-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Sanitation and Hygiene-Specific Risk Factors for Moderate-to-Severe Diarrhea in Young Children in the Global Enteric Multicenter Study, 2007–2011: Case-Control Study
In a matched case-control study, Kelly Baker and colleagues explore the association between sanitation and hygiene exposures and moderate-to-severe diarrhea in young children in Africa and South Asia.
Published in the journal: . PLoS Med 13(5): e32767. doi:10.1371/journal.pmed.1002010
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002010Summary
In a matched case-control study, Kelly Baker and colleagues explore the association between sanitation and hygiene exposures and moderate-to-severe diarrhea in young children in Africa and South Asia.
Introduction
Enteric pathogens cause approximately 1.7 billion episodes of diarrhea per year in children <5 y old and account for ~10%–15% of all deaths in this age group [1–3]. Children in developing countries are exposed early and frequently to fecally contaminated food, water, human hands, soil, and fomites. Frequent exposure to enteric pathogens can result in a high incidence of acute diarrhea and asymptomatic gut infection in young children [4], which can lead to chronic, debilitating sequelae, including environmental enteropathy of the small intestine (previously called tropical enteropathy), malnutrition, and growth stunting [5–9]. Improvements in water, sanitation, and hygiene (WASH) can reduce exposure to enteric pathogens and thereby reduce pediatric diarrheal incidence [10,11]. Access to an improved sanitation facility, in particular, is estimated to reduce a child’s risk of diarrhea by 22% to 36% [12–15], with additional potential impacts on the incidence of asymptomatic infection, enteropathy, and growth stunting [4].
Millennium Development Goal (MDG) Target 7c calls on countries to halve, by 2015, the proportion of people who lack sustainable access to basic sanitation and safe water. To measure global progress, the WHO/United Nations Children's Emergency Fund (UNICEF)_ Joint Monitoring Program (JMP) has defined an improved sanitation facility as one that hygienically separates human excreta from human contact [16]. Acceptable types of facilities include a flush or pour-flush toilet/latrine to piped sewer system or septic tank, a pit latrine, a ventilated improved pit (VIP) latrine, a pit latrine with a slab, or a composting toilet. Facilities of an improved type that are shared by more than one household are considered unimproved because of concerns that they may be less hygienic and accessible than private household facilities. Currently only 68% of the world’s population, and 38% of people living in the least developed countries, meet criteria for access to an improved sanitation facility. An estimated 946 million people still practice open defecation, and 2.4 billion use unimproved facilities [16]. In 2015, an estimated 638 million people relied on shared sanitation facilities, 398 million in urban areas and 240 million in rural areas. This represents an increase of 361 million users since 1990—from 5% of the global population to 9% in 20 y—and is expected to continue growing [16].
Some have argued that post-2015 JMP guidelines should allow facilities shared by five or fewer households (or <30 persons) to be considered improved if they meet the other criteria for separating human excreta from human contact [17]. This is based on the premise that communal facilities shared by a few households are more likely to be co-owned and may be more accessible and hygienic compared with public facilities shared by strangers. However, individuals practicing open defecation or sharing sanitation facilities experience diarrheal disease, malnutrition, and diarrhea-related mortality more often than individuals using household facilities [18–22]. Sharing a facility was associated with an increase in soil-transmitted helminth infection among young men in Egypt [23], norovirus infection on a cruise ship [24], hospital admission for diarrhea among children 12 to 59 mo of age in Brazil [25], perinatal death in Nigerian [26,27] and Jamaican infants [28], cholera in Kenyan [29] and Zambian [30] refugee camps, and increased infant and maternal mortality death rates in 193 country-level surveys collected by WHO, UNICEF, and the World Bank [31]. Evidence on how number of households sharing a latrine affects these health risks, especially in young children, is limited to one analysis of Demographic Health Surveillance data from 51 countries. This study reported that the prevalence of self-reported diarrhea was slightly increased among those sharing a toilet with 1–5 households and >5 households—even one of improved design—compared to those with private access [32].
The Global Enteric Multicenter Study (GEMS) is a matched case-control study of moderate-to-severe diarrhea (MSD) in children <5 y old in four sites in sub-Saharan Africa and three sites in South Asia [33]. GEMS collected data on, among other things, wealth, household density, and WASH facilities and practices. The primary objective of this analysis is to describe sanitation and hygiene access across the study sites and to assess sanitation and hygiene exposures as risk factors for MSD in children <5 y old enrolled in GEMS, with a specific focus on shared sanitation. To our knowledge, this is the first study to use clinically and laboratory confirmed, rather than self-reported, diarrhea to analyze the risks associated with numbers of households sharing a sanitation facility.
Methods
Ethical Considerations
Written informed consent was obtained from caretakers of enrolled children. The scientific and ethical review committees of each participating organization, including in-country ethics approval, and the Institutional Review Board of the University of Maryland, Baltimore, approved the protocol and consent forms (S1 Table).
Setting
The seven GEMS sites included Basse Sante Su, The Gambia; Nyanza Province, Kenya; Bamako, Mali; Manhiça, Mozambique; Mirzapur, Bangladesh; Kolkata, India; and Karachi (Bin Qasim Town), Pakistan [33]. Two sites are located in urban centers (Mali and India), and four sites are in rural settings (The Gambia, Mozambique, Kenya, and Bangladesh), whereas the study villages in Pakistan, located on the coast approximately 20 km outside Karachi, are considered periurban. Each GEMS site was linked to a defined population under a demographic surveillance system (DSS) that visited every household 2–3 times per year to record births, deaths, and migrations.
Study Design and Health Outcome
The GEMS is a matched case-control study in which cases were children <5 y old seeking care for MSD at one of the sentinel health centers serving the DSS at each site (S2 Table). MSD was defined as passing three or more loose stools within 24 h, in conjunction with clinical signs of moderate-to-severe dehydration (sunken eyes, loss of skin turgor, or administration of IV fluids), dysentery, or admission to a health facility. Stool specimens were collected from all children at enrollment. Control children without diarrhea were randomly selected from the DSS population within 14 d of presentation of the case and matched to the case by age, sex, and neighborhood. Detailed GEMS clinical and epidemiologic methods have been published [33,34].
