-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMortality, Morbidity, and Developmental Outcomes in Infants Born to Women Who Received Either Mefloquine or Sulfadoxine-Pyrimethamine as Intermittent Preventive Treatment of Malaria in Pregnancy: A Cohort Study
In a cohort study, Clara Menéndez and colleagues evaluate mortality, morbidity, and developmental outcomes in infants born to women who were enrolled in a randomized controlled trial of intermittent preventive treatment of malaria in pregnancy
Published in the journal: . PLoS Med 13(2): e32767. doi:10.1371/journal.pmed.1001964
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001964Summary
In a cohort study, Clara Menéndez and colleagues evaluate mortality, morbidity, and developmental outcomes in infants born to women who were enrolled in a randomized controlled trial of intermittent preventive treatment of malaria in pregnancy
Introduction
Malaria infection in pregnancy is a significant public health problem in endemic regions, especially in sub-Saharan Africa, where there are nearly 30 million pregnancies at risk of infection every year [1]. Maternal infection is an important contributor to anemia and low birth weight (LBW), as well as to overall morbidity and mortality in both the mother and the infant [2–15]. To prevent maternal infection in malaria endemic areas in Africa, WHO recommends the use of insecticide-treated bed nets and the administration of intermittent preventive treatment of malaria in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) [16]. Prevention of malaria with IPTp with SP has been shown to reduce LBW deliveries, neonatal mortality, and maternal morbidity [5,6,11,12,16–18]. However, the spread of SP parasite resistance in sub-Saharan Africa has raised concerns regarding its long-term use for IPTp, and alternative drugs are being evaluated.
Despite the emergence of resistance to mefloquine (MQ) in parts of Southeast Asia, MQ retains high antimalarial activity in Africa and has been shown to be effective both for malaria treatment in pregnancy and for preventing malaria infection in all trimesters [19–21]. Concerns that have been raised about its safety in pregnancy have not been confirmed, and MQ is among the very few antimalarials considered safe throughout pregnancy and is recommended by WHO and the US Centers for Disease Control and Prevention for chemoprophylaxis in non-immune pregnant women of all gestational ages traveling to malaria endemic regions [22,23]. It has also been recently reclassified as pregnancy category B by the US Food and Drug Administration and was proved to be safe in pregnancy in a recent systematic review [24,25].
It is recommended that clinical research involving pregnant women include a plan to monitor the outcome of the pregnancy as well as the short - and long-term health status of the child [26]; however, follow-up of children is rarely done in most African settings, mainly because of the difficulties of carrying out adequate monitoring. In the case of malaria, little is known about the effects of preventive interventions against malaria during pregnancy on the infant’s health. Only two trials in Southeast Asia have assessed the effect of MQ administration during pregnancy on the offspring’s health over the first month of life. In one study MQ was compared with placebo for malaria prophylaxis, and in the other trial MQ combined with artesunate (AS) was compared with quinine for treatment of malaria episodes. In neither study was an increased risk of adverse health, growth, or developmental outcomes in infants born to women receiving MQ in pregnancy reported [27,28].
We recently reported the results of a multicenter randomized controlled trial comparing MQ and SP for IPTp and evaluating the tolerability of two different MQ regimens in pregnant women in four sub-Saharan countries [29]. Women taking MQ were found to have fewer episodes of clinical malaria than SP recipients, and pregnancy outcomes and safety profiles were similar in both groups. However, drug tolerability was worse for MQ than for SP, even when splitting MQ doses over 2 d. Infants born to women participating in the trial were followed up until 12 mo of age. We report here the nutritional outcomes, psychomotor development, morbidity, and survival of infants born to women receiving either MQ or SP as IPTp.
Methods
Ethics Statement and Participants’ Safety
As previously described [29], the study protocol and informed consent forms were reviewed and approved by the ethics committee of the Hospital Clinic of Barcelona (Spain), the Comité Consultatif de Déontologie et d’Éthique of the Institut de Recherche pour le Développement (France), and all local regulatory authorities and national ethics review committees of each country participating in the study (S1 Table). The trial was conducted under the provisions of the Declaration of Helsinki and in accordance with good clinical practices guidelines set up by WHO and by the International Conference on Harmonization. An independent data safety monitoring board was created prior to the beginning of the trial and regularly reviewed and monitored the safety data collected. The trial was registered prior to the enrollment of the first participant in both ClinicalTrials.gov (NCT00811421) and the Pan African Clinical Trials Registry (PACTR2010020001429343).
Study Area and Population
The study was conducted between September 2009 and January 2013 in four sub-Saharan countries: Benin (Allada, Sékou, and Attogon), Gabon (Lambaréné and Fougamou), Tanzania (Makole and Chamwino), and Mozambique (Manhiça).
Study Design
Details of the study have been reported elsewhere [29]. A total of 4,749 pregnant women were enrolled into a randomized open-label three-arm trial to compare MQ with SP as IPTp for the prevention of the adverse effects of malaria during pregnancy and to compare the tolerability of two different MQ administration regimens in the context of long-lasting insecticide-treated bed net use. The three study arms were (1) IPTp with SP, (2) IPTp with MQ (15 mg/kg) given once as a full dose, and (3) IPTp with MQ (15 mg/kg) split over 2 d. The primary objective of the trial was to assess the safety and efficacy of the study drugs in pregnancy, independently of the dose regimen, as detailed in the study protocol (S1 Text). There were 4,247 live births born to the trial participants, 1,432 born to women in the SP group and 2,815 born to women in the MQ group; the infants were followed up until 12 mo of age. Assessment of the effect of the administration of MQ versus SP in the mother on the infant’s health and survival was also included as a trial objective.
Study Procedures
Enrollment of pregnant women
All pregnant women attending a participating antenatal clinic for the first time were screened for eligibility to participate in the study. Inclusion criteria were the following: permanent residence in the study area; gestational age ≤ 28 weeks; negative HIV test at recruitment; absence of history of allergy to sulfa drugs or MQ; absence of history of severe renal, hepatic, psychiatric, or neurological disease; and absence of MQ or halofantrine treatment in the preceding 4 wk. Women who met the inclusion criteria and signed an informed consent form were randomized to the SP group and received standard IPTp (three tablets of the fixed combination therapy containing 500 mg of sulfadoxine and 25 mg of pyrimethamine; Malastop, Sterop) or to the MQ group and received 15 mg/kg of MQ (tablets of 250 mg of MQ base; Lariam, Roche). The number of tablets for MQ was calculated according to body weight. For women allocated to the MQ split-dose group, the 15-mg/kg dose was divided into two halves and administered over two consecutive days, with the second half dose administered either at the antenatal clinic or at home by study personnel.
