-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-analysis
In this systematic review and meta-analysis of public sector health data, Ramnath Subbaraman and colleagues estimate how many tuberculosis patients are "lost" and therefore do not receive effective care at each step of the treatment cascade.
Published in the journal: . PLoS Med 13(10): e32767. doi:10.1371/journal.pmed.1002149
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002149Summary
In this systematic review and meta-analysis of public sector health data, Ramnath Subbaraman and colleagues estimate how many tuberculosis patients are "lost" and therefore do not receive effective care at each step of the treatment cascade.
Introduction
India has the world’s largest tuberculosis (TB) epidemic, with 23% of the global burden of incident active TB patients annually and 27% of the world’s “missing” patients, representing about 1 million patients each year who have not been notified to the Government of India’s Revised National TB Control Programme (RNTCP) [1,2]. These patients may not have received health services for TB or may have received potentially suboptimal TB care in India’s private sector [3,4]. In recent years, the RNTCP has changed its policy objective from successfully identifying 70% of new smear-positive patients and completing treatment for 85% of these patients to a broader goal of “universal access to quality care” for all TB patients [5]. Similarly, the World Health Organization (WHO)’s post-2015 “End TB” Strategy hopes to treat “all people with TB, including drug-resistant TB” and to end the global TB epidemic by 2035 [6].
Achieving such an ambitious objective in India will require improvements across various RNTCP activities—including access to services, case finding, linkage of diagnosed patients to treatment, retention in treatment, and coordination with private sector providers—to bring about successful outcomes of treatment completion and, ideally, TB recurrence-free survival for all patients.
The “cascade of care” is a useful model for evaluating care delivery by a health system for a specific disease. This model has been used most extensively to assess the adequacy of HIV care [7,8] but has also been used for other diseases, including type II diabetes and hepatitis C [9,10]. The model defines an outcome that all patients should achieve—for example, virological suppression in the case of HIV. It outlines a series of sequential steps needed to achieve that outcome. Estimates are provided of the number of patients who have successfully achieved each sequential step. In the field of HIV, this model is sometimes referred to as the “continuum of care,” reflecting the idea that, because HIV is a chronic disease, patients can sometimes move “backwards” along these steps during their life course (e.g., dropping out of care after initially being retained in care or developing HIV viremia after initially achieving viral suppression). Since TB is usually not a lifelong disease and can be cured with appropriate therapy, we use the term “cascade” to describe our model, even though TB patients can occasionally also move “backwards” in care along the continuum.
The strength of the cascade model is that it allows policymakers to visualize the biggest gaps in care delivery. They can then identify patient subpopulations who may be at highest risk for adverse outcomes or who may drive the development of incident TB patients. The cascade model may promote more efficient targeting of resources and motivate development of new strategies for addressing shortfalls in care. While the WHO has promoted a similar “onion” model (i.e., assessment of concentric layers of patient attrition) for evaluating local TB programs [11], to date, neither the onion nor the cascade model has been used to understand gaps in care delivery for TB at a national scale.
In this article, we describe the TB cascade of care in the public sector in India for 2013, based on an integrated model that draws from the WHO’s onion model and the cascade model used for HIV. Although about half of India’s TB patients may receive private sector care [12,13], we focus on the public sector alone, given substantial limitations in data on TB outcomes in the private sector. Our primary objective is to estimate the number of patients completing each step of the cascade, and an important secondary objective is to highlight gaps in knowledge in order to guide future research to improve the cascade’s accuracy. We also describe cascades for new smear-positive, retreatment smear-positive, new smear-negative, and multidrug-resistant (MDR) TB patients, to show how this model may shed light on care delivery for specific patient subpopulations.
Methods
Integrating the Cascade Model with the WHO Onion Model
The WHO onion model serves as an excellent starting point for constructing a TB cascade of care; however, this model has a few deficiencies [11]. The WHO model has been primarily promoted as a method for refining estimates of TB incidence. As a result, the stopping point for this model is a programmatic outcome—the total number of TB patients notified to the government—rather than a patient-oriented outcome, such as cure, treatment completion, or recurrence-free survival.
We believe that 1-y TB recurrence-free survival is a useful end outcome of the cascade. This indicator not only reflects whether patients completed treatment but also whether treatment was of high quality, since poor adherence increases the risk of post-treatment TB relapse or death [14]. We assume that most patients with recurrent TB in the RNTCP are experiencing disease relapse (rather than reinfection). Two studies help to justify this assumption. First, a study of TB recurrence in South India that used genotypic techniques to compare the DNA fingerprints of paired mycobacterial isolates from patients at the time of initial diagnosis and the time of TB recurrence found that recurrence was due to exogenous re-infection in only 9% of HIV-uninfected patients as compared to 88% of HIV-infected patients [15]. Since the prevalence of HIV-infection in the RNTCP’s overall population of TB patients is low (about 6%) [16], this suggests that the vast majority of TB recurrence represents relapse of prior disease. Second, another study in the RNTCP found that patients who took TB therapy irregularly were twice as likely to experience TB recurrence, even after adjusting for other risk factors, suggesting that the TB recurrence rate partly reflects medication adherence and the quality of care received by patients [14].
We use a 1-y minimum time period after treatment completion to assess for recurrence-free survival because most TB relapses (91%) occur within 1 y [17]. One-year post-treatment follow-up is recommended by the Standards for TB Care in India [18], and routine 1-y follow-up of all patients may be more feasible for national TB programs than longer follow-up periods.
The WHO onion model focuses on estimating “gaps” (the proportion of patients who did not complete specific steps), while most HIV cascades estimate the absolute number of patients who reach each step [7]. In most cascade models, the “gaps” are implied by each drop-off in the bar graph.
We have therefore integrated the WHO onion model and the cascade model, as represented in Fig 1. Each ring represents the estimated number of TB patients at that step of the cascade; we will refer to each of these rings as a “step.” This integrated model incorporates the following steps: (1) the total number of prevalent active TB patients in India, (2) the number of TB patients who reached and were evaluated at TB diagnostic facilities, (3) the number of patients diagnosed with TB, (4) the number of TB patients registered for treatment, (5) the number of patients who achieved cure or treatment completion, and (6) the number of patients who remained alive and TB recurrence-free as assessed a minimum of 1 y (and no more than 24 mo) after completing therapy. For steps 2–6, we will only include data from public sector RNTCP facilities in our analysis, as discussed further below. The spaces between the rings represent the proportion of patients who fail to complete each step; we will refer to these spaces as “gaps.”
Fig. 1. A model for the tuberculosis cascade of care in India that integrates the WHO onion model with concepts from the HIV cascade of care. 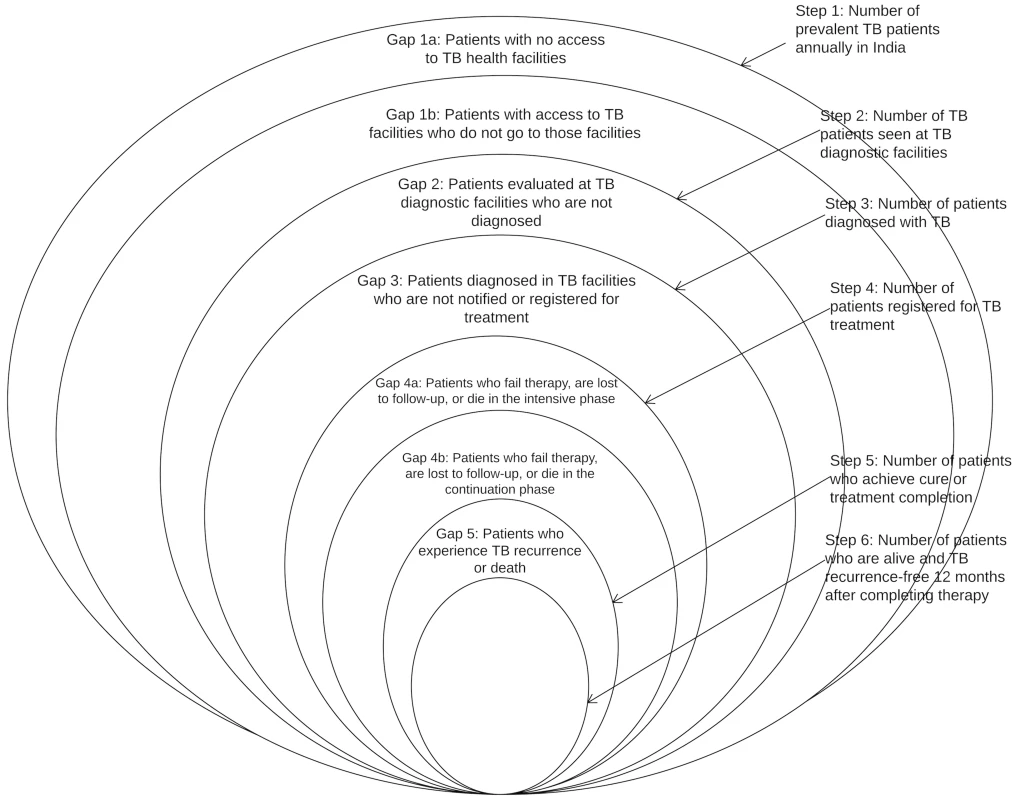
Adapted from WHO, 2009 [11]. The steps and gaps in the model can also be divided into the following parts: accessing care (Steps 1/2, Gaps 1a/1b/2), diagnosis and treatment registration (Steps 3/4, Gap 3), retention during the treatment course (Step 5, Gaps 4a/4b), and recurrence-free survival (Step 6, Gap 5). The steps and gaps are interrelated; in some cases we use data on steps to help estimate subsequent gaps and vice versa.
Data Sources
Detailed information on the data sources and methods used to estimate cascade steps and gaps are presented in Tables 1 and 2. The online database associated with WHO’s 2015 Global TB Report provides the most recently revised estimate of the total number of prevalent TB patients in India in 2013 [2,19]. The 2014 WHO Global TB Report provides an estimate of the total number of multidrug-resistant tuberculosis (MDR TB) patients among notified TB patients in the RNTCP in 2013 [1]. The RNTCP publishes national statistics annually in a document called TB India: RNTCP annual status report [16,20,21]. This report contains statistics on the total number of TB patients registered for treatment in the government program.
Tab. 1. Methods and data sources used to estimate each step of the TB cascade of care for different subpopulations of patients and for the overall population of TB patients in India in 2013. 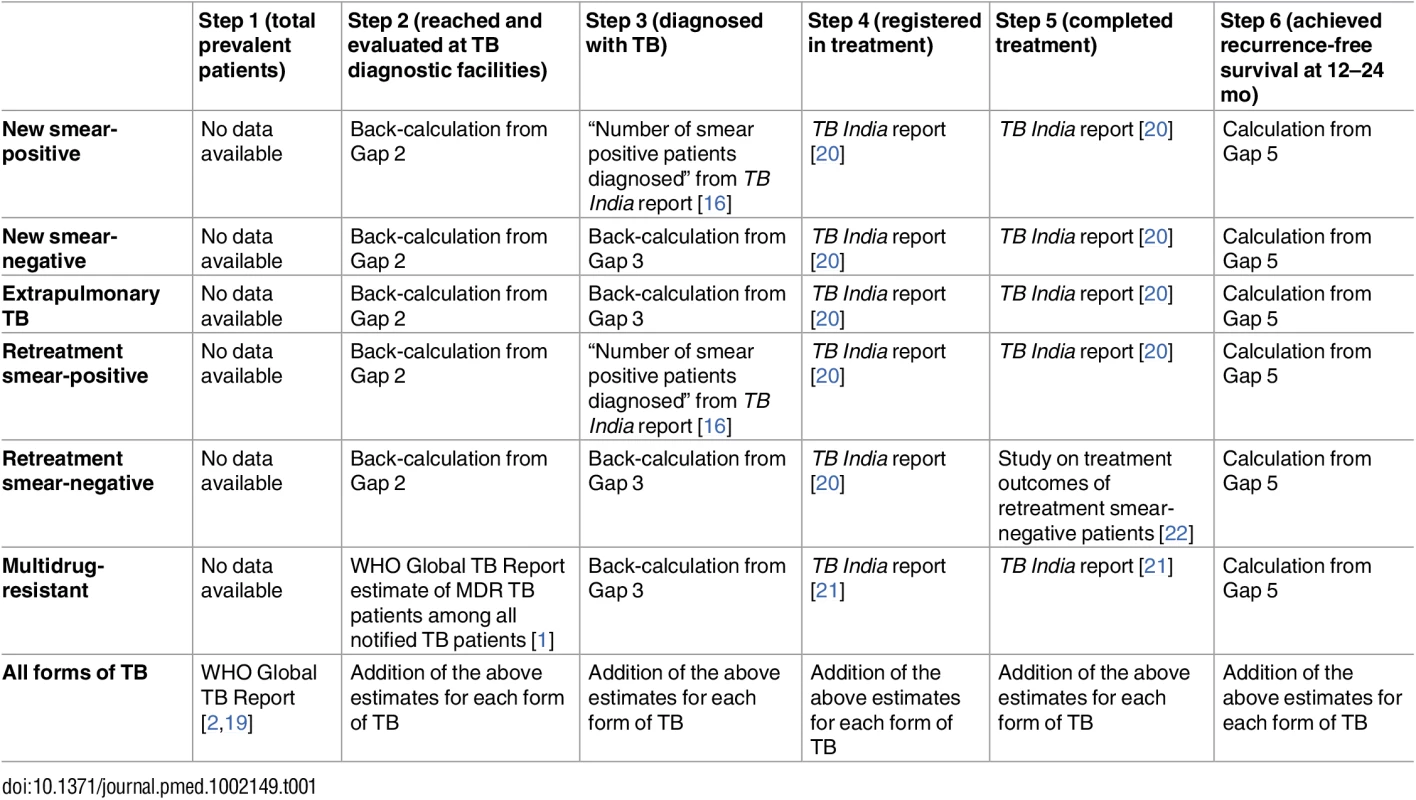
Tab. 2. Methods and data sources used to estimate each gap of the TB cascade of care for different subpopulations of patients and for the overall population of TB patients in India in 2013. 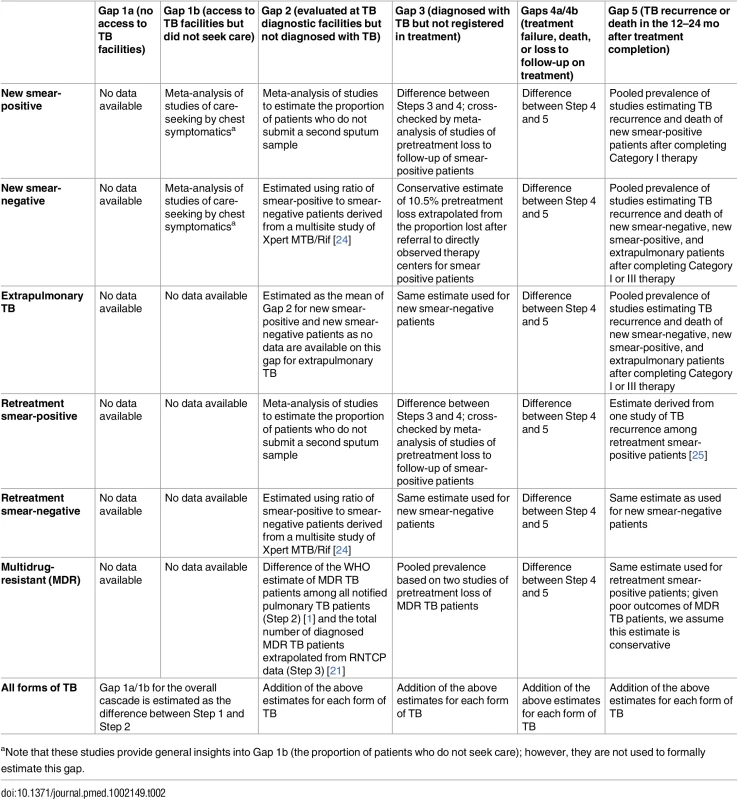
aNote that these studies provide general insights into Gap 1b (the proportion of patients who do not seek care); however, they are not used to formally estimate this gap. Treatment outcomes are provided in the TB India reports for all categories of TB, except retreatment smear-negative patients [20]. Due to the long duration of treatment, final outcomes for MDR TB patients are not reported until 3 y after treatment initiation. Therefore, 2013 is the earliest year for which we are able to construct a TB cascade of care that includes patients with all forms of TB, since treatment outcomes for MDR TB patients registered in 2013 were published in the 2016 TB India report [21].
We conducted targeted systematic reviews and meta-analyses of Indian studies published from 2000 to 2015 to inform or estimate the following gaps in the TB cascade of care: (1) the proportion of individuals in the community with cough >2 wk (i.e., suspected TB patients or “chest symptomatics”) who have not sought medical care (Gap 1b); (2) the proportion of patients who provide one sputum smear at designated microscopy centers but who fail to provide a second smear (sometimes referred to as “lost to follow-up during diagnosis” or “diagnostic default,” used to estimate Gap 2) [23]; (3) the proportion of patients who are lost to follow-up during the multistep diagnostic workup for smear-negative TB (Gap 2); (4) the proportion of smear-positive patients diagnosed at microscopy centers who fail to register for treatment (also known as “pretreatment loss to follow-up” or “initial default,” used to estimate Gap 3); and (5) the proportion of TB patients who experience TB recurrence or death within 12–24 mo after treatment completion (Gap 5). The PRISMA checklist and methods for each of these systematic reviews are presented in detail in S1–S4 Texts.