Data Collection
Case and control enrollment into GEMS took place over 36 mo from December 1, 2007, to March 3, 2011. Demographic information collected about the case or control and his/her household (defined as a group of people who share a cooking fire) included maternal education and household size (including the number of children <5 y old). Building materials and household possessions were documented as potential indicators for constructing a wealth index for each site [33]. WASH data were collected at enrollment from the caretakers of case children presenting at health facilities and at home for matched control children by means of a standardized questionnaire. Approximately 60 (range: 50–90) days after enrollment, a trained field worker visited the household of each case and control to collect follow-up health information and record WASH observations. Information on water sources, facilities to dispose of human fecal waste, and handwashing and other hygiene practices was collected at enrollment, with additional information (including direct observation of hygiene practices, latrines, and toilet facilities) recorded at the 60-d follow-up home visit.
Sociodemographic Variables
Five sociodemographic variables were considered in this analysis as potential confounders (Table 1). A wealth index quintile (WIQ) variable was generated by principal component analysis of 13 household assets. This method has been described elsewhere [33,35]. Access to an improved water source was defined as the main source of drinking water for the household at follow-up as a public or private piped water tap, tube well, borehole, protected dug well, protected spring, or rainwater that was available every day, with a round trip time of 30 min or less to fetch water [16].
Tab. 1. Description of sociodemographic, sanitation, and hygiene variables included in this analysis. 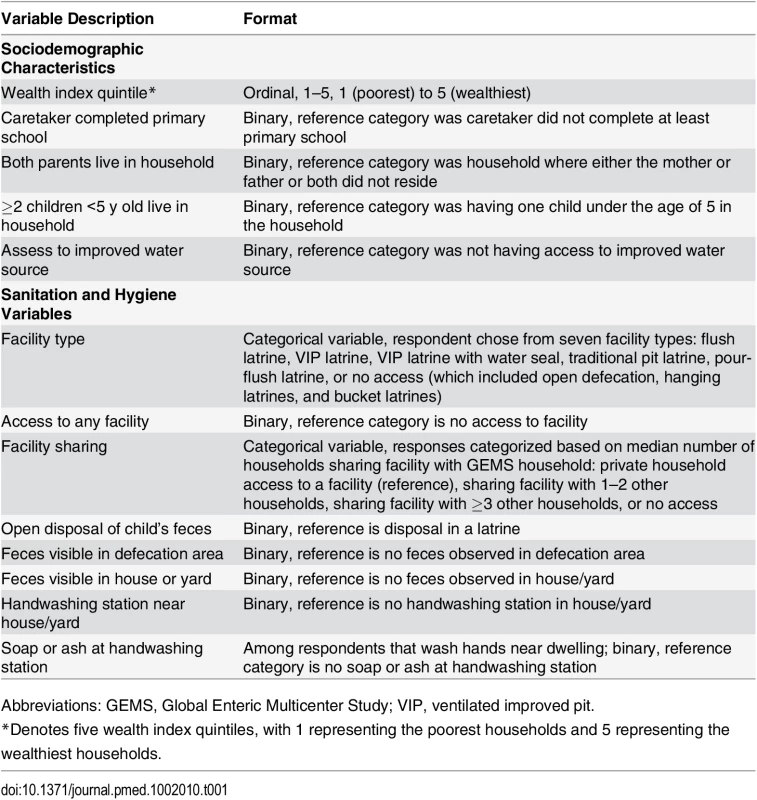
Abbreviations: GEMS, Global Enteric Multicenter Study; VIP, ventilated improved pit. Sanitation and Hygiene Variables
Eight sanitation and hygiene variables were explored in this paper (Table 1), including three self-reported or observed sanitation variables, two directly observed fecal contamination variables, and two directly observed handwashing variables. Sanitation variables included facility type, facility access and sharing, and disposal of child’s feces as reported by respondent. Because of the skewed distribution of the number of households sharing facilities and for comparison across sites, the highest category for numbers of households sharing a sanitation facility was categorized based on the overall median of 3.
Statistical Analysis
Data analysis was limited to subjects for whom complete data were available on sanitation access at enrollment and follow-up. Data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC). Descriptive statistics for sociodemographic and exposure variables were reported as proportions, medians, and ranges. We aimed to describe site-to-site variability in effects; therefore, we present all results stratified by site. The modeling strategy involved estimating unadjusted effects of association between sanitation and hygiene exposures and MSD and assessing two-way interactions between exposures and age, followed by selecting and including consistent sociodemographic confounders across all sites for reporting adjusted estimates of sanitation and hygiene exposures. Site-specific univariable conditional logistic regression models were used to evaluate the relationship between sanitation exposure variables and MSD. Unadjusted matched odds ratios (mORs) and 95% confidence intervals (CIs) are reported. Since risk factors are likely to be different for infants, we assessed two-way interactions between risk factors and age. There were no significant interactions with age; thus, only main effects are reported, and all models still account for the age-, sex-, and geography-matched case-control design. For many of the primary sanitation variables of interest, there were low exposure frequencies, which limited the number of variables that could be included in multivariable conditional logistic models. Therefore, we ran separate multivariable conditional logistic models for each sanitation and hygiene exposure of interest. We considered the following variables as potential confounders: WIQs, caretaker education, parental residence in the household, other young children in the household, and access to an improved water source. We assessed for confounding one at a time in each of the models by identifying significant associations with MSD and effect size changes in our estimates of sanitation and hygiene variables. Based on previous research, we considered a priori that wealth was an important epidemiological factor associated with MSD and should be included in the multivariable models to produce adjusted estimates of sanitation and hygiene exposures [22]. We aimed to present consistent results across sites, so we adjusted for these same parameters in all site-specific multivariable conditional logistic regression models. Multivariable models were assessed for collinearity using condition index diagnostics.
Results
Sociodemographic Characteristics of GEMS Case and Control Households by Site
Between December 2007 and January 2011, 9,439 cases and 13,129 matched controls were enrolled at the seven GEMS sites [36]. Among these, follow-up observations of WASH in the home were available for 8,592 (91.2%) cases and 12,390 (94.4%) controls. The number of households lost to follow-up was highest in Pakistan (15.3%), moderate in Gambia (9.2%), Mali (10.3%), and Mozambique (9.2%), and low in Kenya (2.9%), Bangladesh (1.5%), and India (3.1%). Wealth index was significantly associated with MSD status for three of the seven sites (Mali, India, and Pakistan). Cases in India were more likely to have caretakers with higher education. In all sites, except for India, cases were less likely to have both parents living in the home (Table 2).