Infant follow-up
After delivery, live births were given a study number different from that of the mother in order to be uniquely identified. Women were asked to bring their children to the study health facility when the babies were 1 mo of age or coinciding with the first Expanded Program on Immunization visit at 6 wk, and also at 9 and 12 mo of age. At each visit, the baby’s weight and length were recorded, and psychomotor development was evaluated following a simplified adapted protocol that included assessment of gross and fine motor skills, language, audition, and social skills [30]. Participants who did not attend scheduled study visits were visited at home and encouraged to attend the health facility for completion of study visits. Throughout the follow-up, infants reporting sick at the health facility were seen by study health personnel. Unscheduled outpatient visits and hospital admissions were recorded in standardized questionnaires. A capillary blood sample was taken for malaria parasitemia examination and hemoglobin determination in infants who presented with fever or a history of fever in the last 24 h or who appeared pale. All children who did not attend the visit at 12 mo of age were visited at home to assess the infant’s residence and health status.
Laboratory Methods
Hemoglobin (Hb) was determined using mobile devices in capillary blood samples (HemoCue [Eurotrol] and Hemocontrol [EKF Diagnostics]). Thick and thin blood films were stained and read for Plasmodium species detection according to standard, quality-controlled procedures[31,32].
Data Management, Statistical Methods, and Definitions
The quality of data recorded in the study source documents and case report forms was monitored regularly following good clinical practices principles by the trials’ clinical monitor before the data were sent to the centralized database in Manhiça, Mozambique. Data were double-entered using OpenClinica Enterprise software for clinical data management. The analysis compared infants born to women receiving either SP or MQ, independently of the MQ regimen. The analysis was done on the modified intention-to-treat (mITT) cohort that included all live births born to women who met the inclusion criteria and had data on the outcomes of the main trial. The mITT analyses were adjusted by country. To include seasonality in the adjusted analysis, the duration of women’s recruitment was divided into eight periods, and the interaction terms between period of recruitment and country were included in the model, which allows modeling of the effect of period in each country independently.
Baseline characteristics of newborns at delivery were described using standard statistics. Proportions were compared between mother’s IPTp groups using Fisher’s exact test. Adjustments for covariates and possible confounders were made using logistic regression and robust estimates of the covariance (Huber method) using the method proposed by Zou [33]. Comparisons of proportions are presented as a relative risk (RR) or a reduction in the RR (1 − RR × 100%) if the RR is lower than 1. Continuous variables were compared between groups and adjusted for covariates and possible confounders using ordinary least squares regression. Main outcomes (nutritional outcomes; psychomotor development; incidences of clinical malaria, anemia, outpatient visits, and hospital admissions; and mortality) were compared between all children born to MQ recipients and SP recipients. Results with p < 0.05 were considered statistically significant, without allowance for multiple testing. Variables were transformed to the logarithm scale if normality was thereby improved. Nutritional analysis was adjusted by birth weight. z-Scores were calculated for the four WHO-recommended anthropometric indices to assess nutrition in infants: weight for age (underweight, severe acute malnutrition), height for age (stunting), weight for height (wasting), and mid-upper arm circumference, according to WHO standard definitions [34,35]. Participants with missing data were not included in the analysis. Clinical malaria was defined as fever (≥37. 5°C) or history of fever in the past 24 h or signs suggestive of malaria, reported through direct questioning in scheduled visits and active reporting in unscheduled visits, confirmed by a positive blood smear. Anemia was defined as Hb < 125 g/l in cord blood for neonates and Hb < 110 g/l in peripheral blood in infants. LBW was defined as birth weight < 2,500 g, and preterm as gestational age < 37 wk at delivery. The following psychomotor development milestones were assessed at each age: at 1 mo—(i) movement of four extremities symmetrically, (ii) muscle tone, (iii) following of objects, (iv) response to sounds, and (v) response to smiles; at 9 mo—(i) ability to sit without leaning, (ii) crawling, (iii) standing without help, (iv) walking without support, (v) grasping small objects, (vi) palm grasping, (vii) moving objects from one hand to the other, (viii) turning at voice, (ix) ability to say a word, and (x) bringing solid food to his/her mouth; and at 12 mo—(i) walking, (ii) pincer grasping, (iii) understanding orders, (iv) ability to say some words, and (v) drinking from a cup. Congenital abnormalities, hospital admissions, and deaths were considered serious adverse events (SAEs) and were confirmed and reported in specific questionnaires by a study clinician and notified to the sponsor and the data safety monitoring board. Diagnoses for the SAE reporting were codified using Medical Dictionary for Regulatory Activities preferred term and system organ class coding system [36]. Incidences of clinical malaria, anemia, infant mortality, hospital admissions, and outpatient attendances were estimated as the number of episodes per person over the time at risk. Time at risk was defined as the time from the start of follow-up (day of birth) until the end of follow-up (visit at 12 mo) or withdrawal due to censoring or death, whichever occurred first. In order to avoid counting twice the same episode of clinical malaria, participants did not contribute to the denominator or numerator during an arbitrary period of 28 d after an event of clinical malaria was confirmed. For hospital admissions and outpatient attendances, a maximum of one episode per day was allowed. Infant mortality rate was defined as the number of deaths per 1,000 live births per year at risk. The total number of events was compared between groups using negative binomial regression to take into account a possible extra Poisson variation due to different frailty of the participants. The mortality comparison is expressed as a relative rate. Study completion was defined as attendance to visit 3 at 12 mo of age, independently of completing the other previous study visits. Data analysis was performed using Stata version 13 (Stata Corporation).
Results
Baseline Characteristics of Study Participants
Fig 1 shows the trial profile of the mITT cohort that is the basis of this study. Overall, there were 2,815 and 1,432 live births born to mothers receiving IPTp with MQ and SP, respectively. The study follow-up was completed by 73% (2,054) of babies born to women allocated to the MQ group and by 74% (1,055) of those born to women in the SP group. Reasons for not completing the study were death (4% of total study population), withdrawal (6%), migration (8%), and loss to follow-up (9%). Infants who did not complete the study were similar at birth to those who did, except that they were in a lower proportion from Benin (17%), had a smaller mean head circumference (33.67 cm), a greater median gestational age (40 wk), and a lower proportion of cord blood anemia (8.8%) (S5 Table).
