-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
HIV Shedding from Male Circumcision Wounds in HIV-Infected Men: A Prospective Cohort Study
In this prospective cohort study, Aaron Tobian and colleagues examine the associations between male circumcision wound healing, as well as plasma viral load, and HIV shedding from male circumcision wounds.
Published in the journal: . PLoS Med 12(4): e32767. doi:10.1371/journal.pmed.1001820
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001820Summary
In this prospective cohort study, Aaron Tobian and colleagues examine the associations between male circumcision wound healing, as well as plasma viral load, and HIV shedding from male circumcision wounds.
Introduction
Three randomized trials demonstrated that voluntary medical male circumcision, hereafter referred to as male circumcision (MC), significantly decreases heterosexual HIV acquisition in men by 50%–60% [1–3]. MC of HIV-negative men also reduces herpes simplex virus type 2 (HSV-2) and human papillomavirus (HPV) infection among heterosexual men and provides benefits to female partners, including reduced prevalence of genital ulcer disease (GUD), bacterial vaginosis, Trichomonas vaginalis, and HPV [4–14]. MC may also reduce incident syphilis in both men and their female partners [15]. MC of HIV-infected men reduces GUD and HPV [16,17]. Observational studies showed that MC prior to puberty decreased HIV transmission [18,19]. However, MC of HIV-infected men in a randomized trial had no impact on HPV transmission [20], and it increased HIV transmission to men’s female partners among couples who engaged in sexual intercourse prior to wound healing, though not among couples who delayed resumption of sex [17].
As MC has become more normative, HIV-infected men have sought to become circumcised for multiple reasons, including because of perceived health benefits, because they think they are HIV-negative and are seeking protection against HIV, or to avoid stigma [21]. If they are denied MC through organized programs, some HIV-infected men are likely to seek it from unsafe sources, potentially resulting in post-surgical complications and increasing the risk of HIV transmission [17,22]. Given these concerns, the World Health Organization (WHO) recommends that, although MC should not be promoted for HIV-infected men, these men should not be denied the service if they request it [21].
Even with counseling of post-surgical sexual abstinence, 65% of married men and approximately 30% of all men report resuming sexual intercourse prior to wound healing [23,24]. A better understanding of how MC might increase HIV transmission to female partners and of possible prevention methods in the immediate post-surgical period is urgently needed. A study of HIV-infected men found increased HIV shedding from MC wounds in the first weeks after the procedure [25]. To our knowledge, the association of HIV shedding from MC wounds and plasma viral load (VL), and the potential impact of antiretroviral therapy (ART) on HIV shedding have not been assessed. Here we report the frequency and quantity of penile HIV shedding among HIV-infected men undergoing MC to assess the a priori hypotheses that penile HIV shedding increases immediately after surgery, shedding is associated with plasma VL, and ART suppresses penile HIV shedding.
Methods
Study Population and Design
A prospective study was conducted between June 2009 and April 2012 in Rakai, Uganda. Uncircumcised HIV-infected men aged ≥15 y who requested free MC services and had no contraindication to surgery were invited to participate. All participants provided written informed consent prior to enrollment. All HIV-infected men were referred for HIV care. HIV-negative men were enrolled in a concurrent parallel study of MC wound healing to avoid stigmatization of the HIV-infected participants.
All men were offered the standard health services recommended by WHO prior to MC. These services included (1) free voluntary HIV counseling and testing, though acceptance was not a prerequisite for MC, (2) education on HIV prevention, risks and benefits of MC, wound care, and the need to abstain from intercourse until complete wound healing was certified, and (3) treatment of symptomatic sexually transmitted infections. Men were clinically assessed for contraindications to surgery (e.g., hypospadiasis, genital infections, hemoglobin <8.0 g/dl) by trained medical or clinical officers. Conditions such as urethral discharge or GUD were treated and resolved prior to MC.
At enrollment, information on sociodemographics, self-reported current ART use, and sexual behaviors was collected using structured questionnaires. Venous blood for HIV serology, plasma VL testing, and CD4 count was collected prior to surgery. MCs were conducted by trained clinical officers (96%) and medical officers (4%) by excising the foreskin using the dorsal slit method under aseptic conditions. All participants were followed weekly for 6 wk and then at 8 and 12 wk. Information on sexual behaviors and blood for plasma VL testing were obtained at each follow-up visit. Wound healing was assessed via direct observation starting at the 3-wk post-operative visit by clinical officers. Certified complete wound healing was defined as an intact scar with no scabs, sutures, or stitch sinuses.