Tab. 2. Sociodemographic characteristics of GEMS case and control caretakers and their children in African and Asian sites, 2007–2011. 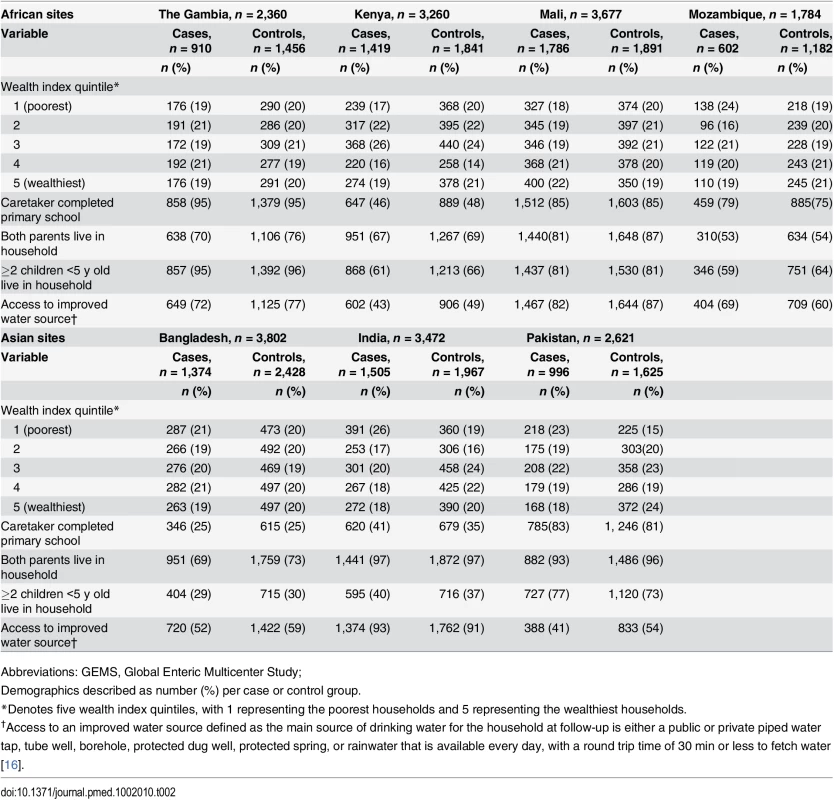
Abbreviations: GEMS, Global Enteric Multicenter Study; Access to Sanitation Facilities
The majority of households (>93%) in Gambia, Mali, Mozambique, Bangladesh, India, and Pakistan reported access to a sanitation facility (Table 3). Kenya had the highest proportion of households without access to any facility (30% case and 29% control households). Traditional pit latrines were the most frequently reported facility type for African sites, while pour-flush toilets were more common among Asian sites. While facility types varied across sites, no specific facility type was significantly associated with MSD in any of the sites. Thus, subsequent analysis focuses on access to any type of facility and is categorized based on the number of households sharing a facility with the GEMS family.
Tab. 3. Sanitation facility characteristics in the households of case and control children enrolled in GEMS by site, 2007–2011. 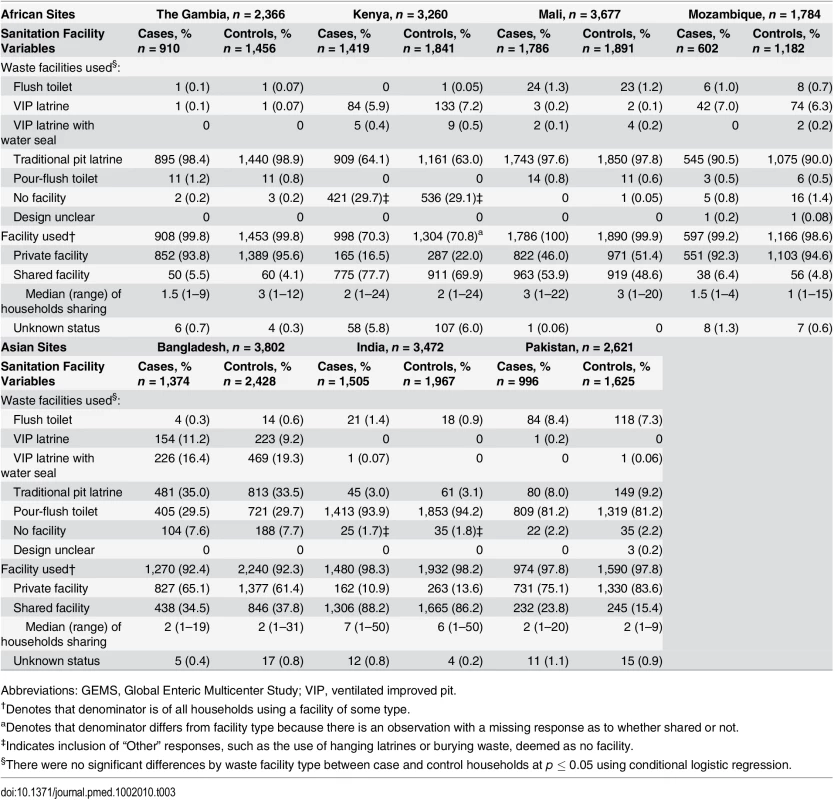
Abbreviations: GEMS, Global Enteric Multicenter Study; VIP, ventilated improved pit. The proportion of households that shared a facility with other households differed between sites. Overall, the proportion of households that shared a facility among those with access to a facility was lowest in The Gambia (6% case and 4% control households) and Mozambique (6% case and 5% control households), which are both rural sites. The highest proportions of households reporting the use of shared facilities were in the urban Indian site (87% case and 85% control households) and the rural Kenyan site (55% case and 49% control households), followed by the urban Mali site (54% case and 49% control households). Among households sharing a sanitation facility, the median number of households sharing a facility with the GEMS family was three or less for all sites, except India, where the median number of additional households sharing a facility was seven (range 1–50) for case households and six (range 1–50) for control households (Table 3).