Fig. 1. Trial profile (modified intention-to-treat cohort). 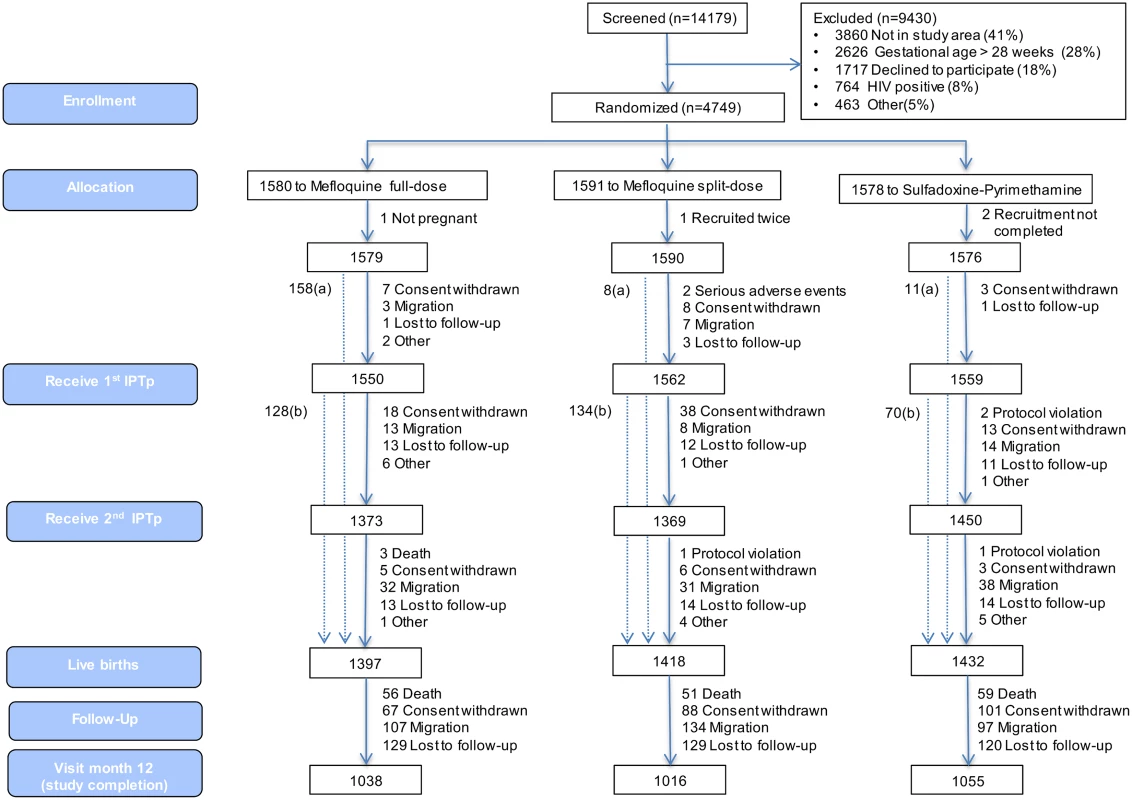
aNumber of women recruited at first antenatal visit who did not attend the rest of the study visits but delivered at the maternity ward and for whom information on pregnancy outcome is available. bNumber of women who delivered before receiving second IPTp dose. Baseline characteristics were similar for both study groups. Overall, mean prevalence of cord blood anemia, malaria parasitemia, and LBW was 10.6%, 0.3%, and 12.0%, respectively, with no significant differences between groups. Median gestational age at birth was 39 wk (interquartile range 38.4; 40.8), and congenital abnormalities were found in 46 (1.1%) live births (1.2% in the MQ group and 1.0% in the SP group); of these congenital abnormalities, 36 were detected at delivery, and ten during follow-up, with no significant differences between groups (Table 1).
Tab. 1. Baseline characteristics of study infants at delivery. 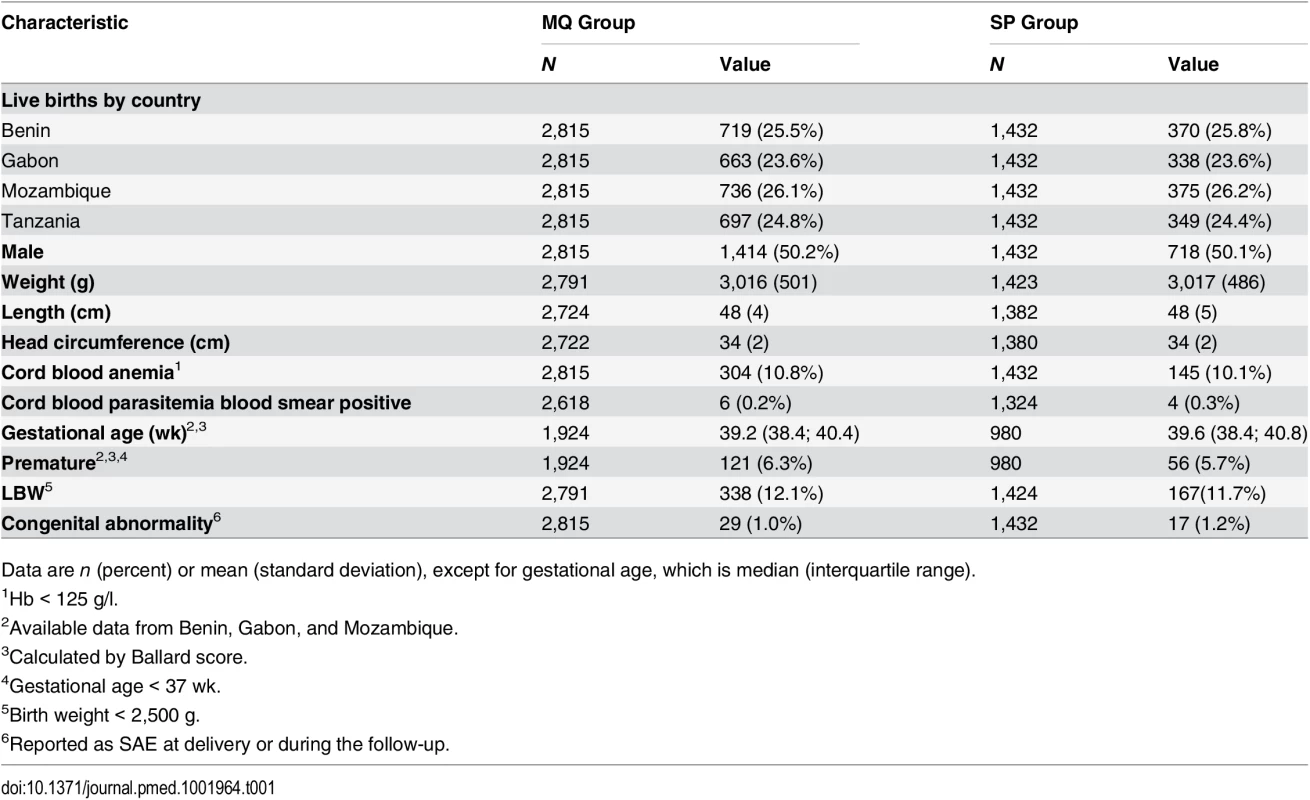
Data are n (percent) or mean (standard deviation), except for gestational age, which is median (interquartile range). Nutritional Outcomes
There were no significant differences between the groups at any of the scheduled study visits in the proportion of children who were stunted, underweight, wasted, or with severely acute malnutrition. There was a non-significant increase from the first study visit at 1 mo of age to the last study visit at 12 mo of age in the prevalence of children who were stunted or wasted. The proportion of children who were underweight at 12 mo (25.7% in the MQ group and 26.1% in the SP group) was four times greater than the proportion who were underweight at 1 mo of age (7.7% in the MQ group and 6.8% in the SP group, odds ratio 4.31, 95% CI 2.28–3.51, p < 0.001) (Table 2).