Analytical Approach
We organize TB patients registered in the RNTCP into the following categories: (1) new smear-positive, (2) new smear-negative, (3) new extrapulmonary, (4) retreatment smear-positive, (5) retreatment smear-negative, and (6) multidrug-resistant (MDR). We define “retreatment smear-positive” patients as including patients falling into the categories of treatment after failure (TAF), treatment after default (TAD), and smear-positive relapse (i.e., patients with TB recurrence) in the TB India reports. We define retreatment smear-negative patients as consisting of patients listed in the category of “retreatment other” in the TB India reports, based on a recent study which found that the vast majority of “retreatment other” patients consist of smear-negative patients with a history of prior TB [22]. For some forms of TB, no data were available from the WHO reports, the TB India reports, or the published literature to estimate a few of the gaps in the cascade. When reasonable, we extrapolated from estimates of the same gap for other forms of TB. In general, we made conservative assumptions that favor better outcomes for the health system.
In 2012, the Government of India issued an order for mandatory private sector notification of TB patients facilitated by a web-based application called E-NIKSHAY. Private sector participation in E-NIKSHAY is low but slowly gaining momentum [26,27]; our study therefore reflects a time period before a substantial increase in private sector notifications, when they had been historically very low. Only 38,596 TB patients were notified to the government by the private sector in 2013 [16], and, since treatment outcome data for these patients are not available, we exclude them from the cascade.
For many of the steps and gaps, uncertainty in the estimated number of patients has been represented using 95% confidence intervals (95%CIs) when appropriate. Most of these 95%CIs derive from the meta-analysis or pooled prevalence estimates that inform specific cascade steps, based on studies identified through the systematic reviews. When appropriate, we have been careful to propagate uncertainty in situations in which we were calculating a chain of estimates involving multiple proportions or numerical estimates, each with its own confidence interval. In such cases, we take the uncertainty of both values into account by multiplying the upper and lower bounds of the confidence interval of the first value by the upper and lower bounds of the confidence interval of the second value in a manner that produces the widest possible confidence interval for the new value.
In addition, when calculating the “gaps” in the cascade, we subtract the upper bound of the confidence interval for a step from the lower bound of the confidence interval for the preceding step to estimate the most conservative lower bound of the confidence interval for the gap between those steps. Similarly, we subtract the lower bound of the confidence interval for a step from the upper bound of the confidence interval for the preceding step to estimate the most conservative upper bound of the confidence interval for the gap between those steps. The sources of uncertainty for the estimates of specific steps in the TB cascade of care are summarized in Table 3.
Tab. 3. Sources of uncertainty for each step in the TB cascade of care in India in 2013. 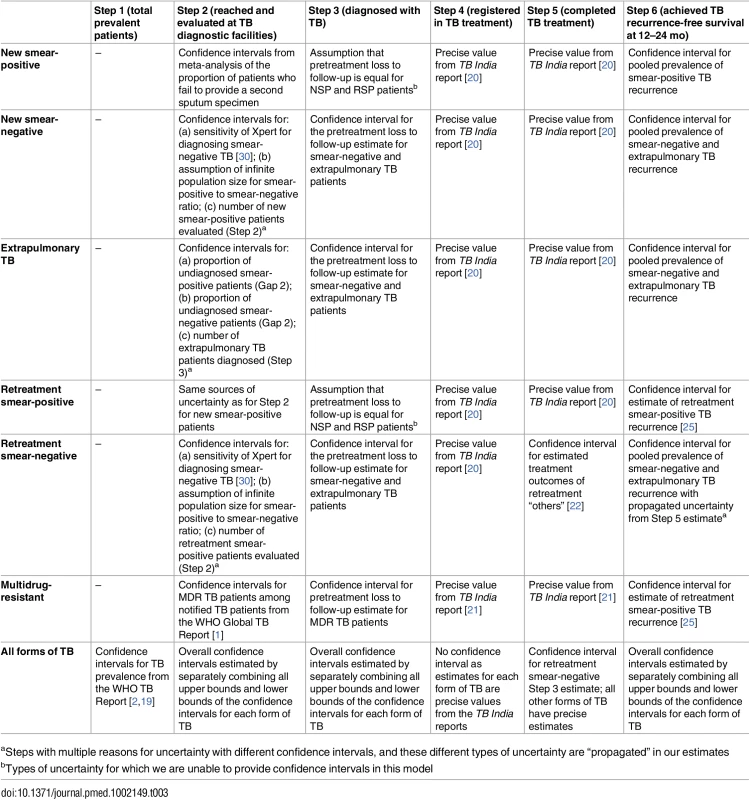
aSteps with multiple reasons for uncertainty with different confidence intervals, and these different types of uncertainty are “propagated” in our estimates Estimating Steps and Gaps in the TB Cascade of Care
Detailed explanations of the assumptions and calculations for every step and gap in the TB cascade of care are included in S5 Text. We summarize the methods used to estimate every step and gap below.
Accessing care
Step 1: We used the revised 2013 WHO prevalence estimate of the overall number of TB patients in India as the starting step of the cascade [2,19] because TB prevalence is estimated partly from population-based surveys. In contrast, estimates of incidence may be more uncertain because they are extrapolated from the number of TB patients notified to the government [28]. Also, TB incidence estimates exclude some types of retreatment TB patients (specifically “failure” and “treatment after default”); these types of TB are relevant to the overall cascade, since such patients require ongoing or modified treatment and may continue to transmit TB.
Gap 1a: The proportion of prevalent TB patients with no access to government TB facilities is difficult to estimate based on existing data. As per the RNTCP, universal district-level coverage with directly observed therapy (DOT) centers was achieved in 2006 [29]; however, recent studies suggest that some subpopulations, especially remote Adivasi (“tribal”) populations, continue to have poor access to government TB services [31]. Unfortunately, national-level data are not available to help quantify this gap, so we are not able to separate Gap 1a from Gap 1b. As a result, Gaps 1a and 1b are presented as a single gap in the cascade. We assume that Gaps 1a and 1b can be estimated together as the difference between Step 1 (total prevalent patients) and Step 2 (the number of TB patients seen at diagnostic facilities) (S5 Text).
Gap 1b: The proportion of patients with access to government TB services who never seek care at these facilities (and remain without care or in private sector care) is also difficult to estimate using existing data. However, there are multiple population-based studies assessing the proportion of people with cough >2–3 wk (i.e., people with suspected TB or “chest symptomatics”) who had not sought medical evaluation at the time of the survey.
This indicator has limitations, since the behavior of chest symptomatics may not fully reflect the behavior of patients who actually have TB, because chronic cough could be secondary to other causes, including chronic obstructive pulmonary disease or other chronic infections. About 5%–10% of patients in India with chronic cough screened at government microscopy facilities and about 2%–5% in community surveys have a positive sputum specimen for TB, which suggests that cough for >2 wk has relatively low positive predictive value for TB [20,32–36]. Screening for cough is also not completely sensitive for pulmonary TB, as a minority of pulmonary TB patients do not have cough but instead have other symptoms such as chest pain, hemoptysis, or fever. In addition, not having sought care at the time of the survey does not necessarily mean that these individuals with suspected TB will never seek care.
Despite these limitations, we systematically reviewed and meta-analyzed studies of care-seeking by chest symptomatics (S2 Text), because these findings still provide some value in understanding TB care-seeking behavior at the population level. However, we did not use the findings of this meta-analysis to calculate Gap 1b. Instead, Gaps 1a and 1b were estimated as the difference between Steps 1 and 2 as noted above (S5 Text).
Step 2 and Gap 2: We estimated Step 2 (the number of TB patients who reach and are evaluated at RNTCP diagnostic facilities) for all forms of TB except MDR TB through back-calculation from estimates for Step 3 (the number of TB patients who are successfully diagnosed). For all forms of TB, we then estimated Gap 2 (the number of patients evaluated at TB diagnostic facilities who remain undiagnosed) as the difference between the estimates for Step 2 and Step 3 (S5 Text).
To estimate Step 2 for new and retreatment smear-positive patients, we assumed that these patients may remain undiagnosed despite being initially evaluated at a diagnostic microscopy center if they are lost to follow-up before providing a second sputum specimen. We therefore estimated the proportion of patients who fail to provide a second sputum specimen through a meta-analysis of previous studies in India (Table F in S3 Text, and Results section, below). We then estimated the number of new and retreatment smear-positive patients who might have remained undiagnosed because they did not provide a second sputum specimen, by extrapolating from findings of a previously published meta-analysis that estimated a 11.9% incremental diagnostic yield of a second sputum specimen for diagnosing smear-positive TB (confidence intervals for this value are not provided in that manuscript) [37].
Based on these two values, we estimated that 1.3% (95%CI: 0.8%–1.8%) of smear-positive patients presenting to TB diagnostic facilities may remain undiagnosed because some patients fail to provide a second sputum specimen for diagnostic evaluation. Using this proportion for Gap 2, we back-calculated an estimate for Step 2 from Step 3 for both new smear-positive and retreatment smear-positive patients (S5 Text).
We estimated Step 2 for new smear-negative and retreatment smear-negative patients as follows: There are multiple reasons why smear-negative TB patients may remain undiagnosed despite presenting to a government TB diagnostic facility. Due to lack of mycobacterial culture and polymerase chain reaction (PCR)-based diagnostic tests such as Xpert MTB/RIF at most facilities, smear-negative TB patients are diagnosed empirically using a multistep workup that includes a trial of broad-spectrum antibiotics and a chest X-ray (Fig B in S3 Text). Studies identified in our systematic review highlight dramatic attrition of smear-negative TB suspects during each step of this workup (Table G in S3 Text) [38–40]; however, none of these studies evaluated this diagnostic algorithm against a gold standard diagnosis of smear-negative TB using mycobacterial culture. Therefore, it is unclear what proportion of “true” smear-negative TB patients fail to get diagnosed.
We therefore used an alternative approach to estimate Step 2 for smear-negative patients. Historical data suggest that the proportion of new smear-positive to new smear-negative TB patients is 1 : 1 [41]; the RNTCP also cites this ratio in its training manual [42]. However, we estimate a more conservative 1.42 : 1 (95%CI: 1.26 : 1 to 1.59 : 1) ratio of smear-positive to smear-negative TB patients, based on data from the recent study evaluating the implementation of Xpert at 18 sites in India (S5 Text) [24].
Using this ratio, we back-calculated the expected number of new smear-negative TB patients being evaluated at diagnostic microscopy facilities (Step 2) from the estimated number of new smear-positive patients being evaluated at these facilities (Step 2) (S5 Text). We also used this ratio to back-calculate the number of retreatment smear-negative patients in Step 2 from the number of retreatment smear-positive patients in Step 2.
To estimate Step 2 for extrapulmonary TB patients, there are no studies we could identify that estimate the proportion of extrapulmonary patients evaluated at RNTCP diagnostic centers who fail to get appropriately diagnosed. One local study suggests that the substantial majority of extrapulmonary TB in India’s public sector is being diagnosed clinically without collection of diagnostic samples for mycobacterial stain, culture, or histopathology [43]. Of the most common forms of extrapulmonary TB, lymphadenitis is relatively easy to diagnose clinically. Diagnosis of TB pleuritis is facilitated by a chest X-ray, and this imaging study may not be available at many RNTCP diagnostic facilities, especially in rural areas [40]. Other forms of TB are considerably more difficult to diagnose without invasive tests.
We therefore assumed that extrapulmonary TB is more challenging to diagnose than smear-positive pulmonary TB, which by definition is easily diagnosable by sputum microscopy. We also conservatively assumed that extrapulmonary TB is easier to diagnose than smear-negative pulmonary TB, as some forms of extrapulmonary TB are more clinically evident (e.g., lymphadenitis) or serious enough to warrant hospitalization (e.g., meningitis). This assumption biases our estimates towards better outcomes for the RNTCP. We therefore estimated the proportion of extrapulmonary TB patients who remain undiagnosed despite presenting to a TB diagnostic facility by taking the average of the proportion of undiagnosed smear-positive TB patients and the proportion of undiagnosed smear-negative TB patients (S5 Text). This estimate for the proportion of undiagnosed extrapulmonary TB patients allowed us to estimate Step 2 for extrapulmonary TB by back-calculation from Step 3 (S5 Text).
To estimate Step 2 for MDR TB patients, we used the WHO estimate of the number of MDR TB patients among notified pulmonary TB patients in 2013 [1]. This estimate is derived from culture-based studies conducted at RNTCP sites in India that estimate the prevalence of MDR TB patients among both new and retreatment patients. In other words, this figure probably provides a relatively accurate estimate of the number of MDR TB patients who undergo diagnostic evaluation at RNTCP microscopy centers. This estimate is not the same as the number of MDR TB patients who are actually diagnosed in the public sector. Because most RNTCP diagnostic facilities still primarily use smear microscopy, many of these patients are not diagnosed with MDR TB and are instead misclassified as new or retreatment TB patients.
We also cross-checked this WHO MDR TB estimate using data from the recent study assessing the pilot implementation of Xpert MTB/RIF at 18 geographically diverse sites in India [24]. This study found that the proportion of TB suspects diagnosed with rifampin-resistant TB, which is presumed to be a marker of MDR TB, increased by 5.82 times between the baseline phase (when Xpert was not being used, but sputum smears were used with drug-susceptibility testing only on high-risk patients) and the intervention phase (when Xpert was used upfront for diagnosis and drug-susceptibility testing of all patients with suspected TB). In that study, 31 out of 10,907 diagnosed TB patients were found to have drug resistance at baseline compared to 1,190 out of 71,754 diagnosed TB patients after implementation of Xpert for upfront screening. Correcting this ratio for the fact that Xpert is only 67% sensitive for diagnosing smear-negative TB [30], we estimate a 6.89 times rate of underdiagnosis of true MDR TB patients evaluated at the vast majority of RNTCP diagnostic facilities relying on smear microscopy alone.
Since there were 9,466 (95%CI: 9,321–9,629) total MDR TB patients diagnosed nationwide in the RNTCP in 2012 (the year the Xpert study was conducted), we multiply this value by 6.89 to estimate that 65,221 (95%CI: 64,221–66,343) true MDR TB patients reached RNTCP diagnostic centers and underwent diagnostic evaluation for TB. Notably, this estimate is very similar to the WHO’s estimate of 64,000 MDR TB patients among all notified pulmonary TB patients in India in 2012 and 61,000 MDR TB patients in 2013. We therefore used the 2013 WHO estimate for Step 2 of the MDR TB cascade.
Diagnosis and registration in treatment
Step 3 and Gap 3: To estimate Step 3 (the number of patients diagnosed with TB) for new smear-positive and retreatment smear-positive TB patients, we extracted the total number smear-positive patients diagnosed in 2013 from the 2014 TB India report [16]. This value does not separate new smear-positive and retreatment smear-positive patients. We therefore assumed that the proportion of new smear-positive and retreatment smear-positive patients among all smear-positive patients diagnosed in Step 3 is the same as the proportion of new smear-positive and retreatment smear-positive patients among all smear-positive patients registered in treatment in Step 4. Based on this assumption, we estimated that 77.6% of all diagnosed smear-positive patients are “new” and 22.4% of all diagnosed smear-positive patients are “retreatment,” which allows us to estimate the number of new smear-positive and retreatment smear-positive patients in Step 3 (S5 Text).
We then estimated Gap 3 (pretreatment loss to follow-up) for new smear-positive and retreatment smear-positive patients by calculating the difference between estimates for Step 3 and Step 4 [16,20]. Estimating pretreatment loss to follow-up in this manner may have shortcomings. It is possible that some patients might submit sputum specimens at multiple microscopy centers, which would lead to overestimation of Gap 3. Alternatively, some patients may migrate or transfer to other centers and get re-registered in care, which could underestimate Gap 3. For this reason, we also crosscheck this estimate by independently estimating the rate of pretreatment loss to follow-up for smear-positive patients through a systematic review and meta-analysis of studies (Table H in S3 Text and results section below).
We could find no studies of pretreatment loss to follow-up among new smear-negative, extrapulmonary, or retreatment smear-negative patients to help inform our estimate of Step 3 and Gap 3 for these forms of TB; however, we estimated Step 3 and Gap 3 for these forms of TB based on our findings on smear-positive pretreatment loss to follow-up. As with smear-positive patients, most patients with these other forms of TB are diagnosed at government TB microscopy centers. After diagnosis, they are referred to directly observed therapy (DOT) centers close to their homes to start treatment. The studies of smear-positive pretreatment loss to follow-up identified in this systematic review suggest that most patients are lost to follow-up during the process of referral from government TB microscopy centers to DOT centers. Specifically, three of the studies in the review provide specific information on patient losses during the referral process, and we used the findings of these studies to estimate the proportion of all pretreatment loss to follow-up patients who are lost during the referral process [44–46].
Based on the overall smear-positive pretreatment loss to follow-up rate, we then estimated the proportion of all smear-positive patients who experience pretreatment loss to follow-up during the referral process. Since most new smear-negative, extrapulmonary, and retreatment smear-negative TB patients also undergo this referral process, we conservatively estimated that the pretreatment loss to follow-up rate (Gap 3) for these patients is the same as the proportion of smear-positive patients experience pretreatment loss to follow-up during the referral process. While some new smear-negative, extrapulmonary, and retreatment smear-negative patients will also be lost to follow-up prior to referral, we assumed that most of these patient losses will be captured in Gap 2 for these forms of TB (loss to follow-up during the diagnostic workup). Using this estimate for Gap 3, we were able to back-calculate Step 3 from Step 4 for new smear-negative, extrapulmonary, and retreatment smear-negative patients.