Lavage samples to assess HIV viral shedding at the coronal sulcus prior to MC and at the MC surgical site were also collected. The lavage involved pouring 5 ml of phosphate buffered saline (pH 7.2) over the coronal sulcus or wound site. The lavage fluid was collected into a sterile cup, aliquoted in 1-ml vials, and stored at −80°C until assay, as described previously [26]. Lavage samples were collected just prior to surgery, intra-operatively immediately after suturing but prior to the routine end-of-surgery wash with normal saline, at 7 d post-MC following removal of the Sofra-Tulle dressing, then weekly during the follow-up visits. Samples at 5 and 8 wk were not evaluated since preliminary data showed essentially no HIV shedding from MC wounds from 5 wk post-MC onwards.
HIV and CD4 Cell Count Testing
HIV status was determined by two enzyme immunoassays: the Murex HIV 1.2.0 followed by Vironostika HIV-1/2 Plus O (Organon Teknika). Discordant and indeterminate samples were subjected to BioMérieux Vitek Western blot confirmation, and to reverse transcription PCR (RT-PCR) when Western blot results were indeterminate. Determination of CD4+ T cell count at baseline used a three-color FACSCalibur (Becton-Dickinson).
Plasma and Lavage HIV Viral Load Testing
Plasma HIV-1 RNA levels (VLs) were determined by an RT-PCR assay (Abbott Laboratories). Men were considered to have an undetectable plasma VL if they had <400 copies/ml at enrollment.
MC lavage samples were assessed using a modified version of the Abbott Laboratories RT-PCR assay with a detection limit of 40 copies/ml. The manufacturer’s instructions were followed with only four exceptions: (1) a total nucleic acid extraction was performed, (2) calibrators were included to perform the assay as a quantitative test, (3) microparticles were increased from 40 μl to 80 μl to increase analytical sensitivity, and (4) an extended drying step (15 min total) was performed to ensure that no ethanol or beads were in the final product.
Statistical Analysis
As described in the prospective analysis plan (see S1 Protocol), we evaluated three hypotheses: (1) penile HIV shedding correlates with wound healing, (2) the frequency and quantity of penile HIV shedding correlate with plasma VL, and (3) ART suppression of HIV plasma VL is associated with decreased penile HIV shedding.
The demographic and clinical characteristics of participants at baseline were tabulated by (1) self-reported not on ART, (2) self-reported ART use with detectable plasma VL, and (3) self-reported ART use with undetectable VL. Differences between groups were estimated using chi-square and Wilcoxon-Mann-Whitney tests for categorical and continuous variables, respectively. The categories for VL and CD4 count were decided using clinically accepted thresholds [27].
The probability of detectable penile HIV shedding at each visit (and 95% CI) for each treatment group was estimated using a modified Poisson regression model with generalized estimating equations (GEE) and a robust variance estimator [28]. This model included main effects for treatment status and weekly visit only. Interaction terms between treatment and visit were not included because no penile shedding was observed at several weekly visits among those who self-reported ART use. While logistic regression is frequently used for binary outcome analyses, modified Poisson regression is the preferred method to estimate relative risk when the outcome is common (>10%) because the odds ratio does not approximate to the relative risk for frequent outcomes [28–30]. However, the results were similar when using either logistic regression or Poisson regression (S1 Fig; S1 Table). We felt that the Poisson estimates were preferable because they are more conservative and more closely approximated the unadjusted risk estimates.
In a separate model, we estimated the prevalence risk ratio (PRR) of detectable lavage HIV-1 relative to baseline among participants not on ART using a modified Poisson regression model with GEE and a robust variance estimator. This secondary analysis included only data from participants not on ART and included only a main effect for visit.
Differences between time to wound healing and time to resumption of sexual intercourse were assessed using the log-rank test for survival data. We also tested for differences in absolute levels of lavage HIV VL (log10 copies/milliliter of lavage fluid) among those with detectable lavage HIV using Wilcoxon-Mann-Whitney tests. We compared groups stratified by study visit, ART/VL group, and plasma VL at the time of lavage.
We also analyzed risk factors for a detectable lavage HIV at weekly visits after MC (1–4, 6, and 12 wk post-MC) using modified Poisson regression models and GEE robust variance estimators. We excluded baseline data from these analyses since risk factors for penile HIV shedding after MC may be different from the risk factors before MC. The adjusted analysis included the primary exposure variable, plasma VL, and potential confounding variables. Confounders included those covariates that were associated with plasma VL and penile HIV shedding after MC in univariate analyses at p<0.10. In addition to plasma VL, the final adjusted model included baseline CD4 count, wound healing status, treatment with co-trimoxazole, and penile HIV shedding status at baseline. Resumption of sex and ART status variables were not included in our adjusted model because of collinearity with wound healing status and plasma VL, respectively. Religion and condom use and number of sex partners in the last year prior to MC were not associated with penile HIV shedding after MC and were not included the final adjusted model. All analyses were conducted in R version 3.0.1 using the “geepack” package.