Tab. 2. Nutritional outcomes of study infants by age and by their mother’s intervention group. 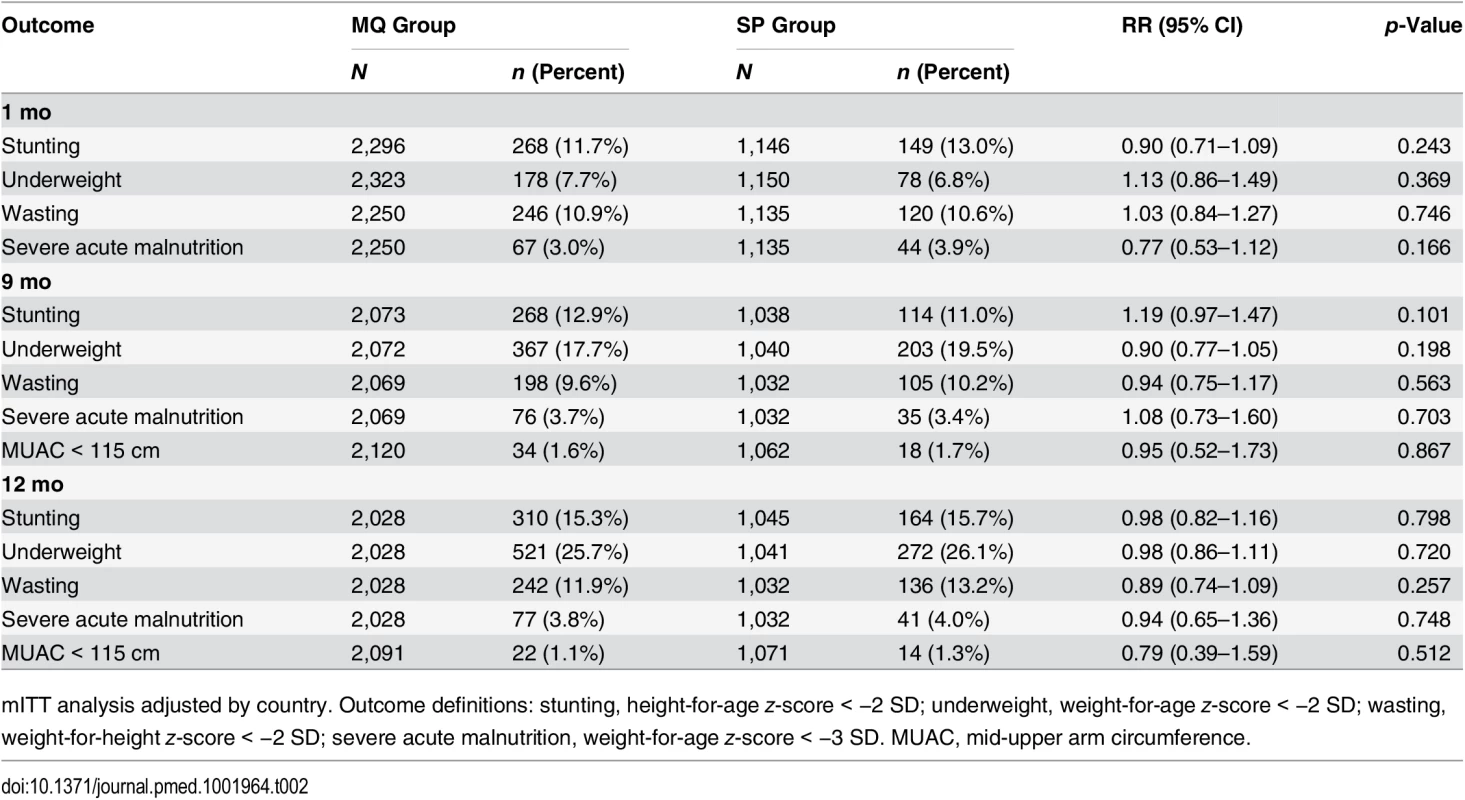
mITT analysis adjusted by country. Outcome definitions: stunting, height-for-age z-score < −2 SD; underweight, weight-for-age z-score < −2 SD; wasting, weight-for-height z-score < −2 SD; severe acute malnutrition, weight-for-age z-score < −3 SD. MUAC, mid-upper arm circumference. Psychomotor Development
Among infants born to women in the MQ group, there was an increased risk of being unable to stand without help, walk without support, and bring solid food to the mouth at 9 mo of age compared to those children born to women in the SP group (RR 1.07, 95% CI 1.00–1.14, p = 0.040; RR 1.10, 95% CI 1.01–1.21, p = 0.039; RR 1.32, 95% CI 1.03–1.70, p = 0.031, respectively), but not at 1 and 12 mo. No other significant differences were observed in the psychomotor development milestones assessed at the study visits (Table 3). Similar differences in the same psychomotor development items at 9 mo were found in both the according-to-protocol (ATP) and mITT analysis. In addition, no other associations were found in the rest of the items assessed or at the other study visits for the ATP group (S6 Table).