To estimate Step 3 and Gap 3 for MDR TB patients, we identified two studies that describe the pretreatment loss to follow-up rate (Gap 3) for patients diagnosed with MDR TB through our systematic review (Table H in S3 Text) [47,48]. We used the pooled prevalence of these findings to estimate Step 3 by back-calculation from Step 4.
Step 4: For all forms of TB, we estimated the number of patients registered in treatment in the RNTCP (Step 4) using values reported in the TB India reports [20,21].
Retention during the treatment course
Gap 4: Gaps 4a (loss to follow-up or death during the intensive phase of therapy) and 4b (loss to follow-up, treatment failure, or death during the continuation phase of therapy) could not be estimated separately since the TB India reports do not provide information on the phases of TB therapy during which these poor treatment outcomes occur. We therefore report Gaps 4a and 4b together as a combined Gap 4. We estimated the proportion of patients who died, failed treatment, or were lost to follow-up during treatment by calculating the difference between Step 4 and Step 5 using data from the TB India reports [16,20].
Step 5: We estimated Step 5 (the number of patients who achieved cure or treatment completion) using data for each form of TB from the TB India reports [16,20]. This estimation includes all forms of TB except for retreatment smear-negative patients. For retreatment smear-negative patients, we estimated Step 5 based on findings from the only Indian study reporting treatment outcomes for these patients, which describes a retreatment smear-negative treatment completion rate of 83.2% (95%CI: 81.0%–85.2%) [22].
Recurrence-free survival
Step 6 and Gap 5: The final outcome of the cascade—the number of patients who are recurrence-free 12 mo after completing TB treatment (Step 6)—was calculated by extrapolating from the estimates for Step 5 (the number of patients who achieve treatment completion) using estimates for Gap 5 (the proportion of patients who experience post-treatment recurrence or death within 12–24 mo) for each form of TB.
We estimated these proportions for Gap 5 based on a systematic review of local studies conducted in India’s public sector (Table K in S4 Text, and Results section, below). We estimated Gap 5 based on the pooled prevalence of findings from two studies of new smear-positive TB patients [14,25], four studies of new smear-negative and extrapulmonary TB patients [49–52], and one study of retreatment smear-positive patients [25]. We assumed that the proportion of patients who experience TB recurrence or death for retreatment smear-negative patients is the same as that for new smear-negative patients, given that these forms of TB have very similar treatment outcomes [22]. Since MDR TB patients have poor treatment outcomes, we made a conservative assumption that their rate of post-treatment TB recurrence and death is similar to that of retreatment smear-positive patients, who have the highest recurrence risk of any form of TB described in our systematic review.
Results
Systematic Reviews Informing Steps and Gaps in the TB Cascade
Chest symptomatics who do not get evaluated at TB diagnostic facilities (Gap 1b)
In the systematic review of care-seeking by people in the community with cough >2 wk (i.e., chest symptomatics), out of 1,679 abstracts found by searching the literature from between January 1, 2000, and October 1, 2015, we identified 219 eligible for full-text review, out of which 8 contain relevant data for extraction (Fig A in S2 Text). All eight studies evaluate the proportion of chest symptomatics who had not seen any provider by the time of the survey [32,53–59]; seven report whether patients sought care from public sector or private sector providers [32,53–56,58,59]; and three report the proportion who had not been screened for TB with a sputum test (Table C in S2 Text) [32,54,58].
Seven studies were conducted in 5 of India’s 36 states [32,53–56,58,59]. In addition, one study collected data from households in 30 districts located in numerous states [57]. Some of India’s poorest states with a high population, such as Uttar Pradesh and Madhya Pradesh, were included. Three studies were conducted in urban areas, three in rural areas, and two in both rural and urban areas (Table C in S2 Text). All of the studies are high quality with regard to sampling strategy and sample size; however, six of the eight studies did not report the proportion of people screened during their population-based data collection (low quality for this criterion), and six studies did not report the proportion of chest symptomatics identified who completed an interview (low quality) (Table C in S2 Text).
Meta-analysis of the eight studies estimating the proportion of individuals in the community with cough >2 wk who report not having visited any medical provider after the onset of cough show that, overall, 39% (95%CI: 30%–49%) of chest symptomatics had not seen any medical provider (Fig 2). Meta-analysis of the seven studies estimating the proportion of individuals with cough >2 wk who report not having visited a public sector provider shows that, overall, 76% (95%CI: 69%–82%) of chest symptomatics had not seen a public sector provider (Fig 3). Meta-analysis of the proportion of individuals with cough >2 wk who report not having visited a private sector medical provider shows that, overall, 65% (95%CI: 60%–70%) of chest symptomatics had not seen a private sector provider (Fig 4). Three studies reported the proportion of patients who had not received screening with sputum microscopy; these studies found that 91%–97% of chest symptomatics had not been screened with sputum microscopy at the time of the survey, even if they had been evaluated by a medical provider (Table C in S2 Text).
Fig. 2. Forest plot of studies estimating the proportion of individuals in the community with cough >2 wk who report not having visited any medical provider after the onset of cough (Gap 1b). 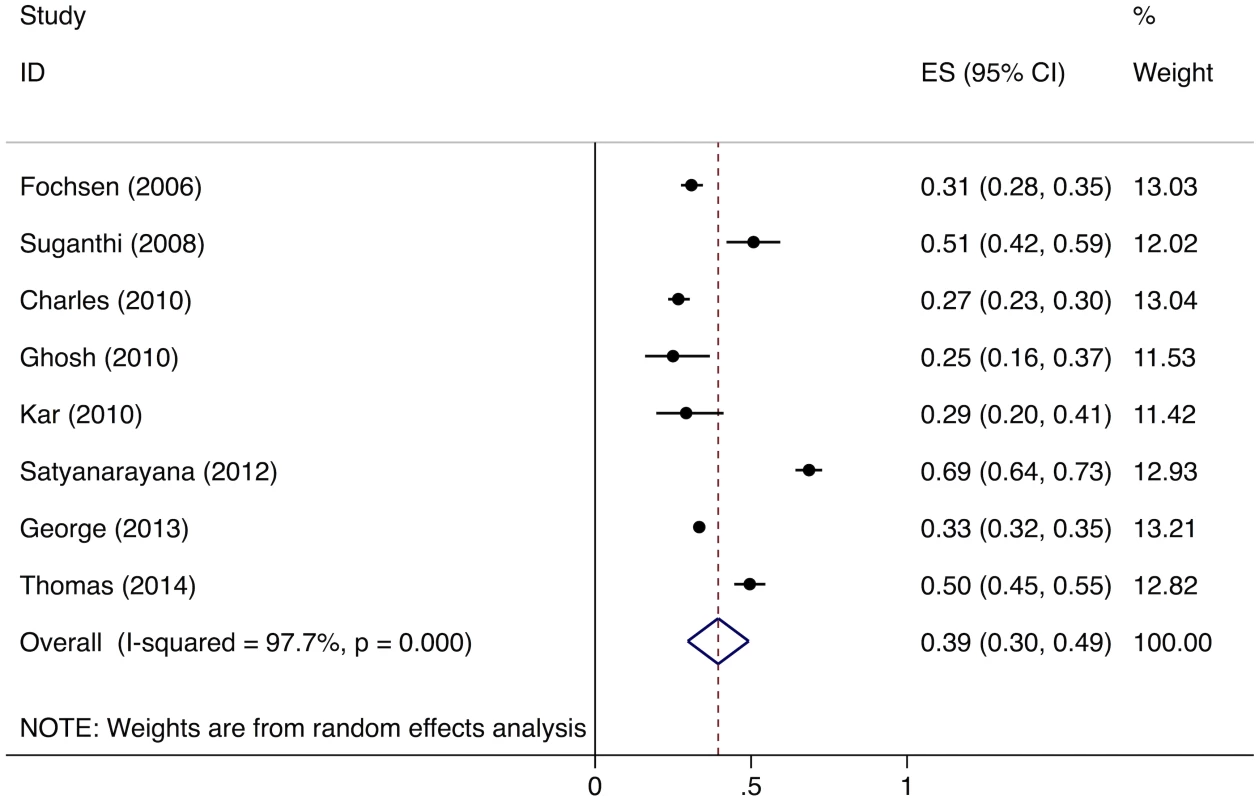
ES, effect size; CI, confidence interval. Fig. 3. Forest plot of studies estimating the proportion of individuals in the community with cough >2 wk who report not having visited a public sector provider after the onset of cough (Gap 1b). 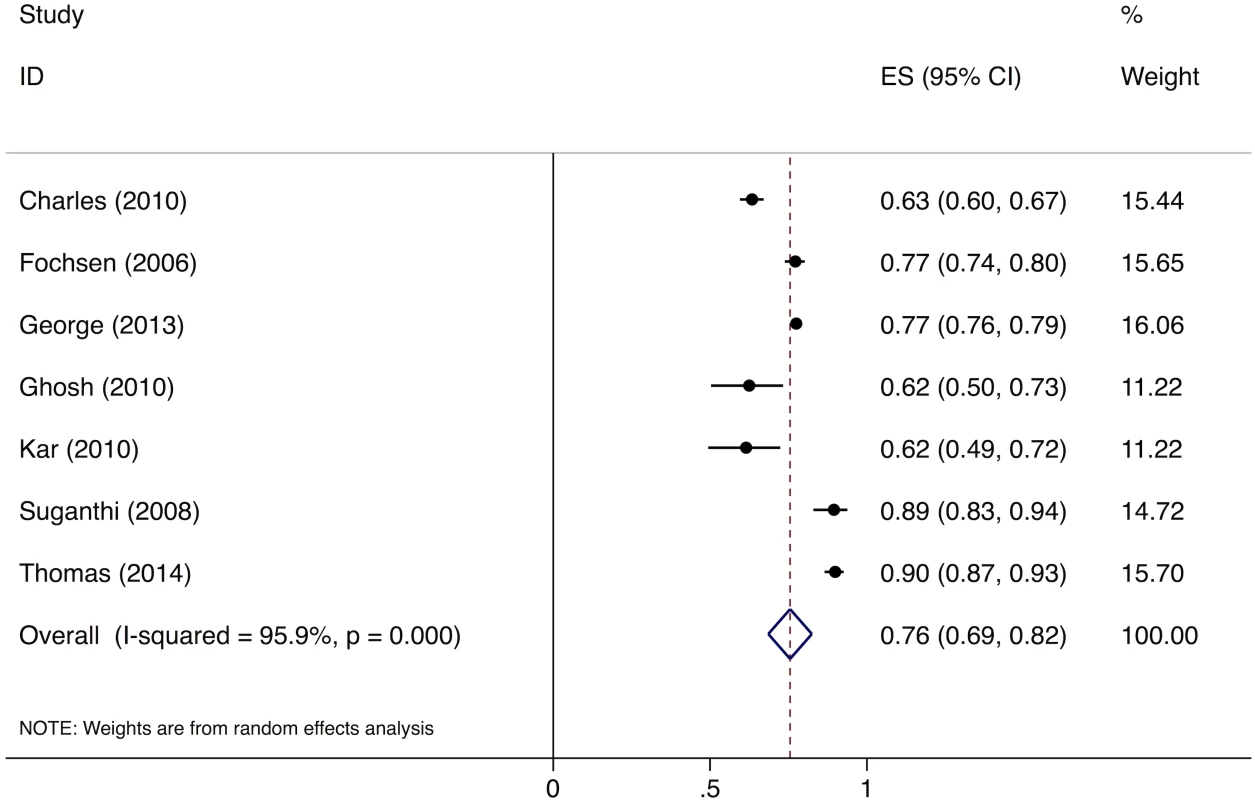
ES, effect size; CI, confidence interval. Fig. 4. Forest plot of studies estimating the proportion of individuals in the community with cough >2 wk who report not having visited a private sector provider after the onset of cough (Gap 1b). 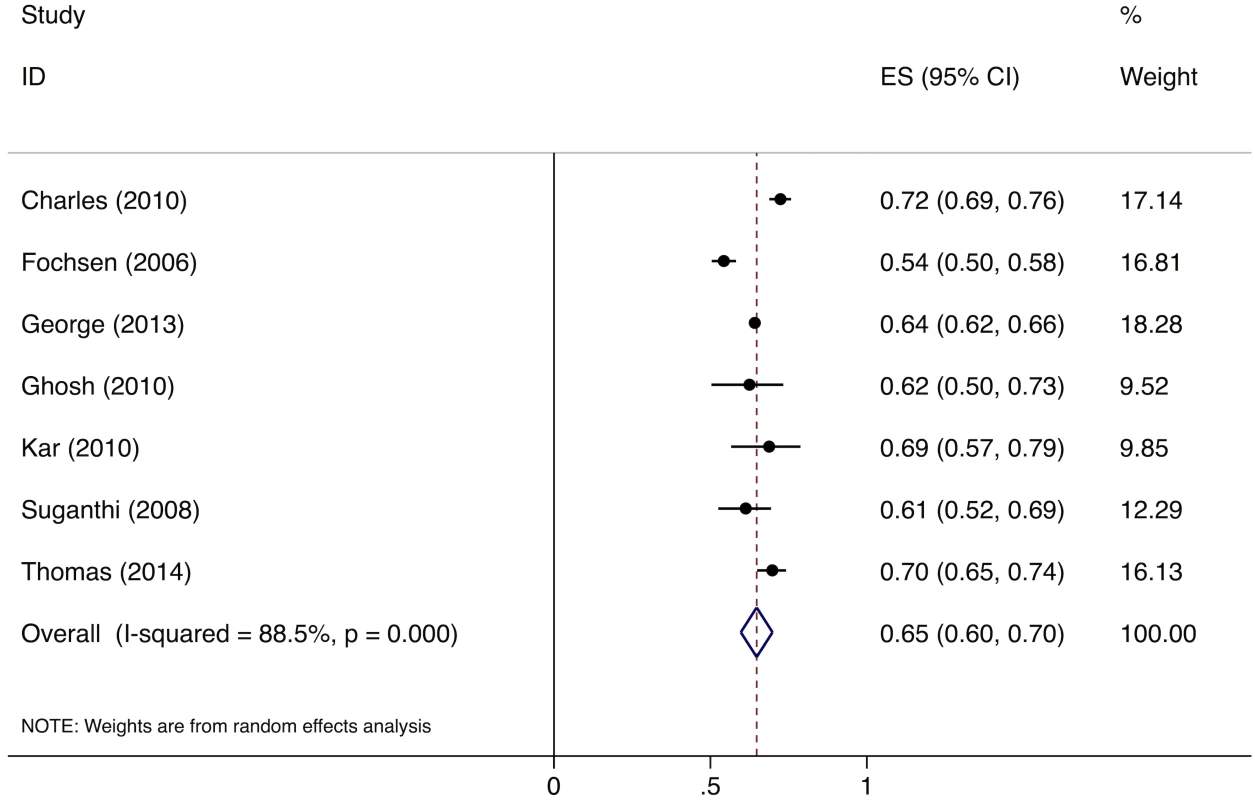
ES, effect size; CI, confidence interval. TB patients evaluated at diagnostic facilities who are not successfully diagnosed (Gap 2)
In the systematic review of studies of TB patients evaluated at diagnostic facilities who fail to get diagnosed with TB, out of 1,704 abstracts found by searching the literature from between January 1, 2000, and February 26, 2015, we identified 26 eligible for full-text review (Fig C in S3 Text). Of these, six studies contain relevant data on the proportion of chest symptomatics who failed to provide a second sputum sample [23,38,60–63]. Three studies contain data on the proportion of patients undergoing evaluation for smear-negative TB who fail to get a chest X-ray, which is the final step in the diagnostic workup [38–40].
The six studies describing the proportion of patients who failed to provide a second sputum sample were conducted in 7 out of India’s 36 states, with two studies focused solely on urban populations, two studies focused solely on rural populations, and two studies focused on both (Table F in S3 Text). The three studies describing the proportion of patients who completed the diagnostic workup for smear-negative TB were conducted in three different states, with one study conducted solely in a rural location and two studies conducted in both urban and rural locations (Table G in S3 Text).
For the studies evaluating the proportion of patients who failed to submit two sputum specimens, one study was medium quality because it was conducted at only one microscopy center at an urban tertiary care hospital; the studies were high quality on other indicators (Table F in S3 Text). One of the studies evaluating loss to follow-up during the smear-negative diagnostic workup was excluded due to a small sample size of <150 patients (Table G in S3 Text) [38].
The meta-analysis of the six studies estimating the proportion of patients who fail to provide a second sputum sample shows that, overall, 11% (95%CI: 7%–15%) of chest symptomatics fail to provide a second sputum sample (Fig 5). Since there were fewer than five studies evaluating the proportion of patients who fail to complete the workup for smear-negative TB, we did not conduct a formal meta-analysis; however, these studies suggest that a large proportion (61%–80%) of TB suspects fail to complete the multistep diagnostic workup for smear-negative TB (Table G in S3 Text) [38–40].