Associations between Sanitation and Hygiene and MSD in Young Children
None of the potential confounders assessed impacted the effect size estimates for the sanitation and hygiene variables. Two sociodemographic variables, WIQ and whether both parents resided in the home, were significantly associated with MSD in at least three of the seven sites and were included in all the adjusted models. Compared to households with private sanitation, those sharing a sanitation facility with 1–2 households demonstrated increased odds of MSD in Kenya (adjusted mOR = 1.41; 95% CI: 1.11–1.79), Mali (adjusted mOR = 1.23; 95% CI: 1.02–1.48), Bangladesh (adjusted mOR = 0.83; 95% CI: 0.70–0.99), and Pakistan (adjusted mOR = 1.58; 95% CI: 1.19–2.09) sites (Table 4). In nearly all sites, with the exception of Bangladesh, effect sizes for sharing with ≥3 households were larger than estimates of sharing with only 1–2 households. Sharing with ≥3 households was statistically significant in Kenya (adjusted mOR = 1.63; 95% CI: 1.26–2.12), Mali (adjusted mOR = 1.68; 95% CI: 1.40–2.03), Mozambique (adjusted mOR = 8.83; 95% CI: 1.01–77.17) and Pakistan (adjusted mOR = 1.82; 95% CI: 1.25–2.66). Having no access to a sanitation facility was most frequently reported in rural Kenya and was a statistically significant risk factor for MSD in children <5 y old (adjusted mOR = 1.48; 95% CI: 1.15–1.90) (Table 4).
Tab. 4. Sanitation and hygiene-specific risk factors for MSD in young children in GEMS, 2007–2011. 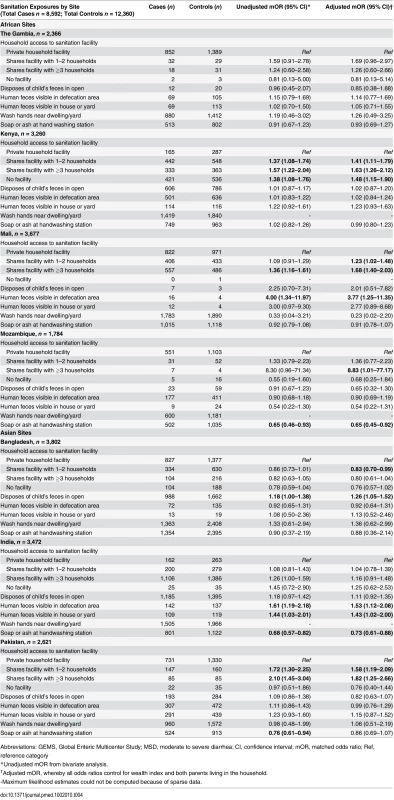
Abbreviations: GEMS, Global Enteric Multicenter Study; MSD, moderate to severe diarrhea; CI, confidence interval; mOR, matched odds ratio; Ref, reference category Disposal of children’s feces in open areas around the home was not associated with increased risk of MSD among children <5 y old, except in Bangladesh (adjusted mOR = 1.26; 95% CI: 1.05–1.52) (Table 4). Having a defecation site with observed fecal material visible was statistically associated with MSD among children <5 y old in Mali (adjusted mOR = 3.77; 95% CI: 1.25–11.35) and India (adjusted mOR = 1.53; 95% CI: 1.12–2.08). Having human feces visible in the house/yard was a statistically significant risk factor in India (adjusted mOR = 1.43; 95% CI: 1.02–2.00).
Among those who reported that they washed their hands in or near the dwelling/yard, keeping soap or ash at the handwashing area demonstrated a protective effect against MSD in children <5 y old in Mozambique (adjusted mOR = 0.65; 95% CI: 0.45–0.92) and India (adjusted mOR = 0.73; 95% CI: 0.61–0.88) (Table 4).
Discussion
In monitoring global progress in access to improved sanitation, the WHO/UNICEF JMP classifies shared sanitation facilities as “unimproved sanitation,” based upon the premise that hygiene conditions in these types of facilities may fail to hygienically separate human excreta from human contact [16,17]. This is a controversial topic, as communal facilities are the most economical and feasible solution for providing sanitation access to the 2.5 billion people without a private facility [16,37]. They are often used in densely populated urban areas, where space is at a premium. Furthermore, shared sanitation may still represent an improvement in hygiene conditions relative to open defecation [16,17]. However, for policy makers weighing options for investing in sanitation improvement, it is equally important to understand whether shared facilities effectively achieve global health targets compared to investment in private facilities.
The limited evidence on whether shared facilities protect users from exposure to human excreta is conflicting. Shared latrines in Tanzania contained lower concentrations of Escherichia coli, higher concentrations of flies, and equal concentrations of helminth ova compared to private latrines [38]. Other studies found that facilities shared by even a few households are dirtier than private facilities [39–41]. A recent meta-analysis found that shared sanitation was associated with an increased risk of adverse health outcomes, including diarrhea [18]. However, shared sanitation includes many different arrangements, from just a few closely related households to publicly run facilities that serve entire neighborhoods. Some of these facilities may be better maintained than others. In acknowledgement that risks may differ among shared facilities, the JMP recently deliberated whether facilities shared by ≤5 households or ≤30 users are likely to provide hygiene conditions that could be considered “improved.” Only one other study has explored this question, and they found a slightly elevated risk of self-reported diarrhea for children in households sharing a latrine with few and with many households [32]. Otherwise, little is known about how the number of households sharing a facility influences disease risks.