Tab. 3. Psychomotor development of children by age and by their mother’s study group. 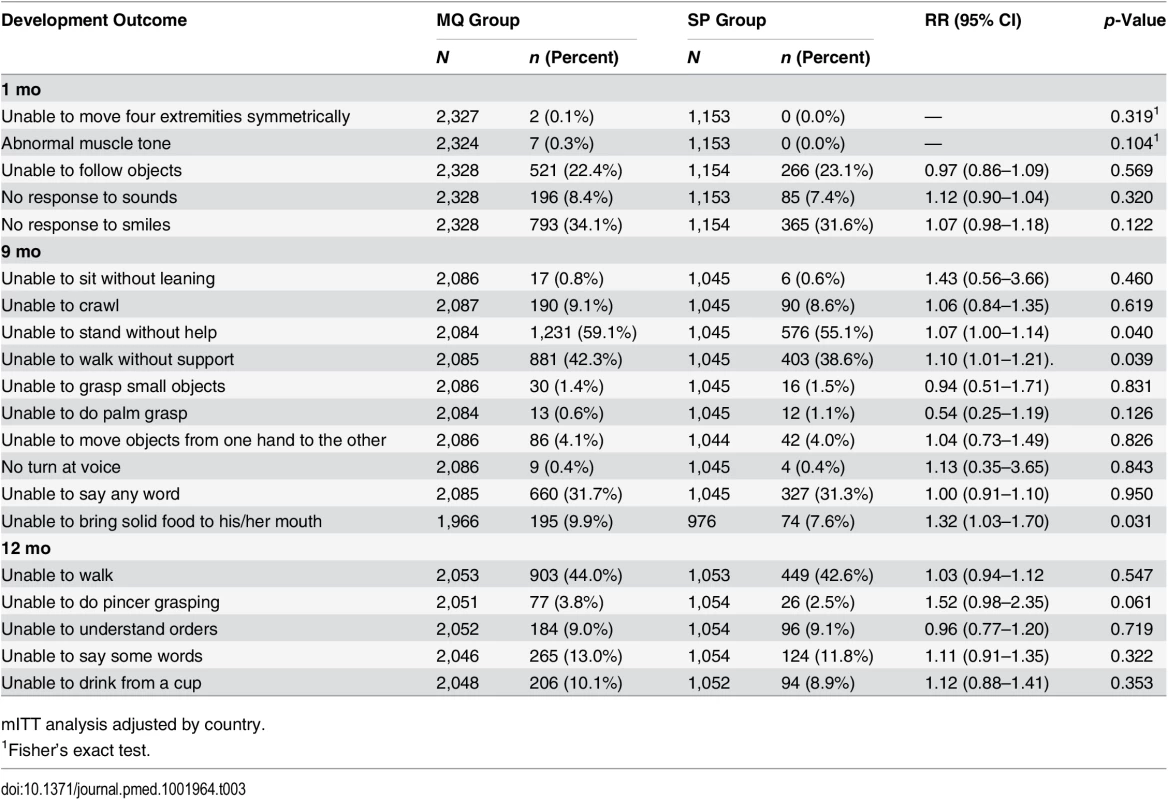
mITT analysis adjusted by country. Morbidity and Mortality
Throughout the study follow-up, the most common SAEs reported were infectious illnesses, and among these, the most frequently reported were malaria, pneumonia, and neonatal sepsis, with no significant differences between the two groups (S2 and S3 Tables). Nor was there any significant difference between the study groups in the incidence of clinical malaria (0.14 and 0.15 episodes per person per year at risk in the MQ and SP groups, respectively, p = 0.594). The incidence of outpatient visits was 0.65 episodes per person per year at risk in both groups. Hospital admissions were also very similar in children born to women who received MQ versus SP, with incidences of 0.09 and 0.10 admissions per person per year at risk, respectively. The infant mortality rate was 26.9 deaths per 1,000 live births per year at risk in both groups (Table 4). Overall, 61% of infant deaths occurred in the neonatal period, of which 43% took place in the first week of life, with no significant difference between the MQ and SP groups. The most common causes of death, with similar proportions in both groups, were non-cause-specific disorders (29%), infectious diseases (25%), respiratory diseases (21%), perinatal complications (9%), blood disorders (6%), congenital abnormalities (5%), and neurological diseases (3%) (S4 Table).
Tab. 4. Incidence of clinical malaria, hospital admissions, outpatient visits, and mortality in study infants by their mother’s intervention group. 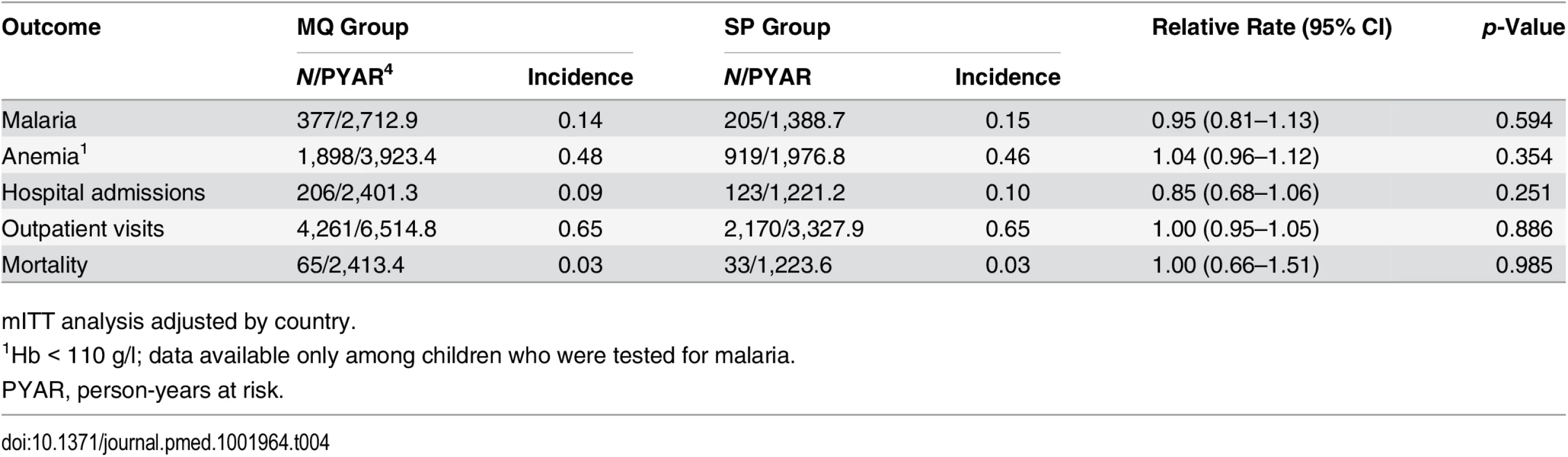
mITT analysis adjusted by country. Discussion
This study assessed the effects of MQ as IPTp compared to that of SP on health outcomes in cohorts of infants from four African countries (Benin, Gabon, Mozambique, and Tanzania). Nearly 4,000 infants born to women participating in a large randomized controlled trial assessing the safety and efficacy of IPTp with MQ compared to SP were followed until 12 mo of age. At all study visits, the prevalence of undernutrition in infants was similar between the study groups. Being able to stand without help, walk without support, and bring solid food to the mouth when assessed at 9 mo of age were the only developmental items found to be less frequently done by infants in the MQ group compared to those in the SP group. No significant differences were observed in any of the other developmental items assessed in the study visits at 1 mo, 9 mo, and 12 mo of age. Whether this result could be a chance finding due to multiple testing or related to maternal exposure to MQ during pregnancy would need to be further investigated. No significant differences were observed in the incidences of clinical malaria, anemia, hospital admissions, outpatient visits, or mortality between the groups.
There is very limited data on the impact of MQ in pregnancy on the infant’s health status. Previous trials evaluating the safety and efficacy of MQ in pregnancy in the African region assessed only pregnancy outcomes or followed up children only until 1 mo after birth [37–39]. Only two trials—one carried out in the Thai–Burmese border area comparing MQ with placebo for malaria prophylaxis in pregnant Karen women and the other, in the same area, comparing MQ combined with AS with quinine for malaria treatment—have reported children’s outcomes for the first 24 and 12 mo of life, respectively [27,28]. However, in the first study, MQ was given at a prophylactic dosage (2.5 mg of base/kg/wk), making it difficult to extrapolate the findings to higher treatment dosages. In the other study, the small sample size was insufficient to appropriately assess differences between groups. In the first trial, weight and weight gain at months 1, 3, 6, and 12 were similar in children born to women who received MQ and those born to women who received placebo [27]. In the second trial, infant growth outcomes were not assessed. An analysis of a case series of 72 American female soldiers who took weekly MQ prophylaxis without prior knowledge of their pregnancy status found that two out of the 13 live-born infants with available data were small for their ages [40]. Other than these studies, most of the safety data in children born to women who took MQ while pregnant come from the post-marketing surveillance system of the manufacturer (dominated by exposure as chemoprophylaxis) and from studies in Southeast Asia, none of which assessed the impact of MQ during pregnancy in children older than 1 mo of age [40–44].