Fig. 5. Forest plot of studies estimating the proportion of patients who fail to provide a second sputum smear (i.e., “diagnostic default”), which allows estimation of the proportion of smear-positive TB patients who might be “missed” at TB diagnostic facilities (Gap 2). 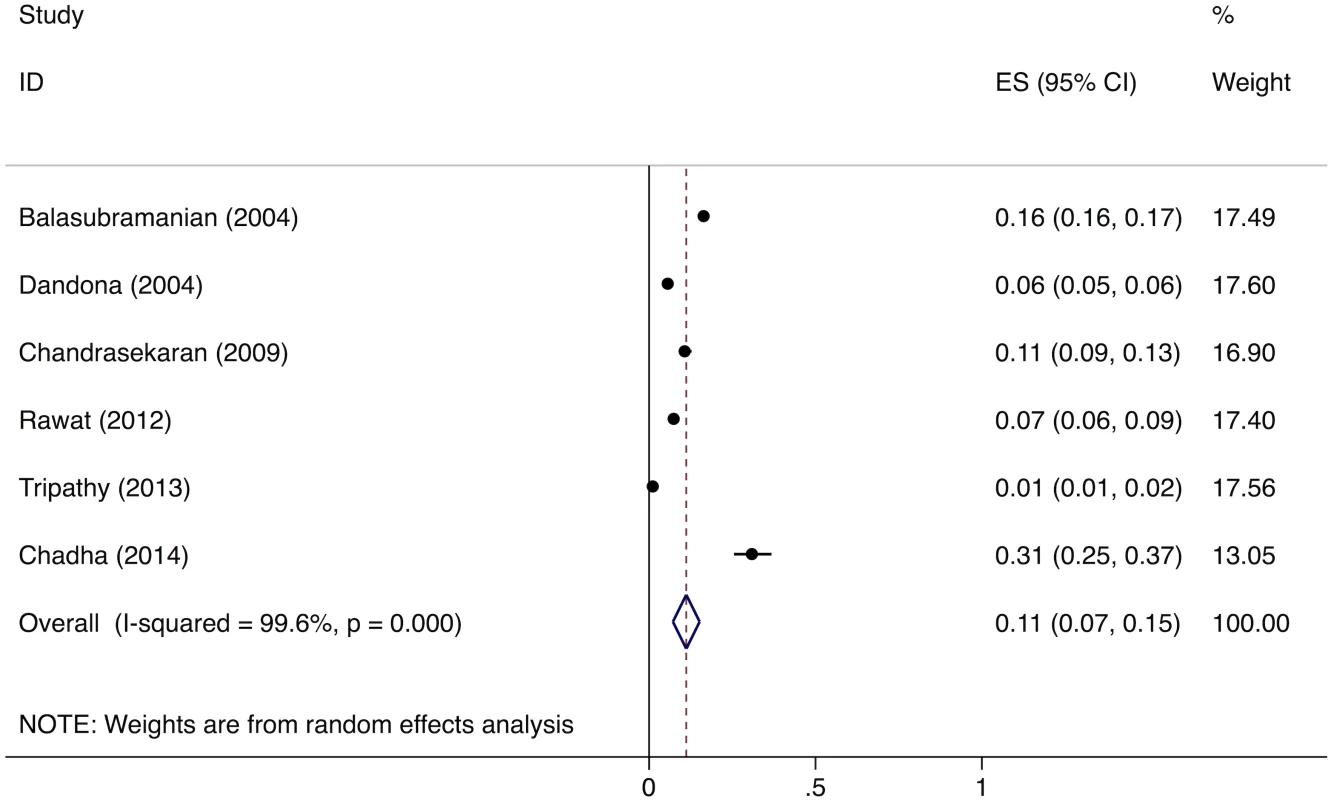
ES, effect size; CI, confidence interval. Pretreatment loss to follow-up of smear-positive patients (Gap 3)
In the systematic review of studies of TB patients who get appropriately diagnosed but fail to get registered in treatment (i.e., pretreatment loss to follow-up), out of 1,704 abstracts found by searching the literature from between January 1, 2000, and February 26, 2015, we identified 26 studies eligible for full-text review (Fig C in S3 Text). Of these, 14 contain relevant data on pretreatment loss to follow-up of smear-positive TB patients [44–46,60,62–71], and two studies contain data on pretreatment loss to follow-up of MDR TB patients [47,48].
The studies of pretreatment loss to follow-up of smear-positive patients were conducted in 10 different Indian states. Four of these studies were conducted solely in rural areas; four were conducted solely in urban areas; and six were conducted in both (Table H in S3 Text). The studies of pretreatment loss to follow-up of MDR TB patients were conducted in both urban and rural settings in two different states (Table H in S3 Text).
Of all 16 pretreatment loss to follow-up studies, four are medium quality for sample selection, because they collected data from single microscopy centers, potentially limiting their representativeness at the local level (Table H in S3 Text). In addition, some studies were low quality for study methodology (five studies) and time frame of evaluation (11 studies) (Table H in S3 Text).
The meta-analysis of the 14 studies of smear-positive patients shows an overall pretreatment loss to follow-up rate of 16% (95%CI: 12%–20%) (Fig 6). Three of these studies provide specific information on the proportion of pretreatment loss to follow-up patients who are lost during the referral process. These studies report that 98/120 (81.6%) [44], 62/63 (98.4%) [45], and 75/145 (51.7%) [46] of pretreatment loss to follow-up among smear-positive patients occurred during referral, for a pooled prevalence of 235/328 (71.6%, 95%CI: 66.5%–76.3%). The pooled prevalence of the two studies of MDR TB patients shows a pretreatment loss to follow-up rate of 23.0% (95%CI: 21.8%–24.3%) (Table H in S3 Text).
Fig. 6. Forest plot of studies estimating pretreatment loss to follow-up (i.e., “initial default”) of smear-positive TB patients in India (Gap 3). 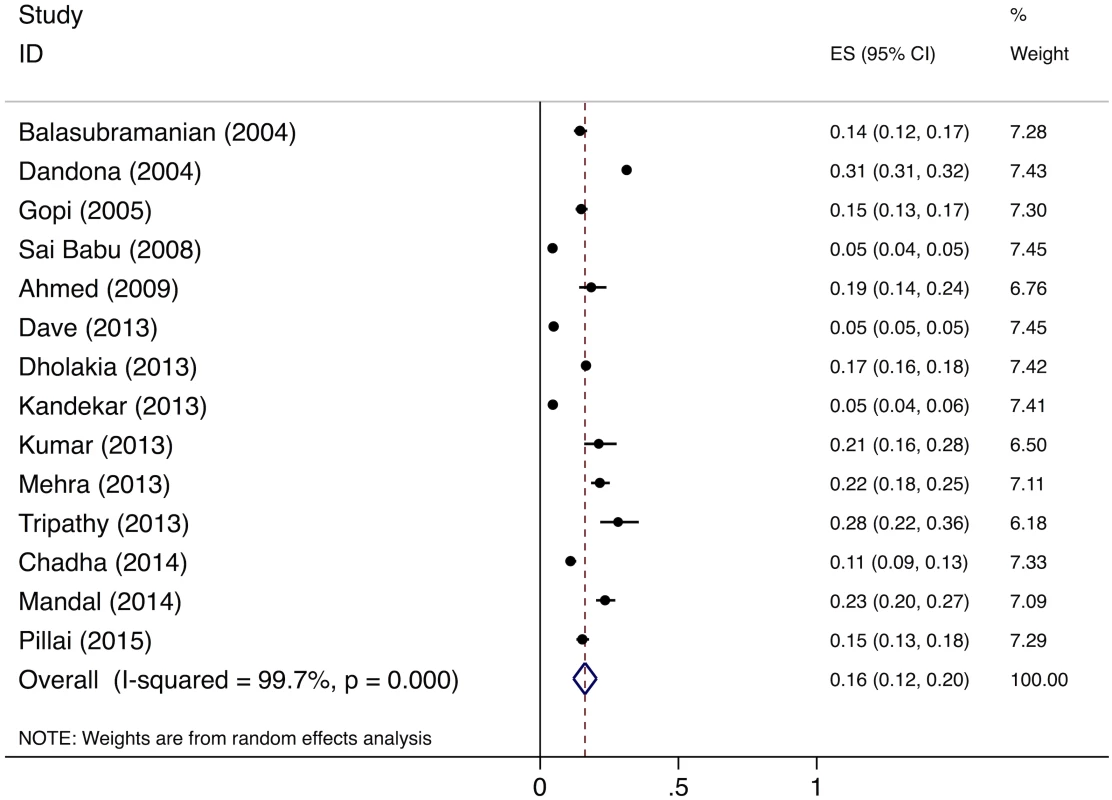
ES, effect size; CI, confidence interval. Post-treatment TB recurrence and death (Gap 5)
In the systematic review of studies evaluating post-treatment TB recurrence and death, out of 2,547 short-listed abstracts found by searching the literature from between January 1, 2000, and October 9, 2015, we identified 30 studies eligible for full-text review (Fig D in S4 Text). Of these, 10 contain data on post-treatment TB recurrence or death in the RNTCP. Three of the studies evaluated predominantly HIV-negative smear-positive pulmonary TB patients who completed category I therapy; three evaluated HIV-infected smear-positive pulmonary TB patients who completed category I therapy; four studies evaluated a mixed population of smear-negative pulmonary, smear-positive pulmonary, and extrapulmonary TB patients who completed category I or III therapy; and one study evaluated retreatment smear-positive TB patients who completed category II therapy (Table K in S4 Text).
Studies were conducted in five Indian states. Eight studies were conducted in urban areas and two in rural areas (Table K in S4 Text). All of the studies are high-quality with regard to sampling strategy; however, two of the studies did not report the proportion of the cohort that was lost to follow-up during the study period [72,73]. Two of the studies describing TB outcomes among HIV-infected individuals are very low in quality because they have a sample size of <100 patients (Table K in S4 Text) [51,74].
We did not conduct a formal meta-analysis of these study findings, as none of these groups had five or more eligible studies; however, we estimated the pooled prevalence of relapse and death for these different types of TB from the limited available data. We excluded studies that did not have complete data for both death and TB recurrence and studies that did not have at least 12 mo and no more than 24 mo of follow-up.
For new smear-positive patients, we found two studies and estimate a pooled prevalence of 16.2% (95%CI: 14.25%–18.5%) for the combined outcome of TB recurrence and death [14,25]. For new smear-negative, extrapulmonary, and retreatment smear-negative patients, we found four studies and estimate a pooled prevalence of 8.8% (95%CI: 6.9%–11.1%) for the combined outcome of TB recurrence and death [49–52]. For retreatment smear-positive patients, we estimate a 27.3% (95%CI: 19.1%–37.4%) combined outcome of post-treatment TB recurrence and death from a single study [25]. Notably, HIV-infected TB patients of all types have more variable and often considerably higher rates of post-treatment TB recurrence and death (ranging from 9%–61%) compared to the general TB population [51,52,74,75]. This suggests that HIV co-infected patients may constitute a higher proportion of retreatment patients and poor outcomes in the RNTCP than would be suggested by their 6% prevalence in the general TB population.
The TB Cascade of Care for 2013
Table 4 and Fig 7 present the tuberculosis cascade of care in the government TB program in India for 2013. Of 2,700,000 (95%CI: 1,800,000–3,800,000) prevalent active TB patients in India, we estimate that 1,049,237 (95%CI: 1,008,775–1,083,243), or 38.9%, were successfully treated in the RNTCP and achieved at least 1 y of TB recurrence-free survival. We divide our discussion of estimates for each step and gap into the following sections: accessing care, diagnosis and registration in treatment, retention during the treatment course, and recurrence-free survival.
Fig. 7. The cascade of care for all forms of tuberculosis in India’s Revised National Tuberculosis Control Programme (RNTCP) in India, 2013. 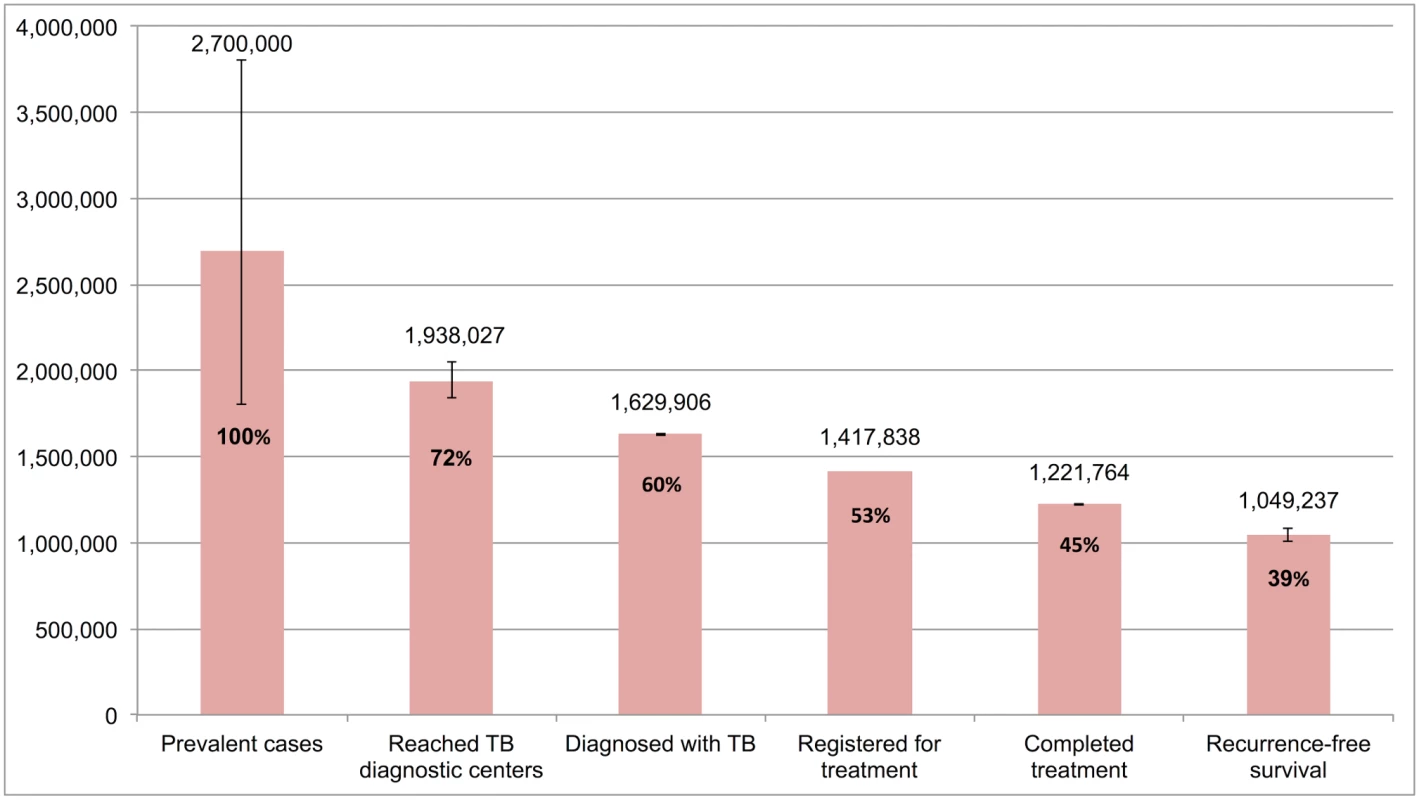
Error bars depict 95% confidence intervals. Tab. 4. Estimates for each step and gap in the cascade of care for different forms of tuberculosis in the Revised National Tuberculosis Control Programme (RNTCP), India, 2013. 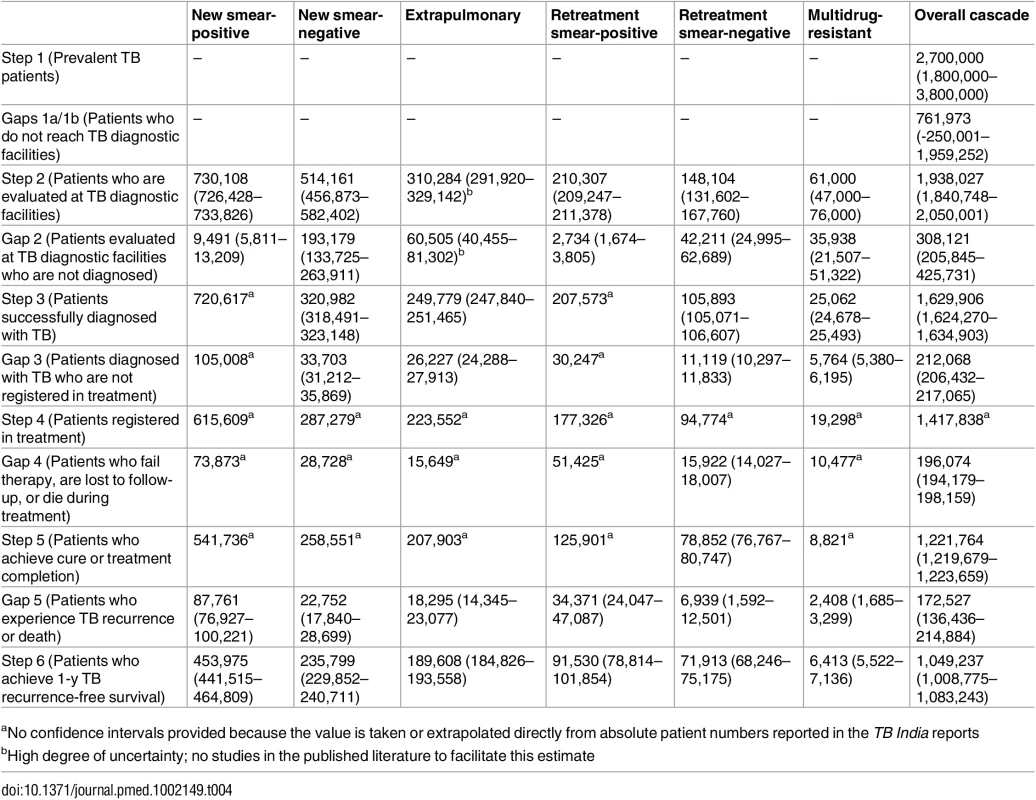
aNo confidence intervals provided because the value is taken or extrapolated directly from absolute patient numbers reported in the TB India reports Accessing care
Of 2,700,000 prevalent active patients with TB (Step 1), we estimate that 1,938,027 (95%CI: 1,840,748–2,050,001), or 71.8%, of TB patients reached and were evaluated at government TB diagnostic facilities (Step 2), based on back-calculation for each form of TB from estimates for Gap 2 and Step 3 (S5 Text). This suggests that about 761,973 (95%CI: -250,001–1,959,252), or 28.2%, of prevalent TB patients did not have access to government TB facilities, never sought TB care, or directly sought TB care in the private sector (Gap 1a/1b).