Epidemiological evidence from the GEMS case-control study offers new insights into the relationship between shared sanitation access, sanitation conditions, and pediatric diarrheal disease in low-income countries. We provide compelling evidence from four of seven GEMS sites that children <5 y old living in households sharing a latrine with ≥3 households, as well as those sharing with just 1–2 households, were more likely to experience MSD than children living in households with private sanitation. While shared sanitation did not reach statistical significance as a risk factor in all sites, this was likely due to our limited statistical power. Despite this limitation, these data demonstrated consistent effects of shared sanitation across most sites as a risk factor with increased odds of MSD at higher levels of sharing. This consistency was not observed for other sanitation and hygiene variables. However, our data in Bangladesh also suggest that there are some situations in which shared sanitation does not pose higher risks than private access and that other sanitation and hygiene factors are more important in diarrheal disease transmission. Global health policy must be based upon known risks to all members of a population, ensuring equity in health for all and doing no harm [42]. While shared sanitation may not be a risk factor in some communities, our study demonstrates that many children around the world do face risks from sharing sanitation facilities, reinforcing the findings of Heijnen et al. and Fuller et al. [32,43]. Without a clearer understanding of the factors that define safe sanitation, classifying shared facilities as “improved” at this point would potentially increase the number of children at risk for sanitation-related disease transmission. We conclude that compared to private sanitation facilities, sharing facilities with 1–2 other households does not prevent children from potential exposure to human excreta, and therefore, all shared facilities should continue to be classified as “unimproved” for monitoring global sanitation access.
More research is needed to understand the risks shared facilities pose and to identify cost-effective strategies to remediate those risks. The relationships between sanitation infrastructure, human behavior, and enteric disease risk in young children are complex and poorly understood. Children could be directly infected by fecal exposure at a sanitation facility—observed fecal material was significantly associated with MSD among children in the densely populated communities in Mali and India. Yet, in some studies direct exposure at a facility was uncommon since very young children did not typically use sanitation facilities [44,45]. Infection risks for children from shared latrines more likely reflect possible exposure pathways outside of the latrine that occur as a consequence of relying on shared facilities. Poor cleanliness of a facility and sharing a facility can increase user dissatisfaction and decrease latrine use [39,40,46–48]. Households relying on shared facilities may be more likely to practice open defecation or open child feces disposal [46]. Open child feces disposal was reported frequently by GEMS caretakers, especially among those who reported shared sanitation. If open defecation or open child feces disposal is amplified in communities relying on shared sanitation, then children may face high risks of exposure to diarrheal pathogens from public domain transmission pathways, such as playing in soil or surface water outside the household [49].
GEMS findings suggest access to private household latrines can provide protective benefits against MSD, even in communities like rural western Kenya, where open defecation and open child feces disposal was common. It is unlikely that private latrine access influences whether children play outside the home or not, so this protective effect may reflect protective benefits of private household latrine access on private (domestic) exposure pathways such as contaminated drinking water, food, household play areas, or hands [49,50]. Handwashing with soap or ash, especially after toileting, is a simple and important means for protecting oneself and others from exposure to fecal matter. In two GEMS sites, the presence of soap in the handwashing area was protective against MSD. Caretakers using shared facilities may be less likely to practice hygienic behaviors, like handwashing after defecation, because of challenges in access, which could increase infection risks for their children [43]. The reverse explanation for this relationship could also be true: households that prioritize safe hygiene practices are more likely to invest in private sanitation facilities than those that do not prioritize hygiene. While GEMS data provide information about the risks of shared sanitation, the evidence remains insufficient to precisely identify fecal transmission pathways associated with shared sanitation access or to distinguish between safe and unsafe shared facilities.
Our analysis has several limitations. Most GEMS households had access to a basic sanitation facility, which limited our ability to compare the risks of shared access to open defecation. While the aim of this paper was to describe sanitation risk factors associated with MSD consistently across sites, it did not address the substantial intersite differences in how facilities were shared. For example, in India most facilities were shared, and the median number of households sharing facilities was much greater than at any other site, with greater variation amongst the households included. The classification of shared facilities did not address variations in household composition and size that are potentially important factors contributing to facility conditions [37]. GEMS did not collect data on how shared sanitation facilities were managed and maintained and whether users themselves or service providers were responsible for maintaining hygienic conditions. We did not document important determinants of facility use, such as the distance between the sanitation facility and the household or handwashing area. Additionally, we did not document how facility septage was contained and managed. Septage from private and communal facilities frequently drains (or is collected by service providers and is dumped) into nearby areas lacking safe containment capabilities, thereby increasing neighborhood-level exposure risks. Each of these factors may be important sanitation-related confounders of the effect of shared sanitation on MSD.
This study also had methodological limitations. Modeling strategies were limited by relatively low frequencies of some of the exposure categories of interest. However, effect sizes remained similar across models, suggesting that our matched study design controlled for the most important sources of bias. We selected two sociodemographic indicators, a wealth quintile index and having two parents in the home. Although wealth indices are widely used in WASH research, it may not be a robust way of adjusting for sociodemographic confounding. While we did not detect any statistically significant interactions with age, we had limited power to detect them with the low exposure frequencies in these study populations. It is possible that differences by age, or age-related etiological differences, exist, but our data were underpowered to detect them. Finally, the observational study design allows us to describe associations but does not allow us to infer causality.
Data from GEMS have highlighted the association between shared and unhygienic sanitation facilities and MSD in children and suggest multiple pathways for enteric disease transmission in households with shared sanitation. Each pathway suggests potential interventions to reduce the risks associated with shared sanitation. A previous GEMS paper found shared sanitation access to be an important contributor to MSD, after adjusting for household drinking water practices, suggesting that combination approaches are necessary [51]. While our data suggest household latrines are the safest option, well-maintained, hygienic shared facilities that consistently ensure safety and privacy and provide access to soap and water for handwashing may motivate facility usage and reduce open defecation. In one urban setting in Brazil, the provision of community-level sanitation services effectively reduced intestinal infections in young children [52]. Complementary social messaging about proper disposal of children’s feces and the importance of handwashing with soap after defecation could also help mitigate risk by reducing exposures outside of the facility. Understanding how shared sanitation affects children’s public and domestic exposure risks could improve the design of interventions, as different strategies are necessary for public versus domestic transmission pathways [49].
The acceptability of shared facilities should be carefully explored in each setting to assess the likelihood of latrine uptake, with the cost–benefit carefully weighed. The benefits for provision of communal facilities may be low, especially if they fail to provide equitable access to safe sanitation across economic and social classes. The poor, women, and children are more likely to depend upon shared facilities or open defecation and are less likely to benefit from latrine promotion programs [32,47,53–55]. The documented lack of equity in sanitation access is consistent with diarrheal disease burden, also inequitably borne by the poorest classes [54]. Sanitation facilities that ensure safe, private, and affordable sanitation access for women—the primary caretakers of children—and a space with water for personal and infant bathing could improve child feces disposal. Understanding how to cost-effectively and equitably deliver safe sanitation worldwide is particularly relevant for policy makers and public health professionals tasked with decreasing diarrhea-related morbidity and mortality in children in developing countries. More research is needed to understand the sanitation-related behaviors, exposures, and conditions that influence disease transmission in developing countries [56]. Given that the risk of disease transmission does in some circumstances increase with the number of households sharing a facility, ensuring an adequate number of functioning and hygienic facilities with ample access to soap and water is particularly critical.
