura F, Lemaitre RN, King IB, Song X, Lichtenstein AH, Matthan NR, et al. Novel circulating fatty acid patterns and risk of cardiovascular disease: the Cardiovascular Health Study. Am J Clin Nutr. 2012;96(6):1252–61. doi: 10.3945/ajcn.112.039990 23097270
16. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diab Endocrinol. 2014;2(10):810–8. doi: 10.1016/S2213-8587(14)70146-9 25107467
17. Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, et al. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med. 2016;13(7):e1002094. doi: 10.1371/journal.pmed.1002094 27434045
18. InterAct Consortium. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54(9):2272–82. doi: 10.1007/s00125-011-2182-9 21717116
19. Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11.
20. Wang L, Summerhill K, Rodriguez-Canas C, Mather I, Patel P, Eiden M, et al. Development and validation of a robust automated analysis of plasma phospholipid fatty acids for metabolic phenotyping of large epidemiological studies. Genome Med. 2013;5(4):39. doi: 10.1186/gm443 23618465
21. Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6(4):407–13. doi: 10.1079/PHN2002439 12795830
22. Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol. 1997;26(Suppl 1):S1–5.
23. Slimani N, Deharveng G, Unwin I, Southgate DAT, Vignat J, Skeie G, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. 2007;61(9):1037–56. doi: 10.1038/sj.ejcn.1602679 17375121
24. Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, Arriola L, et al. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes. 2014;63(12):4378–87. doi: 10.2337/db14-0319 24947364
25. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177 25673413
26. Ehrenberg ASC. Some questions about factor analysis. Statistician. 1962;12(3):191–208.
27. Vigneau E, Qannari EM. Clustering of variables around latent components. Commun Stat Simul Comput. 2003;32(4):1131–50. doi: 10.1081/SAC-120023882
28. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549 21310794
29. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. 2657958
30. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393 19564179
31. Efron B, Tibshirani RJ. Cross-validation and other estimates of prediction error. In: Efron B, Tibshirani RJ, editors. An introduction to the bootstrap. Boca Raton (Florida): Chapman & Hall/CRC, Taylor & Francix Group; 1994. pp. 237–57.
32. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188 15987728
33. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1–10. doi: 10.7326/0003-4819-137-1-200207020-00006 12093239
34. Maldonado G, Greenland S. simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–36. 8256780
35. Jakobsson A, Jörgensen JA, Jacobsson A. Differential regulation of fatty acid elongation enzymes in brown adipocytes implies a unique role for Elovl3 during increased fatty acid oxidation. Am J Physiol Endocrinol Metab. 2005;289(4):E517–26. doi: 10.1152/ajpendo.00045.2005 15855229
36. Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118(14):1467–75. doi: 10.1161/CIRCULATIONAHA.108.793182 18794388
37. Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103(11):1489–98. doi: 10.1172/JCI6223 10359558
38. Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15(5):623–34. doi: 10.1016/j.cmet.2012.03.007 22560215
39. Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96(4):727–34. doi: 10.3945/ajcn.112.038695 22952180
40. Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, et al. Association between low-density lipoprotein cholesterol–lowering genetic variants and risk of type 2 diabetes. JAMA. 2016;316(13):1383. doi: 10.1001/jama.2016.14568 27701660
41. Lemaitre RN, King IB, Rice K, McKnight B, Sotoodehnia N, Rea TD, et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fatty Acids. 2014;91(4):149–53. doi: 10.1016/j.plefa.2014.07.010 25107579
42. Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, et al. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18(7):503–10. doi: 10.1016/j.numecd.2007.04.005 18042359
43. Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62. doi: 10.1016/j.plipres.2011.11.001 22133871
44. Abdullah MMH, Cyr A, Lépine M-C, Labonté M-È, Couture P, Jones PJH, et al. Recommended dairy product intake modulates circulating fatty acid profile in healthy adults: a multi-centre cross-over study. Br J Nutr. 2015;113(3):435–44. doi: 10.1017/S0007114514003894 25609231