No data were available to provide further insights into estimates for Gap 1a. For Gap 1b, as described above, the meta-analysis of studies evaluating care-seeking found that 39% (95%CI: 30%–49%) of individuals with cough >2 wk had not seen any medical provider at the time of the survey; 76% (95%CI: 69%–82%) had not seen a public sector provider; and 91%–97% of had not received evaluation for TB with sputum testing (Figs 2–4).
Diagnosis and registration in treatment
We estimated Gap 2—the proportion of TB patients who remain undiagnosed despite being evaluated at government TB diagnostic facilities—separately for each form of TB. For new smear-positive and retreatment smear-positive patients, we estimate that 11% (95%CI: 7%–15%) of people with suspected TB who present to government microscopy centers are lost to follow-up before submitting a second sputum specimen, based on the meta-analysis of six studies described above (Fig 5). Using an estimate of the incremental yield of a second sputum specimen for diagnosing smear-positive TB [37], we then estimate that 1.3% (95%CI: 0.08%–1.8%) of all smear-positive patients, or 9,491 (95%CI: 5,811–13,209) and 2,734 (95%CI: 1,674–3,805) new smear-positive and retreatment smear-positive patients respectively, remained undiagnosed despite presenting to government diagnostic facilities (S5 Text).
For new smear-negative patients, using the 1.42 : 1 (95%CI: 1.26 : 1 to 1.59 : 1) ratio of new smear-positive to new smear-negative TB patients seeking care in the public sector, we estimate that 514,161 (95%CI: 456,873–582,402) new smear-negative TB patients sought evaluation at government diagnostic facilities (Step 2) (S5 Text). Since 320,982 (95%CI: 318,491–323,148) new smear-negative TB patients were successfully diagnosed (Step 3), we estimate that 193,179 (95%CI: 133,725–263,911), or 37.6%, of patients with new smear-negative TB remained undiagnosed despite evaluation at government facilities (Gap 2). This estimate is consistent with the findings of our systematic review, which identified three studies showing that (61%–80%) of TB suspects fail to complete the multistep diagnostic workup for smear-negative TB, likely resulting in considerable under-diagnosis (Table G in S3 Text) [38–40].
For retreatment smear-negative patients, we also used a 1.42 : 1 (95%CI: 1.26 : 1 to 1.59 : 1) ratio of retreatment smear-positive patients to retreatment smear-negative patients to estimate Gap 2 (S5 Text). Of 148,104 (95%CI: 131,602–167,760) retreatment smear-negative patients evaluated at government diagnostic facilities (Step 2), we estimate that 105,893 (95%CI: 105,071–106,607), or 71.5%, were successfully diagnosed (Step 3) and 42,211 (95%CI: 24,995–62,689), or 28.5%, remained undiagnosed (Gap 2).
For extrapulmonary TB patients, we assume that 19.5% (95%CI: 15.1%–23.6%) of patients evaluated at TB diagnostic facilities remain undiagnosed (Gap 2). This suggests that about 310,284 (95%CI: 291,920–329,142) extrapulmonary TB patients were evaluated at TB diagnostic facilities (Step 2), of whom 249,779 (95%CI: 247,840–251,465) were successfully diagnosed (Step 3) and 60,505 (95%CI: 40,455–81,302) remained undiagnosed (Gap 2) (S5 Text).
For MDR TB patients, we use the WHO estimate of 61,000 (95%CI: 47,000–76,000) MDR TB patients among all notified TB patients in the RNTCP as the number of MDR TB patients who underwent evaluation at government TB diagnostic facilities (Step 2). We estimate that 25,062 (95%CI: 24,678–25,493), or 41.1%, of these patients were successfully diagnosed with MDR TB (Step 3), suggesting that 35,938 (95%CI: 21,507–51,322), or 58.9%, remained undiagnosed or got misdiagnosed with other forms of TB (Gap 2).
Aggregating these findings, we estimate that, of 1,938,027 (95%CI: 1,840,748–2,050,001) patients with all forms of TB who presented for evaluation to government TB diagnostic facilities (Step 2), 1,629,906 (95%CI: 1,624,270–1,634,903), or 84.1%, were appropriately diagnosed (Step 3) and 308,121 (95%CI: 205,845–425,731), or 15.9%, remained undiagnosed (Gap 2).
We also produced separate estimates for each form of TB for Gap 3—the proportion of patients diagnosed with TB who do not get registered in treatment in the government TB program (i.e., pretreatment lost to follow-up).
For Gap 3 among smear-positive TB patients, as noted above, the meta-analysis of findings from 14 studies suggests a pretreatment loss to follow-up rate of 16% (95%CI: 12%–20%) (Fig 6 and Table H in S3 Text). This finding is concordant with our Gap 3 estimate using data from the TB India reports (S5 Text) [16,20]. In that analysis, 928,190 new and retreatment smear-positive TB patients were diagnosed in 2013 (Step 3) and 792,935 (85.4%) new and retreatment smear-positive patients were registered in treatment (Step 4), suggesting there were 135,255 (14.6%) pretreatment loss to follow-up patients (Gap 3). Since this estimate is within the confidence interval of the meta-analysis estimate, we use the more conservative Gap 3 estimate from the TB India reports in the TB cascade.
For Gap 3 among new smear-negative TB patients, using the 10.5% (95%CI: 9.8%–11.1%) rate of pretreatment loss to follow-up, we estimate that 320,982 new smear-negative TB patients were diagnosed (Step 3). Since 287,279 new smear-negative patients were registered in treatment (Step 4), we estimate that 33,703 (95%CI: 31,212–35,869) were diagnosed but did not get registered in treatment (Gap 3) (S5 Text). Using the same rate of pretreatment loss to follow-up for extrapulmonary TB patients, we estimate that 249,779 patients were diagnosed (Step 3). Since 223,552 extrapulmonary TB patients were registered in treatment (Step 4), we estimate that 26,227 (95%CI: 24,288–27,913) were diagnosed but did not get registered in treatment (Gap 3) (S5 Text). Using the same rate of pretreatment loss to follow-up for retreatment smear-negative patients, we estimate that 105,893 patients were diagnosed (Step 3). Since 94,774 retreatment smear-negative patients were registered in treatment (Step 4), we estimate that 11,119 (95%CI: 10,297–11,833) were diagnosed but did not get registered in treatment (Gap 3) (S5 Text).
For MDR TB patients, using the Gap 3 estimate of 23.0% (95%CI: 21.8%–24.3%) from the meta-analysis, we estimate that 25,062 (95%CI: 24,678–25,493) MDR TB patients were diagnosed in 2013 (Step 3), of whom 19,298 (77.0%) got registered in treatment (Step 4).
Aggregating these estimates, we estimate that 1,629,906 (95%CI: 1,624,270–1,634,903) patients with all forms of TB were diagnosed in 2013 (Step 3), but only 1,417,838 (87.0%) patients ultimately got registered in treatment (Step 4), which suggests that 212,068 (95%CI: 206,432–217,065), or 13.0%, of all TB patients in India were diagnosed at government TB diagnostic facilities but lost to follow-up prior to starting treatment (Gap 3).
Retention during the treatment course
The numbers of new smear-positive, new smear-negative, extrapulmonary, and MDR TB patients who completed treatment were extracted from the TB India reports, and the estimates for Step 4, Gap 4, and Step 5 for these forms of TB are presented in Table 4 [20]. For retreatment smear-negative patients, one study describes a 83.2% (95%CI: 81.0%–85.2%) treatment completion rate in this patient population [22]. We therefore estimate that, of 94,774 retreatment smear-negative patients who started TB treatment (Step 4), 78,852 (95%CI: 76,767–80,747) achieved treatment completion (Step 5) and 15,922 (95%CI: 14,027–18,007) failed treatment, were lost to follow-up, or died during the treatment course (Gap 4).
Aggregating these estimates, we estimate that, of the 1,417,838 patients with all forms of TB who registered for treatment in the RNTCP in 2013 (Step 4), 1,221,764 (95%CI: 1,219,679–1,223,659), or 86.2%, successfully completed treatment (Step 5). This suggests that 196,074 (95%CI: 194,179–198,159), or 13.8%, of these patients failed treatment, were lost to follow-up, or died during the treatment course (Gap 4).
Recurrence-free survival
For new smear-positive patients, using the Gap 5 estimate of 16.2% (95%CI: 14.2%–18.5%) from the meta-analysis, we estimate that, of 541,736 patients who completed treatment (Step 5), 453,975 (95%CI: 441,515–464,809) achieved 1-y recurrence-free survival (Step 6) and 87,761 (95%CI: 76,927–100,221) experience TB recurrence or death within 12–24 mo of treatment completion (Gap 5).
For new smear-negative patients, using the Gap 5 estimate of 8.8% (95%CI: 6.9%–11.1%) from the meta-analysis, we estimate that, of 258,551 patients who completed treatment (Step 5), 235,799 (95%CI: 229,852–240,711) achieved 1-y recurrence-free survival (Step 6) and 22,752 (95%CI: 17,840–28,699) experienced post-treatment TB recurrence or death (Gap 5). Using the same 8.8% Gap 5 estimate for extrapulmonary TB patients, we estimate that, of 207,903 patients who completed treatment (Step 5), 189,608 (95%CI: 184,826–193,558) achieved 1-y recurrence free survival (Step 6) and 18,295 (95%CI: 14,345–23,077) experienced post-treatment TB recurrence or death (Gap 5). Using the same 8.8% Gap 5 estimate for retreatment smear-negative patients, we estimate that, of 78,852 patients who completed treatment (Step 5), 71,913 (95%CI: 68,246–75,175) achieved 1-y recurrence-free survival (Step 6) and 6,939 (95%CI: 1,592–12,501) experienced post-treatment TB recurrence or death (Gap 5).
For retreatment smear-positive patients, using the Gap 5 estimate of 27.3% (95%CI: 19.1%–37.4%) from the systematic review [25], we estimate that, of 125,901 patients who completed treatment (Step 5), 91,530 (95%CI: 78,814–101,854) achieved 1-y recurrence-free survival (Step 6) and 34,371 (95%CI: 24,047–47,087) experience post-treatment TB recurrence or death (Gap 5). Using the same 27.3% Gap 5 estimate for MDR TB patients, we estimate that, of 8,821 patients who completed treatment (Step 5), 6,413 (95%CI: 5,522–7,136) achieved 1-y recurrence-free survival (Step 6) and 2,408 (95%CI: 1,685–3,299) experienced post-treatment TB recurrence or death (Gap 5).
Aggregating these estimates, we estimate that of the 1,221,764 patients who successfully completed treatment in the RNTCP in 2013 (Step 5), 1,049,237 (95%CI: 1,008,775–1,083,243), or 85.9%, achieved the optimal outcome of 1-y TB recurrence-free survival (Step 6), and 172,527 (95%CI: 136,436–214,884), or 14.1%, experience TB recurrence or death within 12–24 mo of completing TB therapy (Gap 5).
The TB Cascade of Care in Specific Subpopulations
We present the TB cascade of care for 2013 for specific subpopulations of TB patients. These cascades exclude Step 1 (the number of prevalent patients), since disaggregated prevalence estimates are not available for most forms of TB. Because they start from Step 2 (the number of patients evaluated at diagnostic facilities), these subpopulation cascades reflect the efficiency of RNTCP services in detecting, linking to care, treating, and retaining TB patients.
Fig 8 presents the cascade for new smear-positive TB patients. Of the estimated 730,108 (95%CI: 726,428–733,826) patients who were evaluated at government diagnostic facilities, we estimate that 453,975 (95%CI: 441,515–464,809), or 62.2%, achieved 1-y recurrence-free survival. Moderate attrition of patients occurred at Gap 3 (diagnosed but not started on treatment), Gap 4 (loss to follow-up, failure, or death while on treatment), and Gap 5 (post-treatment TB recurrence or death).
Fig. 8. The tuberculosis cascade of care for new smear-positive tuberculosis patients detected and treated by the Revised National Tuberculosis Control Programme (RNTCP) in India, 2013. 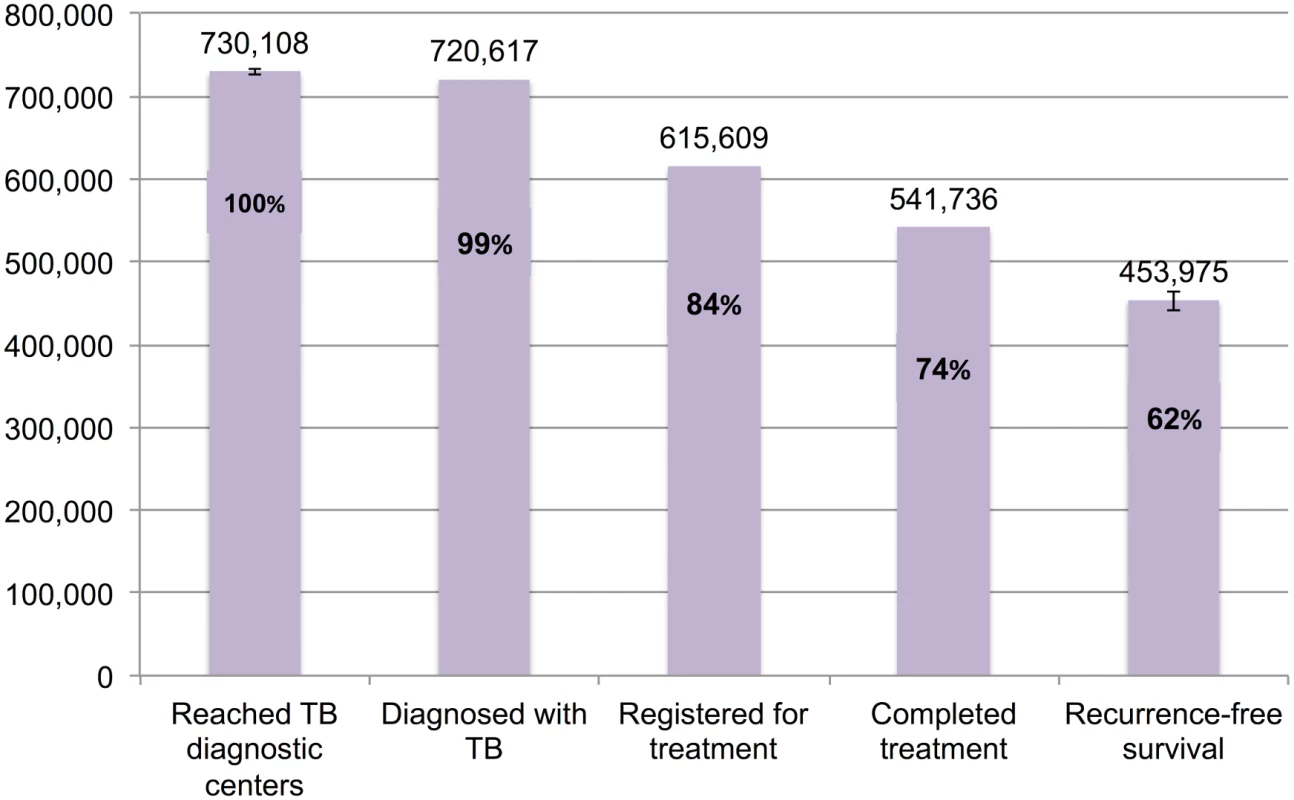
Error bars depict 95% confidence intervals. Fig 9 presents the cascade for new smear-negative TB patients. Of the 514,161 (95%CI: 456,873–582,402) patients who were evaluated at government diagnostic facilities, we estimate that 235,799 (95%CI: 229,852–240,711), or 45.9%, achieved 1-y recurrence-free survival. The most substantial attrition occurred at Gap 2 (evaluated at diagnostic facilities but not diagnosed with TB).