Though controversy exists as to which indicator best defines undernutrition, the use of stunting (low height for age) seems to be the most appropriate, since it is a largely irreversible outcome and has long-term effects in individuals and societies [45]. The prevalence of stunting observed at 12 mo of age in this study is comparable to that found in other studies carried out in African populations [47–49]. More than a quarter of the children in both groups were underweight at 12 mo of age, which is consistent with figures reported from other low-income settings [50–53]. On the other hand, in both groups the prevalence of underweight increased with age from 1 to 12 mo. Although exclusive breast-feeding is considered sufficient to provide adequate nutrition and immunological protection to infants during the first 6 mo, the likely inadequacy, both in quantity and nutritional quality, of weaning foods, together with exposure to infectious diseases, may compromise the infants’ growth onwards [54–57].
While in high-income countries many tools have been developed and validated to evaluate children’s psychomotor development, there is a shortage of appropriate assessment tools for populations living in low-income settings [58,59]. In this study, an adapted and simplified test was used, which might be difficult to compare with that used in other studies [30]. In the two previously described trials carried out in Thailand, no significant differences were found in neurological development assessed by the Denver Developmental Screening Test at 24 mo of age between the children of women who received MQ-AS versus quinine for the treatment of malaria in pregnancy, and mean ages to sit and to crawl were significantly younger in the children of women given MQ versus placebo as malaria prophylaxis [27,28,60]. In contrast, in the current study, there was a higher proportion of children unable to perform certain developmental items at 9 mo of age in the MQ group compared to the SP group, though it is likely that this may be due to multiple testing rather than to true differences between the groups.
Several studies have evidenced an association of maternal malaria, especially placental infection, with increased risk of clinical malaria and overall mortality in infants [1,2,4,6,11,61–63]. Although pregnant women who received IPTp with MQ had a lower incidence of clinical malaria and placental infection than those who received SP, the incidence of malaria and the all-cause mortality rate among their children were similar between the two groups and consistent with results reported from other studies in sub-Saharan Africa and are similar to those estimated in the region for the same age group [2,14,29,38,39,64]. In the only trial assessing the impact of MQ in pregnancy on malaria and all-cause mortality in children, the mortality rate and incidence of malaria episodes were comparable in both groups (malaria prophylaxis with either MQ or placebo) [27].
The main strength of this study is that it provides carefully collected and detailed information on nutritional and developmental outcomes in a large number of African infants born to women who randomly received either MQ or SP as IPTp for malaria prevention. This is of relevance given the limited data available on the effect of MQ in pregnancy on the infant’s health. No previous trials to our knowledge have assessed the effect of MQ administration in African pregnant women on the infant’s health. The results are also important because drug combinations containing MQ are currently recommended for malaria treatment in pregnancy, and MQ alone is recommended for prophylaxis in pregnant women traveling to endemic countries. A limitation of the study was that more than a quarter of infants did not complete the study, though only 9% of them were lost to follow-up. It has been previously noted that loss to follow-up in infants is a potential operational challenge for implementation of preventive programs and for performance of observational studies in this age group in sub-Saharan Africa [65–67]. The proportion of deaths in the first year of life in our study was similar in the two study groups and lower than the 6% UNICEF estimate for the African region [68]. It is common practice in rural Africa for women to move to their parents’ house to give birth, often in a different village. After delivery they move back to their home village, which might explain the migrations during infancy that caused some participants to not complete the study. The baseline characteristics of infants who did not complete the study were similar to those of children who did so, except for the country distribution, which could be explained by the particularities of each site’s follow-up methodology. We did not find any significant difference regarding newborn physical characteristics between those infants who completed the study and those who did not, supporting that infants who did not complete the study did not have an underlying condition or an increased frequency of adverse events that could have influenced the results in one way or another. On the other hand, the open-label design might have led to biases in the assessment of the outcomes, especially of psychomotor development, since MQ is often associated with neurological adverse events. However, it is difficult to rule out or confirm that this could have affected the study results. Children were uniquely identified by a different study number from that of the mother, and were examined by different study personnel, who did not have access to the mother’s study arm allocation. Only study personnel who administered the study interventions to the women were aware of the drug they were providing to the participants, and in general women did not know whether they had received MQ or SP.
In conclusion, these results indicate that administration of 15 mg/kg of MQ as IPTp compared to SP as IPTp in pregnant women is not associated with increased risks of infant mortality, morbidity, and undernutrition. This is of particular relevance considering that antimalarial drug combinations containing MQ are currently recommended for malaria treatment in pregnancy, and MQ alone is recommended for prophylaxis in pregnant women traveling to endemic countries [22,23]. In the current study there was a higher proportion of children unable to perform certain developmental items at 9 mo of age in the MQ group compared to the SP group. Though this finding is interesting and may call for further studies in children whose mothers are exposed to MQ during pregnancy, it cannot be ruled out that it might be explained by the multiple testing or open design of the study.
Supporting Information
Zdroje
1. Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 Suppl):28–35. 11425175
2. Bardaji A, Sigauque B, Sanz S, Maixenchs M, Ordi J, Aponte JJ, et al. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis. 2011;203 : 691–699. doi: 10.1093/infdis/jiq049 21199881
3. Nyirjesy P, Kavasya T, Axelrod P, Fischer PR. Malaria during pregnancy: neonatalmorbidity and mortality and the efficacy of chloroquine chemoprophylaxis. Clin Infect Dis. 1993;16 : 127–132. 8448288
4. Bloland PB, Wirima JJ, Steketee RW, Chilima B, Hightower A, Breman JG. Maternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infection. AIDS. 1995;9 : 721–726. 7546417
5. Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7 : 93–104. 17251080
6. Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatib-N’Jie AB. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Trans R Soc Trop Med Hyg. 1989;83 : 589–594. 2617619
7. Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub - Saharan Africa. Clin Microbiol Rev. 2004;17 : 760–769. 15489346
8. Le Hesran JY, Fievet N, Thioulouse J, Personne P, Maubert B, M’Bidias S, et al. Development of cellular immune responses to Plasmodium falciparum blood stage antigens from birth to 36 months of age in Cameroon. Acta Trop. 2006;98 : 261–269. 16820138
9. Menendez C, D’Alessandro U, ter Kuile FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis. 2007;7 : 126–135. 17251083
10. Menendez C, Mayor A. Congenital malaria: the least known consequence of malaria in pregnancy. Semin Fetal Neonatal Med. 2007;12 : 207–213. 17483042
11. Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181 : 1740–1745. 10823776
12. Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST, Newman RD, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis. 2008;47 : 1017–1025. doi: 10.1086/591968 18781874
13. Shulman CE, Dorman EK, Bulmer JN. Malaria as a cause of severe anaemia in pregnancy. Lancet. 2002;360 : 494.
14. Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55(1 Suppl): 33–41. 8702035
15. van Geertruyden JP, Thomas F, Erhart A, D’Alessandro U. The contribution of malaria inpregnancy to perinatal mortality. Am J Trop Med Hyg. 2004;71(2 Suppl): 35–40. 15331817
16. World Health Organization. A strategic framework for malaria prevention and control during pregnancy in the African region. Geneva: World Health Organization; 2004.