Supporting Information
Zdroje
1. Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–16. Epub 2013/04/16. doi: 10.1016/S0140-6736(13)60222-6 S0140-6736(13)60222-6 [pii]. 23582727.
2. Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. Epub 2010/05/15. S0140-6736(10)60549-1 [pii] doi: 10.1016/S0140-6736(10)60549-1 20466419.
3. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. Epub 2012/05/15. doi: 10.1016/S0140-6736(12)60560-1 S0140-6736(12)60560-1 [pii]. 22579125.
4. Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374(9694):1032–5. Epub 2009/09/22. S0140-6736(09)60950-8 [pii] doi: 10.1016/S0140-6736(09)60950-8 19766883.
5. Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37(4):816–30. Epub 2008/06/24. dyn099 [pii] doi: 10.1093/ije/dyn099 18567626; PubMed Central PMCID: PMC2734063.
6. Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–40. Epub 2008/01/22. S0140-6736(07)61693-6 [pii] doi: 10.1016/S0140-6736(07)61693-6 18206226.
7. Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133(5):1332–8. Epub 2003/05/06. 12730419.
8. Fagundes Neto U, Martins MC, Lima FL, Patricio FR, Toledo MR. Asymptomatic environmental enteropathy among slum-dwelling infants. J Am Coll Nutr. 1994;13(1):51–6. Epub 1994/02/01. 8157854.
9. Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338(8772):907–10. Epub 1991/10/12. 0140-6736(91)91772-M [pii]. 1681266.
10. Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ. 2007;334(7597):782. Epub 2007/03/14. bmj.39118.489931.BE [pii] doi: 10.1136/bmj.39118.489931.BE 17353208; PubMed Central PMCID: PMC1851994.
11. Esrey SA, Feachem RG, Hughes JM. Interventions for the control of diarrhoeal diseases among young children: improving water supplies and excreta disposal facilities. Bulletin of the World Health Organization. 1985;63(4):757–72. Epub 1985/01/01. 3878742; PubMed Central PMCID: PMC2536385.
12. Daniels DL, Cousens SN, Makoae LN, Feachem RG. A case-control study of the impact of improved sanitation on diarrhoea morbidity in Lesotho. Bulletin of the World Health Organization. 1990;68(4):455–63. Epub 1990/01/01. 2208559; PubMed Central PMCID: PMC2393155.
13. Fewtrell L, Colford JM Jr. Water, sanitation and hygiene in developing countries: interventions and diarrhoea—a review. Water science and technology: a journal of the International Association on Water Pollution Research. 2005;52(8):133–42. Epub 2005/11/30. 16312960.
14. Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, Fung IC, et al. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol. 2010;39 Suppl 1:i193–205. Epub 2010/04/02. dyq035 [pii] doi: 10.1093/ije/dyq035 20348121; PubMed Central PMCID: PMC2845874.
15. Esrey SA, Habicht JP. Epidemiologic evidence for health benefits from improved water and sanitation in developing countries. Epidemiol Rev. 1986;8 : 117–28. Epub 1986/01/01. 3533581.
16. UNICEF, WHO. Progress on sanitation and drinking water—2015 update and MDG assessment 2015. http://www.wssinfo.org/fileadmin/user_upload/resources/JMP-Update-report-2015_English.pdf.
17. JMP Technical Task Force Meeting on Sanitation and Methods for Estimating Progress New York, NY, USA: UNICEF; July 2010. http://www.wssinfo.org/fileadmin/user_upload/resources/JMP-Sanitation-Method-Task-Force-Meeting-Report-July-2010-final.pdf.
18. Heijnen M, Cumming O, Peletz R, Chan GK, Brown J, Baker K, et al. Shared Sanitation versus Individual Household Latrines: A Systematic Review of Health Outcomes. PLoS ONE. 2014;9(4):e93300. Epub 2014/04/20. doi: 10.1371/journal.pone.0093300 PONE-D-13-45269 [pii]. 24743336; PubMed Central PMCID: PMC3990518.
19. Lima MC, Motta ME, Santos EC, Pontes da Silva GA. Determinants of impaired growth among hospitalized children: a case-control study. Sao Paulo Med J. 2004;122(3):117–23. Epub 2004/09/28. /S1516-31802004000300008. S1516-31802004000300008 [pii]. 15448810.
20. Clasen TF, Bostoen K, Schmidt WP, Boisson S, Fung IC, Jenkins MW, et al. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev. 2010;(6):CD007180. Epub 2010/06/18. doi: 10.1002/14651858.CD007180.pub2 20556776.
21. Fuentes R, Pfütze T, Seck P. A Logistic Analysis of Diarrhea Incidence and Access to Water and Sanitation 2006. http://hdr.undp.org/en/media/accesswatersanitationanddiarrhea.pdf.
22. Spears D. Effects of Rural Sanitation on Infant Mortality and Human Capital: Evidence from India's Total Sanitation Campaign 2012. http://www.dartmouth.edu/~neudc2012/docs/paper_86.pdf.
23. Curtale F, Shamy MY, Zaki A, Abdel-Fattah M, Rocchi G. Different patterns of intestinal helminth infection among young workers in urban and rural areas of Alexandria Governorate, Egypt. Parassitologia. 1998;40(3):251–4. Epub 1999/06/22. 10376279.
24. Ho MS, Glass RI, Monroe SS, Madore HP, Stine S, Pinsky PF, et al. Viral gastroenteritis aboard a cruise ship. Lancet. 1989;2(8669):961–5. Epub 1989/10/21. 2571872.