Fig. 9. The tuberculosis cascade of care for new smear-negative tuberculosis patients detected and treated by the Revised National Tuberculosis Control Programme (RNTCP) in India, 2013. 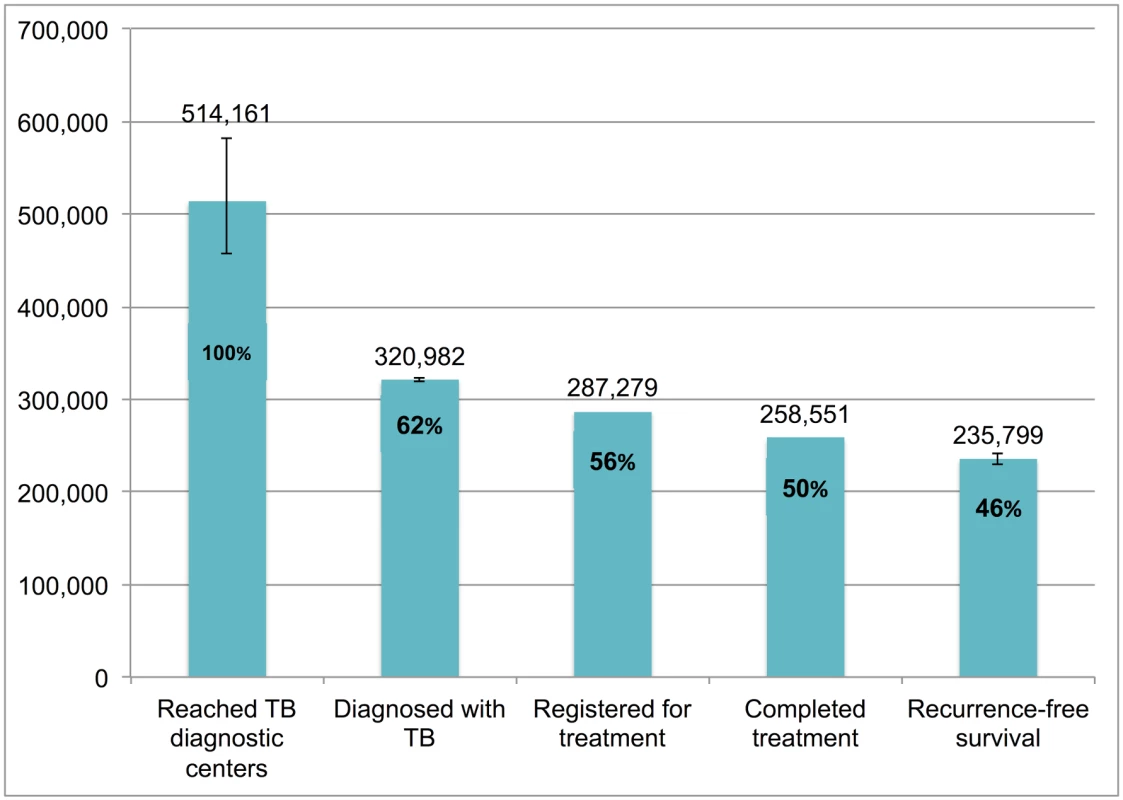
Error bars depict 95% confidence intervals. Fig 10 presents the cascade for retreatment smear-positive TB patients. Of the estimated 210,307 (95%CI: 209,247–211,378) patients who were evaluated at government diagnostic facilities, we estimate that 91,530 (95%CI: 78,814–101,854), or 43.5%, achieved 1-y recurrence-free survival. The most substantial attrition occurred at Gap 4 (loss to follow-up, failure, or death while on treatment) and Gap 5 (post-treatment TB recurrence or death).
Fig. 10. The tuberculosis cascade of care for retreatment smear-positive tuberculosis patients detected and treated by the Revised National Tuberculosis Control Programme (RNTCP) in India, 2013. 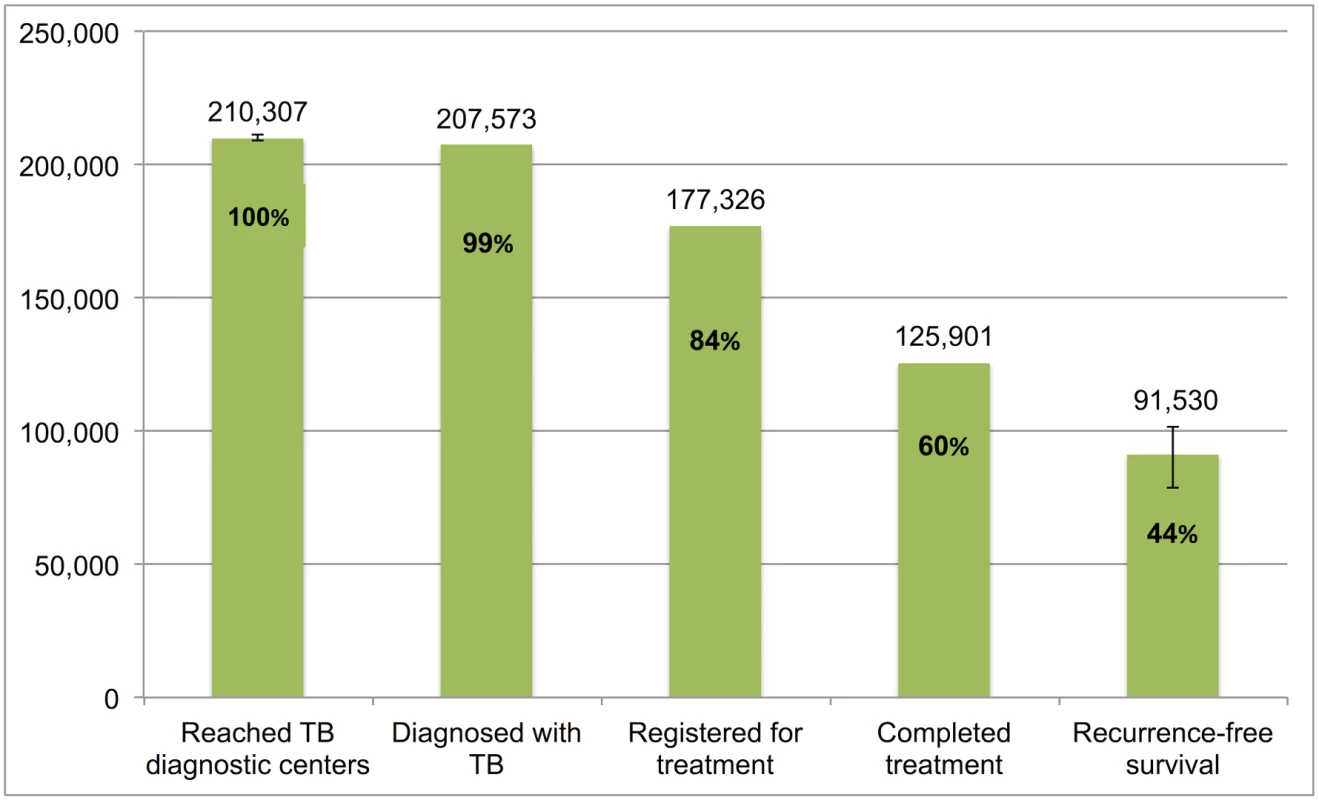
Error bars depict 95% confidence intervals. Fig 11 presents the cascade for MDR TB patients. We estimate that, of the 61,000 (95%CI: 47,000–76,000) MDR TB patients who reached government TB diagnostic facilities, only 8,821 (14.4%) were appropriately diagnosed and completed MDR TB therapy and 6,413 (95%CI: 5,522–7,136), or 10.5%, ultimately achieved 1-y recurrence free survival. While there is considerable attrition at every step of the cascade, Gap 2 (evaluated at diagnostic facilities but not diagnosed with MDR TB) contributed most to the cascade’s poor performance.
Fig. 11. The tuberculosis cascade of care for multidrug-resistant tuberculosis (MDR TB) patients detected and treated by the Revised National Tuberculosis Control Programme (RNTCP) in India, 2013. 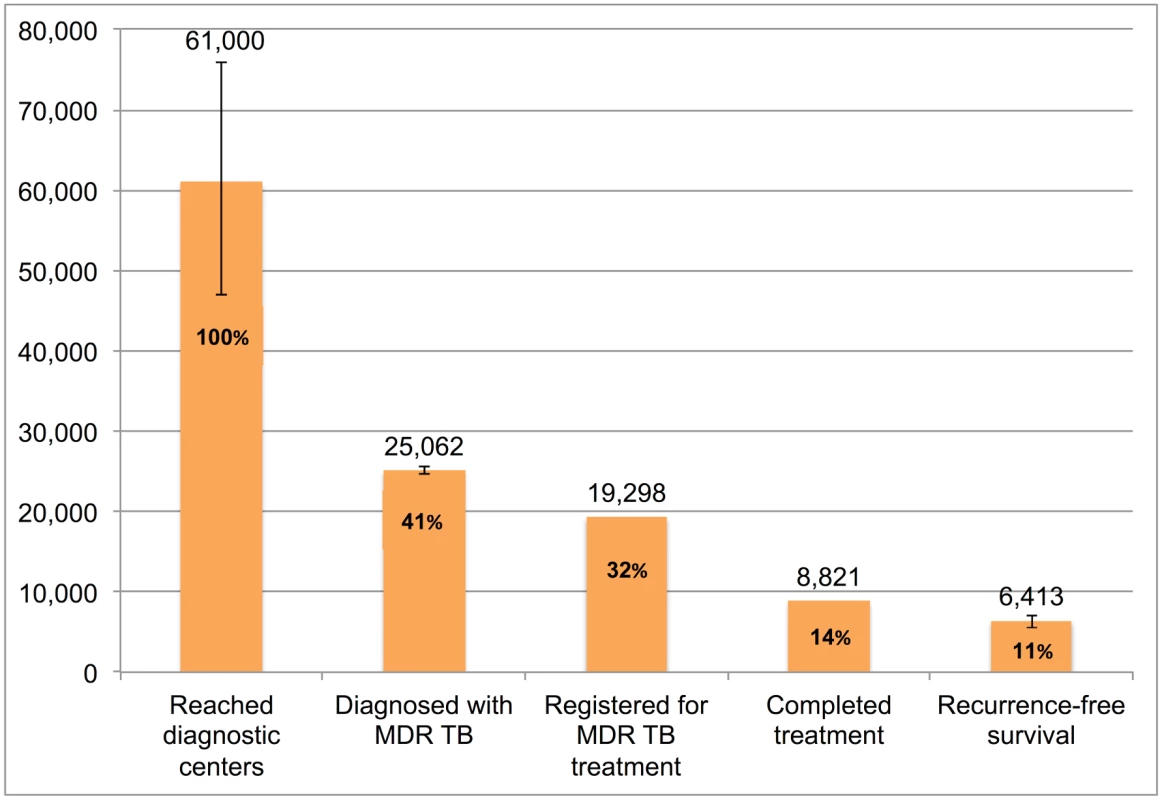
Error bars depict 95% confidence intervals for each estimate. Discussion
We constructed the cascade of TB care in India for 2013 with two different aims—(1) to identify critical points of patient attrition that may guide TB control interventions and (2) to identify knowledge gaps for different steps of the cascade in order to guide future research to improve its accuracy.
Critical Points of Attrition in the Cascade
For the overall cascade including all forms of TB, the most substantial point of attrition is Gap 1a/1b, which suggests that more than one-quarter of prevalent TB patients in India (or about 760,000 patients annually) are not evaluated at government TB diagnostic centers. In addition, our estimates for Gaps 2 and 3 together suggest that nearly one-fifth of prevalent TB patients (or about 520,000 patients annually) interface with public sector TB services but are either not successfully diagnosed or are diagnosed with TB but lost to follow-up before starting treatment. Together, Gaps 1, 2, and 3 indicate that nearly 1.3 million TB patients in India do not receive therapy in the public sector—a finding that roughly corroborates the WHO’s estimate of “missing” TB patients in India [1,12,13].
National-level surveys suggest that a considerable proportion of these “missing” patients in Gaps, 1, 2, and 3 are being treated by private sector providers [12,13]. The private sector in India is very heterogeneous, and studies suggest that, in some locations, the majority of care is provided by practitioners of Indian medical traditions (e.g., Ayurveda, Yoga, Unani, Siddha, and Homeopathy [AYUSH]) or informal providers, who often have no formal medical degrees [76]. The few available data suggest lower quality of care [3,4] and poorer treatment completion rates (about 50%) [77] in private facilities when compared to the public sector (~85%), even in the context of public–private partnerships aimed at improving private sector care.
To decrease Gap 1a/1b, more research is needed to understand the TB diagnostic and referral practices of private providers, especially AYUSH and informal practitioners who provide most community-level care [76]. Data from Mumbai suggest that, when they do suspect TB, most private providers prefer referring patients to public sector TB services or to private chest physicians with expertise in managing TB [78]. However, a study in Delhi showed that only one-fifth of unannounced standardized patients presenting to private providers with a classic TB history were correctly managed with referral to public or private TB services or ordering of an appropriate TB test (e.g., sputum microscopy) [4].
These findings highlight a need to bridge private providers’ “know–do” gap—the gap between their intentions and actual practice—to increase referrals to the public sector and case notifications. Enhanced engagement of the RNTCP with private providers, through robust continuing medical education initiatives, on-site visits from RNTCP clinicians to review TB patient management strategies, collaborative efforts at patient co-management with provision of free medications from the public sector, and incentives to promote referral of TB patients, may help to reduce this gap. At the same time, the cascade model emphasizes that improving private sector case notification alone is inadequate; it is equally important to routinely collect data on treatment outcomes and 1-y TB recurrence for patients treated in the private sector to monitor this sector’s quality of care.
The proportion of patients who never seek any TB care and remain untreated in Gap 1a/1b remains uncertain, though our meta-analysis shows that about 39% of chest symptomatics had not sought medical care at the time they were surveyed, which at minimum suggests delayed care-seeking behavior. Public education may help to improve care-seeking behavior, which can be monitored using serial population-based surveys. In addition, enhancing the availability and accessibility of RNTCP facilities in hard-to-reach Adivasi (tribal) areas, where case detection rates are very low, may help to reduce the proportion of India’s population without access to TB care [31]. Finally, various strategies of targeted active case finding—especially higher yield strategies focused on repeated screening of household contacts of known TB patients [79], people living with HIV [80,81], and individuals visiting health care facilities [82]—may also help to substantially reduce Gap 1a/1b by identifying patients who may otherwise not seek TB care or do so with substantial delay [83].
Gaps 2 and 3 are notable because they suggest that about one-fifth of all TB patients in India are “lost” despite engaging with government TB services. These patients go undetected during the diagnostic workup at government facilities (possibly due to heavy reliance on insensitive diagnostics such as smear microscopy) or fail to start TB treatment despite being successfully diagnosed. Since this population of half a million patients is willing to engage with public sector services in some capacity, they present a major opportunity for strengthening TB control. Improving care at public sector facilities may help to retain these patients—by increasing case detection (e.g., through use of more sensitive and rapid molecular tests), accelerating the time to treatment initiation, improving linkage of diagnosed patients to care, and making TB services more patient-friendly. In addition, since most diagnosed patients in Gap 2 are lost during the process of referral from TB microscopy centers to local DOT centers [44–46], strengthening patient tracking and improving the accountability of the health system for these patients may help address this gap.
While the RNTCP emphasizes the importance of treatment completion rates, our model suggests that the gap immediately after treatment—post-treatment TB recurrence and death (Gap 5)—may be an equally important contributor to the poor performance of the cascade. Routine follow-up of all patients for 1 y after treatment may have substantial benefits for TB control by promoting early detection of patients with recurrent TB, who are more likely to have drug resistant disease. The yield of this approach for detecting new patients may be comparable or superior to other strategies of case finding, such as household contact tracing [79,84].
The cascades for different subpopulations highlight different patterns of attrition for each form of TB. For new smear-positive patients, multiple steps—pretreatment loss to follow-up, loss to follow-up on treatment, and post-treatment TB recurrence or death—are equally important points of attrition.
In contrast, for new smear-negative patients, failure to detect patients who present to diagnostic facilities (Gap 2) is the major point of attrition. In fact, we probably underestimate the size of Gap 2 for these patients, because studies suggest that non-adherence of providers to the diagnostic algorithm for smear-negative TB may be resulting in considerable overdiagnosis [85]. As a result, patients with other pulmonary diseases might be incorrectly treated as having smear-negative TB, a problem that we were not able to correct for in this model. Concurrent use of alternative or more sensitive diagnostic tests (e.g., Xpert MTB/RIF or culture) may reduce this gap. In addition, an emerging revision to the RNTCP smear-negative diagnostic algorithm, in which chest X-ray is used upfront along with sputum smears, may reduce this gap [86].
For retreatment smear-positive patients, poor outcomes while taking TB therapy and high post-treatment recurrence and death rates are major points of attrition. Both of these points of attrition highlight a need for improved care of these patients after they register for treatment, potentially through better access to drug-susceptibility testing, enhanced counseling, and closer adherence monitoring. While HIV co-infected TB patients were not analyzed separately in the cascade, the results of our systematic review of TB recurrence suggests that these patients may also have substantially poorer treatment outcomes and higher rates of post-treatment TB recurrence and death [51,52,74,75]. For MDR TB patients, the cascade suggests that the vast majority of MDR TB patients who present to government diagnostic facilities are not diagnosed with drug resistance, highlighting an urgent need to move towards universal drug-susceptibility testing, as recommended by the End TB Strategy [6].
Limitations and Improving the Accuracy of the Cascade
A major limitation of this study is the substantial uncertainty in the estimate of prevalent TB patients (Step 1), since India has not had a nationally representative TB prevalence survey conducted in decades. This figure also represents an estimate of point prevalence and is possibly an underestimate of the 1-y period prevalence of TB. Also, prior TB prevalence studies conducted in India have usually screened individuals in the community for TB symptoms before collecting sputum specimens [33,35,36]; this approach may miss a considerable proportion of patients without symptoms, thereby underestimating TB prevalence [87]. In addition, a recent study estimating the number of TB patients treated in India’s private sector suggests that the overall burden of TB disease in India may be underestimated [88]. While the uncertainty in the overall estimate of TB prevalence may impact interpretation of subsequent cascade steps, analysis of the cascade can also be performed starting from Step 2 (the number of TB patients evaluated at diagnostic facilities) and still provide substantial insights into the efficiency and functioning of the RNTCP.