17. Menendez C, Bardaji A, Sigauque B, Romagosa C, Sanz S, Serra-Casas E, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS ONE. 2008;3:e1934. doi: 10.1371/journal.pone.0001934 18398460
18. Menendez C, Bardaji A, Sigauque B, Sanz S, Aponte JJ, Mabunda S, et al. Malaria prevention with IPTp during pregnancy reduces neonatal mortality. PLoS ONE. 2010;5:e9438. doi: 10.1371/journal.pone.0009438 20195472
19. Ramharter M, Wernsdorfer WH, Kremsner PG. In vitro activity of quinolines against Plasmodium falciparum in Gabon. Acta Trop. 2004;90 : 55–60. 14739023
20. Aubouy A, Fievet N, Bertin G, Sagbo JC, Kossou H, Kinde-Gazard D, et al. Dramatically decreased therapeutic efficacy of chloroquine and sulfadoxine-pyrimethamine, but not mefloquine, in southern Benin. Trop Med Int Health. 2007;12 : 886–894. 17596256
21. Uhlemann AC, Ramharter M, Lell B, Kremsner PG, Krishna S. Amplification of Plasmodium falciparum multidrug resistance gene 1 in isolates from Gabon. J Infect Dis. 2005;192 : 1830–1835. 16235185
22. World Health Organization. Guidelines for the treatment of malaria, 3rd edition. Geneva: World Health Organization; 2015.
23. US Centers for Disease Control and Prevention. Update: new recommendations for mefloquine use in pregnancy. 2011 Oct 3 [cited 31 Jul 2013]. Available: http://www.cdc.gov/malaria/new_info/2011/mefloquine_pregnancy.html.
24. Department of Health and Human Services, US Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling: final rule. Docket number FDA-2006-N-0515. Silver Spring (Maryland): US Food and Drug Administration; 2008. Available: http://wwwgpogov/fdsys/pkg/FR-2008-05-29/pdf/E8-11806pdf.
25. Gonzalez R, Hellgren U, Greenwood B, Menendez C. Mefloquine safety and tolerability in pregnancy: a systematic literature review. Malar J. 2014;13 : 75. doi: 10.1186/1475-2875-13-75 24581338
26. Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Geneva: Council for International Organizations of Medical Sciences; 2002.
27. Nosten F, ter Kuile F, Maelankiri L, Chongsuphajaisiddhi T, Nopdonrattakoon L, Tangkitchot S, et al. Mefloquine prophylaxis prevents malaria during pregnancy: a double - blind, placebo-controlled study. J Infect Dis. 1994;169 : 595–603. 8158032
28. Bounyasong S. Randomized trial of artesunate and mefloquine in comparison with quinine sulfate to treat P. falciparum malaria pregnant women. J Med Assoc Thai. 2001;84 : 1289–1299. 11800303
29. Gonzalez R, Mombo-Ngoma G, Ouedraogo S, Kakolwa MA, Abdulla S, Accrombessi M, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV - negative women: a multicentre randomized controlled trial. PLoS Med. 2014;11:e1001733. doi: 10.1371/journal.pmed.1001733 25247709
30. Lissauer T, Clayden G. Illustrated textbook of paediatrics, 4th edition. Philadelphia: Mosby; 2012.
31. Swysen C, Vekemans J, Bruls M, Oyakhirome S, Drakeley C, Kremsner P, et al. Development of standardized laboratory methods and quality processes for a phase III study of the RTS, S/AS01 candidate malaria vaccine. Malar J. 2011;10 : 223
32. Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg. 2001;65 : 599–602. 15033648
33. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159 : 702–706. 15033648
34. World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Methods and development. Geneva: World Health Organization; 2006.
35. Vidmar S, Carlin J, Hesketh K, Cole T. Standardizing anthropometric measures in children and adolescents with new functions for egen. Stata J. 2004;4 : 50–55.
36. (ICH) ICoH. MedDRA Data retrieval and presentation: points to consider. ICH-Endorsed Guide for MedDRA Users on Data Output. Geneva: IFPMA; 2012.
37. Sowunmi A, Oduola AM, Ogundahunsi OA, Fehintola FA, Ilesanmi OA, Akinyinka OO, et al. Randomised trial of artemether versus artemether and mefloquine for the treatment of chloroquine/sufadoxine-pyrimethamine-resistant falciparum malaria during pregnancy. J Obstet Gynaecol. 1998; 18 : 322–327. 15512100.
38. Briand V, Bottero J, Noel H, Masse V, Cordel H, Guerra J, et al. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect Dis. 2009; 200 : 991–1001. doi: 10.1086/605474 19656069.
39. Denoeud-Ndam L, Clement MC, Briand V, Akakpo J, Agossou VK, Atadokpede F, et al. Tolerability of mefloquine intermittent preventive treatment for malaria in HIV-infected pregnant women in Benin. J Acquir Immune Defic Syndr. 2012;61 : 64–72. 22706291
40. Smoak BL, Writer JV, Keep LW, Cowan J, Chantelois JL. The effects of inadvertentexposure of mefloquine chemoprophylaxis on pregnancy outcomes and infants of US Army servicewomen. J Infect Dis. 1997;176 : 831–833. 9291347
41. Vanhauwere B, Maradit H, Kerr L. Post-marketing surveillance of prophylactic mefloquine (Lariam) use in pregnancy. Am J Trop Med Hyg. 1998;58 : 17–21. 9452285
42. Schlagenhauf P, Blumentals WA, Suter P, Regep L, Vital-Durand G, Schaerer MT, et al. Pregnancy and fetal outcomes after exposure to mefloquine in the pre - and periconception period and during pregnancy. Clin Infect Dis. 2012;54:e124–e131. doi: 10.1093/cid/cis215 22495078
43. Newman RD, Parise ME, Slutsker L, Nahlen B, Steketee RW. Safety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan Africa. Trop Med Int Health. 2003;8 : 488–506. 12791054
44. Nosten F, McGready R, d’Alessandro U, Bonell A, Verhoeff F, Menendez C, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1 : 1–15. 18690910
45. United Nations Children’s Fund. Improving child nutrition. The achievable imperative for global progress. New York: United Nations Children’s Fund; 2013.