25. Sobel J, Gomes TA, Ramos RT, Hoekstra M, Rodrigue D, Rassi V, et al. Pathogen-specific risk factors and protective factors for acute diarrheal illness in children aged 12–59 months in Sao Paulo, Brazil. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;38(11):1545–51. Epub 2004/05/25. doi: 10.1086/420822 CID32736 [pii]. 15156440.
26. Ezeh OK, Agho KE, Dibley MJ, Hall J, Page AN. The impact of water and sanitation on childhood mortality in Nigeria: evidence from demographic and health surveys, 2003–2013. Int J Environ Res Public Health. 2014;11(9):9256–72. doi: 10.3390/ijerph110909256 25198687; PubMed Central PMCID: PMCPMC4199018.
27. Ezeh OK, Agho KE, Dibley MJ, Hall J, Page AN. Determinants of neonatal mortality in Nigeria: evidence from the 2008 demographic and health survey. BMC Public Health. 2014;14 : 521. doi: 10.1186/1471-2458-14-521 24886517; PubMed Central PMCID: PMCPMC4049428.
28. Golding J, Greenwood R, McCaw-Binns A, Thomas P. Associations between social and environmental factors and perinatal mortality in Jamaica. Paediatr Perinat Epidemiol. 1994;8 Suppl 1 : 17–39. Epub 1994/04/01. 8072899.
29. Shultz A, Omollo JO, Burke H, Qassim M, Ochieng JB, Weinberg M, et al. Cholera outbreak in Kenyan refugee camp: risk factors for illness and importance of sanitation. The American journal of tropical medicine and hygiene. 2009;80(4):640–5. Epub 2009/04/07. 80/4/640 [pii]. 19346392.
30. Sasaki S, Suzuki H, Igarashi K, Tambatamba B, Mulenga P. Spatial analysis of risk factor of cholera outbreak for 2003–2004 in a peri-urban area of Lusaka, Zambia. The American journal of tropical medicine and hygiene. 2008;79(3):414–21. Epub 2008/09/12. 79/3/414 [pii]. 18784235.
31. Cheng JJ, Schuster-Wallace CJ, Watt S, Newbold BK, Mente A. An ecological quantification of the relationships between water, sanitation and infant, child, and maternal mortality. Environ Health. 2012;11 : 4. doi: 10.1186/1476-069X-11-4 22280473; PubMed Central PMCID: PMCPMC3293047.
32. Fuller JA, Clasen T, Heijnen M, Eisenberg JN. Shared Sanitation and the Prevalence of Diarrhea in Young Children: Evidence from 51 Countries, 2001–2011. The American journal of tropical medicine and hygiene. 2014. Epub 2014/05/29. 13–0503 [pii] ajtmh.13-0503 [pii] doi: 10.4269/ajtmh.13-0503 24865679.
33. Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55 Suppl 4:S232–45. Epub 2012/11/28. doi: 10.1093/cid/cis753 cis753 [pii]. 23169936; PubMed Central PMCID: PMC3502307.
34. Farag TH, Nasrin D, Wu Y, Muhsen K, Blackwelder WC, Sommerfelt H, et al. Some epidemiologic, clinical, microbiologic, and organizational assumptions that influenced the design and performance of the Global Enteric Multicenter Study (GEMS). Clin Infect Dis. 2012;55 Suppl 4:S225–31. Epub 2012/11/28. doi: 10.1093/cid/cis787 cis787 [pii]. 23169935; PubMed Central PMCID: PMC3502315.
35. McKenzie DJ. Measuring Inequality with Asset indicators. Journal of Population Economics. 2005;18(2):229–60.
36. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013. Epub 2013/05/18. S0140-6736(13)60844-2 [pii]. doi: 10.1016/S0140-6736(13)60844-2 23680352.
37. Rheinlander T, Konradsen F, Keraita B, Apoya P, Gyapong M. Redefining shared sanitation. Bulletin of the World Health Organization. 2015;93(7):509–10. doi: 10.2471/BLT.14.144980 26170511; PubMed Central PMCID: PMCPMC4490809.
38. Exley JL, Liseka B, Cumming O, Ensink JH. The sanitation ladder, what constitutes an improved form of sanitation? Environmental science & technology. 2014. Epub 2014/12/17. doi: 10.1021/es503945x 25513885.
39. Nelson KB, Karver J, Kullman C, Graham JP. User perceptions of shared sanitation among rural households in Indonesia and Bangladesh. PLoS ONE. 2014;9(8):e103886. doi: 10.1371/journal.pone.0103886 25090096; PubMed Central PMCID: PMCPMC4121202.
40. Kwiringira J, Atekyereza P, Niwagaba C, Gunther I. Descending the sanitation ladder in urban Uganda: evidence from Kampala Slums. BMC Public Health. 2014;14 : 624. Epub 2014/06/21. doi: 10.1186/1471-2458-14-624 1471-2458-14-624 [pii]. 24948084; PubMed Central PMCID: PMC4071028.
41. Kwiringira J, Atekyereza P, Niwagaba C, Gunther I. Gender variations in access, choice to use and cleaning of shared latrines; experiences from Kampala Slums, Uganda. BMC Public Health. 2014;14(1):1180. Epub 2014/11/20. doi: 10.1186/1471-2458-14-1180 1471-2458-14-1180 [pii]. 25407788; PubMed Central PMCID: PMC4247598.
42. Bioethics GNoWCCf. Global Health Ethics Key Issues. World Health Organization, 2015.
43. Heijnen M, Routray P, Torondel B, Clasen T. Shared Sanitation Versus Individual Household Latrines in Urban Slums: A Cross-Sectional Study in Orissa, India. The American journal of tropical medicine and hygiene. 2015;93(2):263–8. doi: 10.4269/ajtmh.14-0812 26123953; PubMed Central PMCID: PMC4530745.
44. Curtis V, Kanki B, Mertens T, Traore E, Diallo I, Tall F, et al. Potties, pits and pipes: explaining hygiene behaviour in Burkina Faso. Soc Sci Med. 1995;41(3):383–93. Epub 1995/08/01. 027795369400341P [pii]. 7481932.
45. Miller-Petrie MK, Voigt L, McLennan L, Cairncross S, Jenkins MW. Infant and Young Child Feces Management and Enabling Products for Their Hygienic Collection, Transport, and Disposal in Cambodia. Am J Trop Med Hyg. 2015. doi: 10.4269/ajtmh.15-0423 26598568.