A second major limitation of this study is the lack of data on the number of TB patients who are detected, treated, and cured in the private sector. Similar to the WHO’s estimate of “missing” TB patients, our model assumes that these patients are essentially undiagnosed and untreated. In reality, a considerable proportion of these patients have likely sought diagnosis and treatment in the private sector [12,13,89]. As such, we overestimate the gap in case detection and underestimate subsequent steps of the cascade. It is critical that the RNTCP strengthen private sector notification and, perhaps more importantly, routinely assess treatment outcomes for private sector patients.
A third limitation is the fact that we do not adequately account for possible misclassification of patients who may have diagnoses other than TB or who may have other forms of TB. We did assume, for example, that most MDR TB patients in Step 2 are subsequently misclassified and treated as either “new” or “retreatment” patients. However, due to limited data, we did not account for potential misdiagnosis with TB of patients who actually have other pulmonary processes, including non-tuberculous mycobacterial infection [90]. Due to poor adherence to the diagnostic algorithm, misdiagnosis with TB may especially be a problem for patients who have negative sputum smears [85]. In addition, one study suggests that as many as 9%–13% of new smear-positive patients started on treatment have a history of previous TB that was missed by providers, which means that these patients should have been screened for MDR TB and treated as retreatment smear-positive patients [91].
A fourth general limitation of our study is the inability to exclude duplicate patient records, since we used aggregate data from the RNTCP to construct the cascade. A small percentage of patients may have submitted sputum specimens at multiple facilities or have been registered for treatment at multiple DOT centers, thereby leading to overestimation of certain steps and underestimation of gaps. Preliminary analysis using computerized RNTCP records in Gujarat by one of this manuscript’s co-authors (KR) suggests that the percentage of duplicate records for smear-positive patients is likely very small (about 0.02%) [92]. The ongoing rollout of E-NIKSHAY, the RNTCP’s computerized case notification system, and India’s ongoing Unique ID (Aadhar) card program may facilitate more accurate nationwide estimates of duplicate records in the future.
A fifth general limitation is that time delays between steps are not well captured. This problem is especially relevant to patient care-seeking and case detection, since time delays (and not just the gaps alone) contribute to TB transmission. A recent systematic review suggests a median of nearly 2 mo delay in TB patients in India getting diagnosed after development of symptoms [93]. In addition, understanding the timing of loss to follow-up during TB treatment may help to target interventions for retaining patients on therapy.
A sixth general limitation is that we do not disaggregate the different types of poor outcomes in the cascade—loss to follow-up, death, treatment failure, and misclassification of patients. Since these are collapsed into single gaps in our current model, this limits understanding of the transmission risks associated with specific gaps, as some poor outcomes (e.g., treatment failure or loss to follow-up) may be associated with higher transmission than others (e.g., death). Incorporating a detailed description of poor outcomes into future cascades may help guide the development of interventions to reduce these gaps. A seventh general limitation is that we do not present longitudinal trends in the cascade. As the RNTCP is currently undergoing multiple transitions, including scale-up of Xpert MTB/RIF and switching from thrice weekly to daily TB therapy for all patients, longitudinal evaluation of the cascade in the future may help to measure changes in the performance of the overall program.
Other study limitations are specific to each step of the cascade and are summarized in Table 5, with suggested strategies for improving the accuracy of each estimate. For example, Gap 1b—the proportion of TB patients who never seek care—is very difficult to estimate; however, this indicator may be assessed indirectly by using serial surveys of care-seeking behavior by chest symptomatics or by conducting nationally-representative verbal autopsy studies that assess whether patients who died of TB ever sought care. Alternatively, strengthening the medical certification process for documenting causes of death, with subsequent audits of TB deaths to see whether patients had sought care, may be preferable. Gap 2—the proportion of TB patients seen at government diagnostic facilities who are not diagnosed—could best be assessed for smear-negative and MDR TB patients through studies in which mycobacterial culture is used to identify “true” TB patients, so that diagnostic attrition rates can be more accurately estimated.
Tab. 5. Limitations of current estimates and recommendations for improving the accuracy of the TB cascade of care for India. 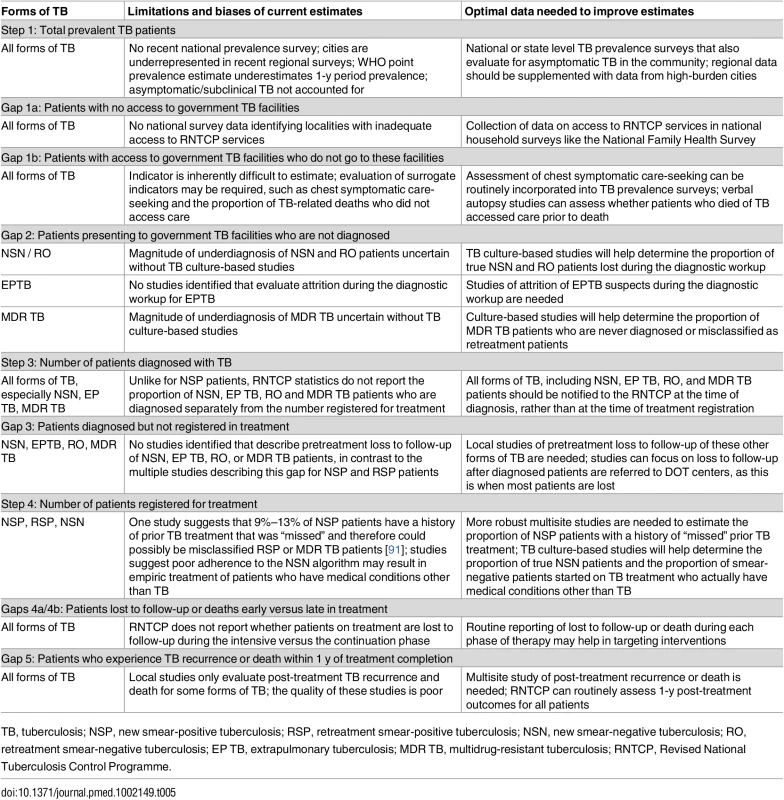
TB, tuberculosis; NSP, new smear-positive tuberculosis; RSP, retreatment smear-positive tuberculosis; NSN, new smear-negative tuberculosis; RO, retreatment smear-negative tuberculosis; EP TB, extrapulmonary tuberculosis; MDR TB, multidrug-resistant tuberculosis; RNTCP, Revised National Tuberculosis Control Programme. To best estimate Gaps 4a and 4b, the RNTCP should routinely collect and report data on when patients are lost or die while taking TB treatment, as this will help to focus resources for patient retention earlier or later in the treatment course. For Gap 6—the proportion of patients who experience post-treatment TB recurrence or death—higher quality studies are needed that estimate this indicator for all forms of TB, especially since routine follow-up of patients post-treatment may be an efficient strategy for early detection of recurrent and potentially drug-resistant TB patients.
The most recent year for which we could construct a complete cascade that included all forms of TB was 2013. Since the treatment course for MDR TB is prolonged (often >18 mo), treatment outcomes are not reported until 2–3 y after these patients are registered for treatment. Since 2013, MDR TB case detection has improved in India, due to increased use of Xpert MTB/RIF and line probe assays at select RNTCP sites to diagnose drug resistance, especially in patients with HIV or a prior history of TB [24]. Indeed, recent TB India reports suggest a considerable increases in the number of MDR TB patients registered for treatment from 12,285 in 2013 to 20,087 in 2014 and 26,966 in 2015 [16,20,21]. Therefore, the cascade presented here may provide baseline data against which emerging national efforts to improve MDR TB case detection and treatment can be evaluated.
Finally, if our model of the TB cascade is applied to other settings, it may require considerable modification to adjust for local differences in TB epidemics. For example, in using recurrence-free survival as the end outcome of the cascade, we assume that most TB recurrence is secondary to relapse of the same strain of TB [15]. However, this may be a less reliable end outcome in settings with a high prevalence of HIV infection, since a higher proportion of TB recurrences may be due to exogenous reinfection with a new strain of TB rather than relapse, which means that recurrences are less likely to reflect the quality of prior TB care received by the patient [94,95]. In addition, our cascade model will have to be modified in settings where Xpert is the predominant diagnostic test, as our analysis assumes the smear microscopy is the most common method of diagnosis.
Conclusions
We describe the TB cascade of care in India for 2013 and estimate that about 39% of prevalent TB patients are treated in the government system and achieve an optimal outcome of 1-y recurrence free survival. Cascades constructed for subpopulations of TB patients highlight substantial attrition and poorer outcomes among retreatment smear-positive and MDR TB patients, and there are differences in the critical points of attrition for each type of TB.
TB control efforts in India are currently in the midst of a dramatic transition. The rollout of E-NIKSHAY, the RNTCP’s electronic data management system, may help to improve private sector case notification. Implementation of novel public–private partnerships in major Indian cities may increase case detection, improve private sector quality of care, and capture data on private sector treatment outcomes. Increased use of new diagnostic tests such as Xpert may reduce the considerable gaps in diagnosis of both smear-negative and MDR TB. Newer patient-centered models of care using electronic observation of therapy (e.g., through cell phones or electronic pillboxes) have the potential to improve patient retention throughout the treatment course.
In this context of rapid change, greater commitment of resources for TB control at the international, national, and state levels will be crucial for improving the outcomes of the TB cascade. The cascade model could help to identify gaps in care in a manner that might inform the most cost-effective targeting of resources in the public sector TB program. As with the Joint United Nations Programme on HIV/AIDS (UNAIDS)’ “90-90-90” global strategy for HIV, which is based on the HIV cascade model, future multisite studies of the TB cascade in India could be used to longitudinally measure TB control outcomes in a manner that accelerates progress towards the goals of the End TB Strategy [6]. In addition, since substantial numbers of India’s TB patients seek care in the private sector [12,88], a cascade of care analysis is urgently needed to identify critical gaps in patient care-seeking, diagnosis, retention in treatment, and disease recurrence in the private sector.
Supporting Information
Zdroje
1. World Health Organization (WHO). Global tuberculosis report. Geneva: World Health Organization, 2014 Contract No.: WHO/HTM/TB/2014.08.
2. World Health Organization (WHO). Global tuberculosis report. Geneva: WHO, 2015 Contract No.: WHO/HTM/TB/2015.22.
3. Satyanarayana S, Subbaraman R, Shete P, Gore G, Das J, Cattamanchi A, et al. Quality of tuberculosis care in India: a systematic review. Int J Tuberc Lung Dis. 2015;19(7):751–63. doi: 10.5588/ijtld.15.0186 26056098.
4. Das J, Kwan A, Daniels B, Satyanarayana S, Subbaraman R, Bergkvist S, et al. Use of standardised patients to assess quality of tuberculosis care: a pilot, cross-sectional study. Lancet Infect Dis. 2015;15(11):1305–13. doi: 10.1016/s1473-3099(15)00077-8 26268690.
5. Sachdeva KS, Kumar A, Dewan P, Kumar A, Satyanarayana S. New vision for Revised National Tuberculosis Control Programme (RNTCP): Universal access—"reaching the un-reached". Indian J Med Res. 2012;135(5):690–4. 22771603.
6. World Health Organization (WHO). Global strategy and targets for tuberculosis prevention, care, and control after 2015. Geneva: WHO, 2013 Contract No.: EB134/12.
7. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243 21367734.
8. Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14(1):40–9. doi: 10.1016/s1473-3099(13)70254-8 24076277.
9. Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med. 2014;161(10):681–9. doi: 10.7326/m14-0019 25402511.
10. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS ONE. 2014;9(7):e101554. doi: 10.1371/journal.pone.0101554 24988388.
11. World Health Organization (WHO). TB impact measurement: policy and recommendations for how to assess the epidemiological burden of TB and the impact of TB control. Geneva: WHO, 2009 Contract No.: WHO/HTM/TB/2009.416.
12. Satyanarayana S, Nair SA, Chadha SS, Shivashankar R, Sharma G, Yadav S, et al. From where are tuberculosis patients accessing treatment in India? Results from a cross-sectional community based survey of 30 districts. PLoS ONE. 2011;6(9):e24160. doi: 10.1371/journal.pone.0024160 21912669.
13. Hazarika I. Role of Private Sector in Providing Tuberculosis Care: Evidence from a Population-based Survey in India. J Glob Infect Dis. 2011;3(1):19–24. doi: 10.4103/0974-777x.77291 21572604.
14. Thomas A, Gopi PG, Santha T, Chandrasekaran V, Subramani R, Selvakumar N, et al. Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int J Tuberc Lung Dis. 2005;9(5):556–61. 15875929.
15. Narayanan S, Swaminathan S, Supply P, Shanmugam S, Narendran G, Hari L, et al. Impact of HIV infection on the recurrence of tuberculosis in South India. J Infect Dis. 2010;201(5):691–703. doi: 10.1086/650528 20121433.
16. Central TB Division. TB India 2014: Revised National TB Control Programme annual status report. New Delhi: Ministry of Health and Family Welfare, 2014.
17. Nunn AJ, Phillips PP, Mitchison DA. Timing of relapse in short-course chemotherapy trials for tuberculosis. Int J Tuberc Lung Dis. 2010;14(2):241–2. 20074418.
18. WHO Country Office for India. Standards for TB Care in India. New Delhi: World Health Organization, 2014.
19. World Health Organization (WHO). WHO TB burden estimates [dataset]. 2016 Mar 22 [cited 2016 June 20]. WHO. Available from: https://extranet.who.int/tme/generateCSV.asp?ds=estimates
20. Central TB Division. TB India 2015: Revised National TB Control Programme annual status report. New Delhi: Ministry of Health and Family Welfare, 2015.
21. Central TB Division. TB India 2016: Revised National TB Control Programme annual status report. New Delhi: Ministry of Health and Family Welfare, 2016.
22. Srinath S, Sharath B, Santosha K, Chadha SS, Roopa S, Chander K, et al. Tuberculosis 'retreatment others': profile and treatment outcomes in the state of Andhra Pradesh, India. Int J Tuberc Lung Dis. 2011;15(1):105–9. 21276305.
23. Rawat J, Biswas D, Sindhwani G, Kesharwani V, Masih V, Chauhan BS. Diagnostic defaulters: an overlooked aspect in the Indian Revised National Tuberculosis Control Program. J Infect Dev Ctries. 2012;6(1):20–2. 22240423.
24. Sachdeva KS, Raizada N, Sreenivas A, Van't Hoog AH, van den Hof S, Dewan PK, et al. Use of Xpert MTB/RIF in Decentralized Public Health Settings and Its Effect on Pulmonary TB and DR-TB Case Finding in India. PLoS ONE. 2015;10(5):e0126065. doi: 10.1371/journal.pone.0126065 25996389.
25. Sadacharam K, Gopi PG, Chandrasekaran V, Eusuff SI, Subramani R, Santha T, et al. Status of smear-positive TB patients at 2–3 years after initiation of treatment under a DOTS programme. Indian J Tuberc. 2007;54(4):199–203. 18072535.
26. Thomas BE, Velayutham B, Thiruvengadam K, Nair D, Barman SB, Jayabal L, et al. Perceptions of Private Medical Practitioners on Tuberculosis Notification: A Study from Chennai, South India. PLoS ONE. 2016;11(1):e0147579. doi: 10.1371/journal.pone.0147579 26820750.
27. Kundu D, Chopra K, Khanna A, Babbar N, Padmini TJ. Accelerating TB notification from the private health sector in Delhi, India. Indian J Tuberc. 2016;63(1):8–12. doi: 10.1016/j.ijtb.2016.02.002 27235938.
28. Cowling K, Dandona R, Dandona L. Improving the estimation of the tuberculosis burden in India. Bull World Health Organ. 2014;92(11):817–25. doi: 10.2471/blt.13.129775 25378743.
29. Central TB Division. TB India 2008: Revised National TB Control Programme annual status report. New Delhi: Ministry of Health and Family Welfare, 2008.
30. Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:Cd009593. doi: 10.1002/14651858.CD009593.pub3 24448973.
31. Muniyandi M, Rao VG, Bhat J, Yadav R. Performance of Revised National Tuberculosis Control Programme (RNTCP) in tribal areas in India. Indian J Med Res. 2015;141(5):624–9. 26139780
32. Fochsen G, Deshpande K, Diwan V, Mishra A, Diwan VK, Thorson A. Health care seeking among individuals with cough and tuberculosis: a population-based study from rural India. Int J Tuberc Lung Dis. 2006;10(9):995–1000. 16964790.
33. Bhat J, Rao VG, Gopalan PG, Yadav R, Nagamiah S, Tiwari AK, et al. Prevalence of pulmonary tuberculosis among tribal population of Madhya Pradesh, central India. Int J Epidemiol. 2009;38 : 1026–32. doi: 10.1093/ije/dyp222 19509182
34. Gopi PR, Subramani R, Narayanan PR. Evaluation of different types of chest symptoms for diagnosing pulmonary tuberculosis cases in community surveys. Indian J Tuberc. 2008;55 : 116–21. 18807742
35. Chadha VK, Kumar P, Anjinappa SM, Singh S, Narasimhaiah S, Joshi MV, et al. Prevalence of pulmonary tuberculosis among adults in a rural sub-district of South India. PLoS ONE. 2012;7(8):e42625. doi: 10.1371/journal.pone.0042625 22956993.
36. Rao VG, Bhat J, Yadav R, Gopalan GP, Nagamiah S, Bhondeley MK, et al. Prevalence of pulmonary tuberculosis—a baseline survey in central India. PLoS ONE. 2012;7(8):e43225. doi: 10.1371/journal.pone.0043225 22952651.
37. Mase SR, Ramsay A, Ng V, Henry M, Hopewell PC, Cunningham J, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2007;11(5):485–95. 17439669.