46. Engebretsen IM, Tylleskar T, Wamani H, Karamagi C, Tumwine JK. Determinants ofinfant growth in Eastern Uganda: a community-based cross-sectional study. BMC Public Health. 2008;8 : 418. doi: 10.1186/1471-2458-8-418 19102755
47. Masibo PK, Makoka D. Trends and determinants of undernutrition among young Kenyan children: Kenya Demographic and Health Survey; 1993, 1998, 2003 and 2008–2009. Public Health Nutr. 2012;15 : 1715–1727. doi: 10.1017/S1368980012002856 22694984
48. Jones AD, Ickes SB, Smith LE, Mbuya MN, Chasekwa B, Heidkamp RA, et al. World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Matern Child Nutr. 2014;10 : 1–17. doi: 10.1111/mcn.12070 23945347
49. Engebretsen IM, Jackson D, Fadnes LT, Nankabirwa V, Diallo AH, Doherty T, et al. Growth effects of exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa: the cluster-randomised PROMISE EBF trial. BMC Public Health. 2014;14 : 633. doi: 10.1186/1471-2458-14-633 24950759
50. Medhin G, Hanlon C, Dewey M, Alem A, Tesfaye F, Worku B, et al. Prevalence and predictors of undernutrition among infants aged six and twelve months in Butajira, Ethiopia: the P-MaMiE Birth Cohort. BMC Public Health. 2010;10 : 27. doi: 10.1186/1471-2458-10-27 20089144
51. Matanda DJ, Mittelmark MB, Kigaru DM. Child undernutrition in Kenya: trend analyses from 1993 to 2008–09. BMC Pediatr. 2014;14 : 5. doi: 10.1186/1471-2431-14-5 24410931
52. Abubakar A, Uriyo J, Msuya SE, Swai M, Stray-Pedersen B. Prevalence and risk factors for poor nutritional status among children in the Kilimanjaro region of Tanzania. Int J Environ Res Public Health. 2012;9 : 3506–3518. doi: 10.3390/ijerph9103506 23202759
53. Beiersmann C, Bountogo M, Tiendrebeogo J, Louis VR, Gabrysch S, Ye M, et al. Malnutrition in young children of rural Burkina Faso: comparison of survey data from 1999 with 2009. Trop Med Int Health. 2012;17 : 715–721. doi: 10.1111/j.1365-3156.2012.02985.x 22519807
54. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;8:CD003517. doi: 10.1002/14651858.CD003517.pub2 22895934
55. Robinson S, Fall C. Infant nutrition and later health: a review of current evidence. Nutrients. 2012;4 : 859–874. 23016121
56. Przyrembel H. Timing of introduction of complementary food: short - and long-term health consequences. Ann Nutr Metab. 2012;60(Suppl 2):8–20. doi: 10.1159/000336287 22555185
57. Wright CM, Parkinson KN, Drewett RF. Why are babies weaned early? Data from a prospective population based cohort study. Arch Dis Child. 2004;89 : 813–816. 15321854
58. Aina OF, Morakinyo O. The validation of Developmental Screening Inventory (DSI) on Nigerian children. J Trop Pediatr. 2001;47 : 323–328. 11827298
59. Abubakar A, Holding P, van Baar A, Newton CR, van de Vijver FJ. Monitoring psychomotor development in a resource-limited setting: an evaluation of the Kilifi Developmental Inventory. Ann Trop Paediatr. 2008;28 : 217–226. doi: 10.1179/146532808X335679 18727851
60. Frankenburg WK. The Denver Developmental Screening Test. Dev Med Child Neurol. 1969;11 : 260–262. 5787726
61. Greenwood AM, Armstrong JR, Byass P, Snow RW, Greenwood BM. Malaria chemoprophylaxis, birth weight and child survival. Trans R Soc Trop Med Hyg. 1992;86 : 483–485. 1475810
62. Luxemburger C, McGready R, Kham A, Morison L, Cho T, Chongsuphajaisiddhi T, et al. Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. Am J Epidemiol. 2001;154 : 459–465. 11532788
63. Verhoeff FH, Le Cessie S, Kalanda BF, Kazembe PN, Broadhead RL, Brabin BJ. Post - neonatal infant mortality in Malawi: the importance of maternal health. Ann Trop Paediatr. 2004;24 : 161–169. 15186545
64. Nosten F, Vincenti M, Simpson J, Yei P, Thwai KL, de Vries A, et al. The effects of mefloquine treatment in pregnancy. Clin Infect Dis. 1999;28 : 808–815. 10825043
65. Sidze LK, Faye A, Tetang SN, Penda I, Guemkam G, Ateba FN, et al. Different factors associated with loss to follow-up of infants born to HIV-infected or uninfected mothers: observations from the ANRS 12140-PEDIACAM study in Cameroon. BMC Public Health. 2015;15 : 228. doi: 10.1186/s12889-015-1555-2 25886161
66. Kalembo FW, Zgambo M. Loss to followup: a major challenge to successful implementation of prevention of mother-to-child transmission of HIV-1 programs in sub-Saharan Africa. ISRN AIDS. 2012;2012 : 589817. doi: 10.5402/2012/589817 24052879
67. Sibanda EL, Weller IV, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27 : 2787–2797. doi: 10.1097/QAD.0000000000000027 24056068
68. United Nations Children’s Fund, World Health Organization, World Bank Group, United Nations. Levels and trends in child mortality: report 2015. New York: United Nations Children’s Fund; 2015.
Štítky
Interní lékařství
Článek 2015 Reviewer Thank You
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 2- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- 2015 Reviewer Thank You
- The Future of Diabetes Prevention: A Call for Papers
- The Case for Reforming Drug Naming: Should Brand Name Trademark Protections Expire upon Generic Entry?
- The Health Care Consequences Of Australian Immigration Policies
- Microenvironmental Heterogeneity Parallels Breast Cancer Progression: A Histology–Genomic Integration Analysis
- Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination
- Estimated Effects of Different Alcohol Taxation and Price Policies on Health Inequalities: A Mathematical Modelling Study
- Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Uganda
- Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Nigeria
- When Children Become Adults: Should Biobanks Re-Contact?
- Transforming Living Kidney Donation with a Comprehensive Strategy
- A Time for Global Action: Addressing Girls’ Menstrual Hygiene Management Needs in Schools
- The Rise of Consumer Health Wearables: Promises and Barriers
- Risk of Injurious Fall and Hip Fracture up to 26 y before the Diagnosis of Parkinson Disease: Nested Case–Control Studies in a Nationwide Cohort
- Mortality, Morbidity, and Developmental Outcomes in Infants Born to Women Who Received Either Mefloquine or Sulfadoxine-Pyrimethamine as Intermittent Preventive Treatment of Malaria in Pregnancy: A Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination
- A Time for Global Action: Addressing Girls’ Menstrual Hygiene Management Needs in Schools
- Transforming Living Kidney Donation with a Comprehensive Strategy
- The Rise of Consumer Health Wearables: Promises and Barriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