46. Biran A, Jenkins MW, Dabrase P, Bhagwat I. Patterns and determinants of communal latrine usage in urban poverty pockets in Bhopal, India. Tropical medicine & international health: TM & IH. 2011;16(7):854–62. Epub 2011/03/19. doi: 10.1111/j.1365-3156.2011.02764.x 21414114.
47. Kwiringira J, Atekyereza P, Niwagaba C, Gunther I. Gender variations in access, choice to use and cleaning of shared latrines; experiences from Kampala Slums, Uganda. BMC Public Health. 2014;14 : 1180. Epub 2014/11/20. doi: 10.1186/1471-2458-14-1180 1471-2458-14-1180 [pii]. 25407788; PubMed Central PMCID: PMC4247598.
48. Tumwebaze IK, Orach CG, Niwagaba C, Luthi C, Mosler HJ. Sanitation facilities in Kampala slums, Uganda: users' satisfaction and determinant factors. International journal of environmental health research. 2013;23(3):191–204. Epub 2012/08/10. doi: 10.1080/09603123.2012.713095 22873693.
49. Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Tropical medicine & international health: TM & IH. 1996;1(1):27–34. 8673819.
50. Azage M, Haile D. Factors associated with safe child feces disposal practices in Ethiopia: evidence from demographic and health survey. Arch Public Health. 2015;73 : 40. doi: 10.1186/s13690-015-0090-z 26504520; PubMed Central PMCID: PMCPMC4620604.
51. Baker KK, Sow SO, Kotloff KL, Nataro JP, Farag TH, Tamboura B, et al. Quality of piped and stored water in households with children under five years of age enrolled in the Mali site of the Global Enteric Multi-Center Study (GEMS). American Journal of Tropical Medicine and Hygiene. 2013;89(2):214–22. doi: 10.4269/ajtmh.12-0256 23836570
52. Barreto ML, Genser B, Strina A, Teixeira MG, Assis AM, Rego RF, et al. Impact of a citywide sanitation program in Northeast Brazil on intestinal parasites infection in young children. Environ Health Perspect. 2010;118(11):1637–42. Epub 2010/08/14. doi: 10.1289/ehp.1002058 20705544; PubMed Central PMCID: PMC2974706.
53. Rodgers AF, Ajono LA, Gyapong JO, Hagan M, Emerson PM. Characteristics of latrine promotion participants and non-participants; inspection of latrines; and perceptions of household latrines in Northern Ghana. Trop Med Int Health. 2007;12(6):772–82. Epub 2007/06/07. TMI1848 [pii] doi: 10.1111/j.1365-3156.2007.01848.x 17550475.
54. Rheingans R CO, Anderson J, Showalter J. Estimating inequities in sanitation-related disease burden and estimating the potential impacts of pro-poor targeting London: London School of Hygiene and Tropical Medicine; 2012. http://r4d.dfid.gov.uk/PDF/Outputs/sanitation/EquityResearchReport.pdf.
55. Caruso BA, Sevilimedu V, Fung IC, Patkar A, Baker KK. Gender disparities in water, sanitation, and global health. Lancet. 2015;386(9994):650–1. doi: 10.1016/S0140-6736(15)61497-0 26334153.
56. Eisenberg JN, Trostle J, Sorensen RJ, Shields KF. Toward a systems approach to enteric pathogen transmission: from individual independence to community interdependence. Annu Rev Public Health. 2012;33 : 239–57. Epub 2012/01/10. doi: 10.1146/annurev-publhealth-031811-124530 22224881; PubMed Central PMCID: PMC3360960.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 5- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study
- Steroid-Based Therapy and Risk of Infectious Complications
- How Much Can the USA Reduce Health Care Costs by Reducing Smoking?
- Interpreting the Global Enteric Multicenter Study (GEMS) Findings on Sanitation, Hygiene, and Diarrhea
- Health Research and the World Humanitarian Summit—Not a Thousand Miles Apart
- A Public Health Paradox: The Women Most Vulnerable to Malaria Are the Least Protected
- Toward a Common Secure Future: Four Global Commissions in the Wake of Ebola
- The Clinical Challenge of Sepsis Identification and Monitoring
- All-Cause Mortality of Low Birthweight Infants in Infancy, Childhood, and Adolescence: Population Study of England and Wales
- Smoking Behavior and Healthcare Expenditure in the United States, 1992–2009: Panel Data Estimates
- Estimating the Risk of Chronic Pain: Development and Validation of a Prognostic Model (PICKUP) for Patients with Acute Low Back Pain
- Initiating Antiretroviral Therapy for HIV at a Patient’s First Clinic Visit: The RapIT Randomized Controlled Trial
- Prioritizing Surgical Care on National Health Agendas: A Qualitative Case Study of Papua New Guinea, Uganda, and Sierra Leone
- Effectiveness of and Financial Returns to Voluntary Medical Male Circumcision for HIV Prevention in South Africa: An Incremental Cost-Effectiveness Analysis
- Risk of Advanced Neoplasia in First-Degree Relatives with Colorectal Cancer: A Large Multicenter Cross-Sectional Study
- Common Infections in Patients Prescribed Systemic Glucocorticoids in Primary Care: A Population-Based Cohort Study
- Sanitation and Hygiene-Specific Risk Factors for Moderate-to-Severe Diarrhea in Young Children in the Global Enteric Multicenter Study, 2007–2011: Case-Control Study
- A Revolution in Treatment for Hepatitis C Infection: Mitigating the Budgetary Impact
- Nondisclosure of Financial Interest in Clinical Practice Guideline Development: An Intractable Problem?
- Financial Relationships between Organizations That Produce Clinical Practice Guidelines and the Biomedical Industry: A Cross-Sectional Study
- Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Estimating the Risk of Chronic Pain: Development and Validation of a Prognostic Model (PICKUP) for Patients with Acute Low Back Pain
- Prioritizing Surgical Care on National Health Agendas: A Qualitative Case Study of Papua New Guinea, Uganda, and Sierra Leone
- A Revolution in Treatment for Hepatitis C Infection: Mitigating the Budgetary Impact
- Toward a Common Secure Future: Four Global Commissions in the Wake of Ebola
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