38. Chadha VK, Praseeja P, Hemanthkumar NK, Shivshankara BA, Sharada MA, Nagendra N, et al. Implementation efficiency of a diagnostic algorithm in sputum smear-negative presumptive tuberculosis patients. Int J Tuberc Lung Dis. 2014;18(10):1237–42. doi: 10.5588/ijtld.14.0218 25216839.
39. Thomas A, Gopi PG, Santha T, Jaggarajamma K, Charles N, Prabhakaran E, et al. Course of action taken by smear negative chest symptomatics: a report from a rural area in South India. Indian J Tuberc. 2006;53 : 4–6.
40. Sarkar J, Murhekar MV. Factors associated with low utilization of X-ray facilities among the sputum negative chest symptomatics in Jalpaiguri District (West Bengal) 2009. Indian J Tuberc. 2011;58 : 208–11. 22533172
41. Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(2):97–107. 10694086.
42. Central TB Division. Training course for programme managers (module 1–4). New Delhi: Ministry of Health and Family Welfare, 2011.
43. Chander V, Raina SK, Bhardwaj AK, Kashyap AK, Gupta A, Sood A. Is diagnostic protocol a cause of overestimation of extra-pulmonary tuberculosis in Himachal Pradesh? A report from a high-prevalence tuberculosis unit. Ann Trop Med PH. 2013;6(2):193–6.
44. Mehra D, Kaushik RM, Kaushik R, Rawat J, Kakkar R. Initial default among sputum-positive pulmonary TB patients at a referral hospital in Uttarakhand, India. Trans R Soc Trop Med Hyg. 2013;107(9):558–65. doi: 10.1093/trstmh/trt065 23920324.
45. Khandekar J, Acharya AS, R TH, Sharma A. Do patients with tuberculosis referred from a tertiary care referral centre reach their peripheral health institution? Natl Med J India. 2013;26(6):332–4. 25073989.
46. Pillai D, Purty A, Prabakaran S, Singh Z, Soundappan G, Anandan V. Initial default among tuberculosis patients diagnosed in select medical colleges of Puducherry: issues and possible interventions. Int J Med Sci Public Health. 2015;4(7):1–4.
47. Chadha SS, Sharath BN, Reddy K, Jaju J, Vishnu PH, Rao S, et al. Operational challenges in diagnosing multi-drug resistant TB and initiating treatment in Andhra Pradesh, India. PLoS ONE. 2011;6(11):e26659. doi: 10.1371/journal.pone.0026659 22073182.
48. Shringarpure KS, Isaakidis P, Sagili KD, Baxi RK. Loss-To-Follow-Up on Multidrug Resistant Tuberculosis Treatment in Gujarat, India: The WHEN and WHO of It. PLoS ONE. 2015;10(7):e0132543. doi: 10.1371/journal.pone.0132543 26167891.
49. Dandekar RH, Jagannath VD. The fate of tuberculosis cases after two years of DOTS chemotherapy in Aurangabad city, Maharasthra. Natl J Community Med. 2014;5(2):174–8.
50. Prasad R, Verma SK, Shrivastava P, Kant S, Kushwaha RA, Kumar S. A follow up study on Revised National Tuberculosis Control Programme (RNTCP): results from a single centre study. Lung India. 2008;25(4):142–4. doi: 10.4103/0970-2113.45277 21264079.
51. Tripathy S, Anand A, Inamdar V, Manoj MM, Khillare KM, Datye AS, et al. Clinical response of newly diagnosed HIV seropositive & seronegative pulmonary tuberculosis patients with the RNTCP Short Course regimen in Pune, India. Indian J Med Res. 2011;133 : 521–8. 21623038.
52. Vashishtha R, Mohan K, Singh B, Devarapu SK, Sreenivas V, Ranjan S, et al. Efficacy and safety of thrice weekly DOTS in tuberculosis patients with and without HIV co-infection: an observational study. BMC Infect Dis. 2013;13 : 468. doi: 10.1186/1471-2334-13-468 24099345.
53. Charles N, Thomas B, Watson B, Raja Sakthivel M, Chandrasekeran V, Wares F. Care seeking behavior of chest symptomatics: a community based study done in South India after the implementation of the RNTCP. PLoS ONE. 2010;5(9):e12379. doi: 10.1371/journal.pone.0012379 20862219.
54. George O, Sharma V, Sinha A, Bastian S, Santha T. Knowledge and behaviour of chest symptomatics in urban slum populations of two states in India towards care-seeking. Indian Journal of Tuberculosis. 2013;60 : 95–106.
55. Ghosh S, Sinhababu A, Taraphdar P, Mukhopadhyay DK, Mahapatra BS, Biswas AB. A study on care seeking behavior of chest symptomatics in a slum of Bankura, West Bengal. Indian J Public Health. 2010;54(1):42–4. doi: 10.4103/0019-557x.70553 20859052.
56. Kar M, Logaraj M. Awareness, attitude and treatment seeking behaviour regarding tuberculosis in a rural area of Tamil Nadu. Indian J Tuberc. 2010;57(4):226–9. 21141344.
57. Satyanarayana S, Nair SA, Chadha SS, Sharma G, Yadav S, Mohanty S, et al. Health-care seeking among people with cough of 2 weeks or more in India. Is passive TB case finding sufficient? Public Health Action. 2012;2(4):157–61. doi: 10.5588/pha.12.0019 26392977
58. Suganthi P, Chadha VK, Ahmed J, Umadevi G, Kumar P, Srivastava R, et al. Health seeking and knowledge about tuberculosis among persons with pulmonary symptoms and tuberculosis cases in Bangalore slums. Int J Tuberc Lung Dis. 2008;12(11):1268–73. 18926036.
59. Thomas BE, Charles N, Watson B, Chandrasekaran V, Senthil Kumar R, Dhanalakshmi A, et al. Prevalence of chest symptoms amongst brick kiln migrant workers and care seeking behaviour: a study from South India. J Public Health (Oxf). 2014. doi: 10.1093/pubmed/fdu104 25538142.
60. Balasubramanian R, Garg R, Santha T, Gopi PG, Subramani R, Chandrasekaran V, et al. Gender disparities in tuberculosis: report from a rural DOTS programme in south India. Int J Tuberc Lung Dis. 2004;8(3):323–32. 15139471.
61. Chandrasekaran V, Ramachandran R, Cunningham J, Balasubramanian R, Thomas A, Sudha G, et al. Factors leading to tuberculosis diagnostic drop-out and delayed treatment initiation in Chennai, India. Int J Tuberc Lung Dis. 2005;9(Supplement 1):S172.
62. Dandona R, Dandona L, Mishra A, Dhingra S, Venkatagopalakrishna K, Chauhan LS. Utilization of and barriers to public sector tuberculosis services in India. Natl Med J India. 2004;17(6):292–9. 15736548.
63. Tripathy JP, Srinath S, Naidoo P, Ananthakrishnan R, Bhaskar R. Is physical access an impediment to tuberculosis diagnosis and treatment? A study from a rural district in North India. Public Health Action. 2013;3(3):235–9. doi: 10.5588/pha.13.0044 26393036.
64. Ahmed J, Chadha VK, Singh S, Venkatachalappa B, Kumar P. Utilization of RNTCP services in rural areas of Bellary District, Karnataka, by gender, age and distance from health centre. Indian J Tuberc. 2009;56(2):62–8. 19810587.
65. Chadha VK, Praseeja P, Gupta J, Ahmed J, Sharada MA, Srivastava R, et al. A descriptive study of tuberculosis case finding in private health care facilities in a South Indian district. Int J Tuberc Lung Dis. 2014;18(12):1455–8. doi: 10.5588/ijtld.14.0228 25517811.
66. Dave P, Nimavat P, Shah A, Pujara K, Patel P, Modi B. Knowing more about initial default among diagnosed sputum smear-positive pulmonary tuberculosis patients in Gujarat, India [Abstract PC-868-03]. Int J Tuberc Lung Dis. 2013;17 (Suppl 2)(12):S469.
67. Dholakia YN. TB/ HIV coordination through Public Private Partnership: lessons from the field. Indian J Tuberc. 2013;60(1):23–7. 23540085.
68. Gopi PG, Chandrasekaran V, Subramani R, Narayanan PR. Failure to initiate treatment for tuberculosis patients diagnosed in a community survey and at health facilities under a DOTS program in a district of south India. Indian J Tuberc. 2005;52 : 153–6.
69. Kumar S. A retrospective cohort study of the magnitude of initial default among sputum smear-positive TB patients diagnosed at NITRD New Delhi, 4th quarter 2012. India EIS Conferece; 2013 Nov 21–23; New Delhi, India.
70. Mandal A, Basu M, Das P, Mukherjee S, Das S, Roy N. Magnitude and reasons of initial default among new sputum positive cases of pulmonary tuberculosis under RNTCP in a district of West Bengal, India. South East Asia J of Public Health. 2015;4(1):41–7. doi: 10.3329/seajph.v4i1.21839
71. Sai Babu B, Satyanarayana AV, Venkateshwaralu G, Ramakrishna U, Vikram P, Sahu S, et al. Initial default among diagnosed sputum smear-positive pulmonary tuberculosis patients in Andhra Pradesh, India. Int J Tuberc Lung Dis. 2008;12(9):1055–8. 18713504.
72. Mehra RK, Dhingra VK, Nish A, Vashist RP. Study of relapse and failure cases of CAT I retreated with CAT II under RNTCP—an eleven year follow up. Indian J Tuberc. 2008;55(4):188–91. 19295105.
73. Mahishale V, Patil B, Lolly M, Eti A, Khan S. Prevalence of Smoking and Its Impact on Treatment Outcomes in Newly Diagnosed Pulmonary Tuberculosis Patients: A Hospital-Based Prospective Study. Chonnam Med J. 2015;51(2):86–90. doi: 10.4068/cmj.2015.51.2.86 26306303.
74. Swaminathan S, Deivanayagam CN, Rajasekaran S, Venkatesan P, Padmapriyadarsini C, Menon PA, et al. Long term follow up of HIV-infected patients with tuberculosis treated with 6-month intermittent short course chemotherapy. Natl Med J India. 2008;21(1):3–8. 18472696.
75. Sharma SK, Soneja M, Prasad KT, Ranjan S. Clinical profile & predictors of poor outcome of adult HIV-tuberculosis patients in a tertiary care centre in north India. Indian J Med Res. 2014;139(1):154–60. 24604050.
76. Das J, Holla A, Das V, Mohanan M, Tabak D, Chan B. In urban and rural India, a standardized patient study showed low levels of provider training and huge quality gaps. Health Aff (Millwood). 2012;31(12):2774–84. doi: 10.1377/hlthaff.2011.1356 23213162.
77. Floyd K, Arora VK, Murthy KJ, Lonnroth K, Singla N, Akbar Y, et al. Cost and cost-effectiveness of PPM-DOTS for tuberculosis control: evidence from India. Bull World Health Organ. 2006;84(6):437–45. /S0042-96862006000600012. doi: 10.2471/BLT.05.024109 16799727.
78. McDowell A, Pai M. Alternative medicine: an ethnographic study of how practitioners of Indian medical systems manage TB in Mumbai. Trans R Soc Trop Med Hyg. 2016;110(3):192–8. doi: 10.1093/trstmh/trw009 26884500.
79. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(1):140–56. doi: 10.1183/09031936.00070812 22936710.
80. Kranzer K, Lawn SD, Meyer-Rath G, Vassall A, Raditlhalo E, Govindasamy D, et al. Feasibility, yield, and cost of active tuberculosis case finding linked to a mobile HIV service in Cape Town, South Africa: a cross-sectional study. PLoS Med. 2012;9(8):e1001281. doi: 10.1371/journal.pmed.1001281 22879816.
81. Tiam A, Machekano R, Gounder CR, Maama-Maime LB, Ntene-Sealiete K, Sahu M, et al. Preventing tuberculosis among HIV-infected pregnant women in Lesotho: the case for rolling out active case finding and isoniazid preventive therapy. J Acquir Immune Defic Syndr. 2014;67(1):e5–e11. doi: 10.1097/qai.0000000000000209 25118796.
82. Silva DR, Muller AM, Tomasini Kda S, Dalcin Pde T, Golub JE, Conde MB. Active case finding of tuberculosis (TB) in an emergency room in a region with high prevalence of TB in Brazil. PLoS ONE. 2014;9(9):e107576. doi: 10.1371/journal.pone.0107576 25211158.
83. Yuen CM, Amanullah F, Dharmadhikari A, Nardell EA, Seddon JA, Vasilyeva I, et al. Turning off the tap: stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet. 2015. doi: 10.1016/S0140-6736(15)00322-0 26515675
84. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):381–91. doi: 10.1093/cid/cit643 24065336.
85. Chadha VK, Praseeja P, Hemanthkumar NK, Shivshankara BA, Sharada MA, Nagendra N, et al. Are registered sputum smear-negative tuberculosis patients in Karnataka, India, diagnosed by national algorithm? Int J Tuberc Lung Dis. 2014;18(12):1491–5. doi: 10.5588/ijtld.14.0216 25517817.
86. TB Diagnostics Market Analysis Consortium. Market assessment of tuberculosis diagnostics in India in 2013. Int J Tuberc Lung Dis. 2016;20(3):304–13. Appendix Figure A.3. doi: 10.5588/ijtld.15.0571 27046709
87. Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20(9):1128–45. doi: 10.1111/tmi.12534 25943163.
88. Arinaminpathy N, Batra D, Khaparde S, Vualnam T, Maheshwari N, Sharma L, et al. The number of privately treated tuberculosis cases in India: an estimation from drug sales data. Lancet Infect Dis. 2016. Epub 2016 Aug 24. http://dx.doi.org/10.1016/S1473-3099(16)30259-6. 27568356
89. Mistry N, Rangan S, Dholakia Y, Lobo E, Shah S, Patil A. Durations and Delays in Care Seeking, Diagnosis and Treatment Initiation in Uncomplicated Pulmonary Tuberculosis Patients in Mumbai, India. PLoS ONE. 2016;11(3):e0152287. doi: 10.1371/journal.pone.0152287 27018589.
90. Jain S, Sankar MM, Sharma N, Singh S, Chugh TD. High prevalence of non-tuberculous mycobacterial disease among non-HIV infected individuals in a TB endemic country—experience from a tertiary center in Delhi, India. Pathog Glob Health. 2014;108(2):118–22. doi: 10.1179/2047773214y.0000000133 24649868.
91. Atre S, D'Souza B, Dholakia Y, Mistry N. Observations on categorisation of new TB cases: implications for controlling drug resistance. Int J Tuberc Lung Dis. 2007;11(10):1152–53. 17966562
92. Rade K. TB cascade of care final review: discussion on duplicate diagnoses [Internet]. Email message to: Ramnath Subbaraman (ramnath.sub@gmail.com). 2016 March 22–24 [cited 2016 June 8]. [4 paragraphs].
93. Sreeramareddy CT, Qin ZZ, Satyanarayana S, Subbaraman R, Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis. 2014;18(3):255–66. doi: 10.5588/ijtld.13.0585 24670558.
94. Charalambous S, Grant AD, Moloi V, Warren R, Day JH, van Helden P, et al. Contribution of reinfection to recurrent tuberculosis in South African gold miners. Int J Tuberc Lung Dis. 2008;12(8):942–8. 18647455.
95. Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171(12):1430–5. doi: 10.1164/rccm.200409-1200OC 15831840.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Sailing in Uncharted Waters: Carefully Navigating the Polio Endgame
- Core Outcome Set–STAndards for Reporting: The COS-STAR Statement
- Improving the Science of Measles Prevention—Will It Make for a Better Immunization Program?
- Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study
- Population Immunity against Serotype-2 Poliomyelitis Leading up to the Global Withdrawal of the Oral Poliovirus Vaccine: Spatio-temporal Modelling of Surveillance Data
- Conveying Equipoise during Recruitment for Clinical Trials: Qualitative Synthesis of Clinicians’ Practices across Six Randomised Controlled Trials
- How Relevant Is Sexual Transmission of Zika Virus?
- A Holistic, Person-Centred Care Model for Victims of Sexual Violence in Democratic Republic of Congo: The Panzi Hospital One-Stop Centre Model of Care
- Impact on Epidemic Measles of Vaccination Campaigns Triggered by Disease Outbreaks or Serosurveys: A Modeling Study
- The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling
- Circulating Apolipoprotein E Concentration and Cardiovascular Disease Risk: Meta-analysis of Results from Three Studies
- Towards Equity in Service Provision for Gay Men and Other Men Who Have Sex with Men in Repressive Contexts
- Obstetric Facility Quality and Newborn Mortality in Malawi: A Cross-Sectional Study
- Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study)
- Characterization of Novel Antimalarial Compound ACT-451840: Preclinical Assessment of Activity and Dose–Efficacy Modeling
- Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial
- Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study
- Tradeoffs in Introduction Policies for the Anti-Tuberculosis Drug Bedaquiline: A Model-Based Analysis
- Whole Genome Sequence Analysis of a Large Isoniazid-Resistant Tuberculosis Outbreak in London: A Retrospective Observational Study
- Texas and Its Measles Epidemics
- Impacts on Breastfeeding Practices of At-Scale Strategies That Combine Intensive Interpersonal Counseling, Mass Media, and Community Mobilization: Results of Cluster-Randomized Program Evaluations in Bangladesh and Viet Nam
- The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study)
- Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial
- Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study
- Improving the Science of Measles Prevention—Will It Make for a Better Immunization Program?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