45. Weitkunat K, Schumann S, Nickel D, Hornemann S, Petzke KJ, Schulze MB, et al. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr. 2017;105(6):1544–51. doi: 10.3945/ajcn.117.152702 28424190
46. Jenkins BJ, Seyssel K, Chiu S, Pan P-H, Lin S-Y, Stanley E, et al. Odd chain fatty acids; new insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Sci Rep. 2017;7 : 44845. doi: 10.1038/srep44845 28332596
47. Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38(5):613–9. doi: 10.1016/j.ypmed.2003.11.027 15066364
48. Murase T, Misawa K, Minegishi Y, Aoki M, Ominami H, Suzuki Y, et al. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2011;300(1):E122–33. doi: 10.1152/ajpendo.00441.2010 20943752
49. Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37(2):569–86. doi: 10.2337/dc13-1203 24459154
50. Jacobs S, Kroger J, Floegel A, Boeing H, Drogan D, Pischon T, et al. Evaluation of various biomarkers as potential mediators of the association between coffee consumption and incident type 2 diabetes in the EPIC-Potsdam Study. Am J Clin Nutr. 2014;100(3):891–900. doi: 10.3945/ajcn.113.080317 25057154
51. Vinknes KJ, Elshorbagy AK, Nurk E, Drevon CA, Gjesdal CG, Tell GS, et al. Plasma stearoyl-CoA desaturase indices: association with lifestyle, diet, and body composition. Obesity. 2013;21(3):E294–302. doi: 10.1002/oby.20011 23404690
52. Imamura F, Micha R, Wu JHY, de Oliveira Otto MC, Otite FO, Abioye AI, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13(7):e1002087. doi: 10.1371/journal.pmed.1002087 27434027
53. Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr. 2008;87(3):627–37. 18326601
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Improving tuberculosis diagnosis: Better tests or better healthcare?
- Fitness cost and benefit of antimicrobial resistance in : Multidisciplinary approaches are needed
- When cost-effective interventions are unaffordable: Integrating cost-effectiveness and budget impact in priority setting for global health programs
- Regional initiatives for malaria elimination: Building and maintaining partnerships
- Firearm-Related Injury and Death: A U.S. Health Care Crisis in Need of Health Care Professionals
- Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case–control study
- Decommissioning care: The need for rigorous multifaceted evaluations of decisions to withdraw health services
- Tuberculosis detection and the challenges of integrated care in rural China: A cross-sectional standardized patient study
- Estimating the fitness cost and benefit of cefixime resistance in to inform prescription policy: A modelling study
- Elevated blood pressure and risk of mitral regurgitation: A longitudinal cohort study of 5.5 million United Kingdom adults
- A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study
- The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: A primary care cohort study
- Quantifying underreporting of law-enforcement-related deaths in United States vital statistics and news-media-based data sources: A capture–recapture analysis
- Impact of disinvestment from weekend allied health services across acute medical and surgical wards: 2 stepped-wedge cluster randomised controlled trials
- Benefit and harm of intensive blood pressure treatment: Derivation and validation of risk models using data from the SPRINT and ACCORD trials
- Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: A phase I randomised trial
- Intergenerational diabetes and obesity—A cycle to break?
- Associations between an IgG3 polymorphism in the binding domain for FcRn, transplacental transfer of malaria-specific IgG3, and protection against malaria during infancy: A birth cohort study in Benin
- Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis
- A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): A randomized, controlled non-inferiority trial
- Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: The MAL-ED longitudinal birth cohort study
- Effectiveness of cervical screening after age 60 years according to screening history: Nationwide cohort study in Sweden
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis
- Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case–control study
- A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study
- Quantifying underreporting of law-enforcement-related deaths in United States vital statistics and news-media-based data sources: A capture–recapture analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