Study Power
Based on preliminary observations, the study was designed to detect whether post-MC HIV wound shedding was associated with plasma VL. Among a proposed population of 131 HIV-infected men, we expected that 116 men would have a detectable plasma VL at the time of MC. The study was designed to be able to detect with 80% power and two-sided alpha = 0.05 whether men with detectable plasma VL were 4.4 times more likely to have HIV wound shedding post-surgery compared to those without detectable HIV plasma VL. In addition, we expected that HIV shedding from MC wounds would decrease with wound healing and would depend on pre-surgical HIV plasma VL. Assuming a standard deviation of log10 plasma VL of 0.74 (from preliminary data during the grant submission), the study was designed to detect with 80% power and two-sided alpha = 0.05 a risk ratio of 0.7 with a one-unit increase in log10 plasma VL. While the study was initially powered to evaluate 131 HIV-infected men, other outcomes (e.g., safety of MC and wound healing) required a larger sample size and a longer follow-up interval. Thus, we included all consenting HIV-infected men in this study rather than randomly sampling the study population to include 131 HIV-infected men as originally planned.
Results
Study Participants
There were 712 HIV-negative and HIV-infected men invited to participate. HIV-negative men were enrolled in a parallel study to mask HIV status in the HIV-infected cohort. There were 332 HIV-infected men who agreed to participate in the study (Fig 1). The first 96 HIV-infected men who agreed to participate were excluded since no demographic or CD4 count data were collected at the beginning of the study. Of the 236 men who completed enrollment, 195 (82.6%) were not on ART, and 183 (93.8%) of these men had a baseline and at least one follow-up lavage sample. Among the 41 men (17.4%) who reported ART use, 40 (97.6%) had a baseline and at least one-follow-up lavage sample. Of these 40 men, 29 (72.5%) had undetectable plasma VL and 11 (27.5%) had a detectable plasma VL at enrollment. Thus, there were 223 men included in the analysis.
Fig. 1. Study flow diagram. 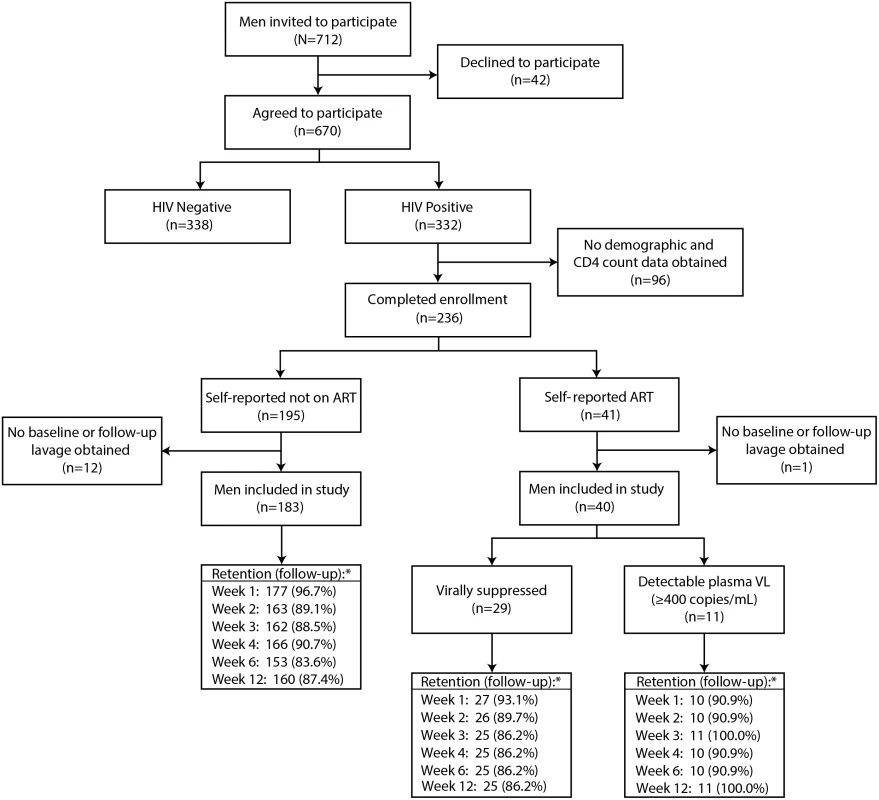
The retention (asterisk) indicates the number (percent) of men at each follow-up visit. Penile HIV shedding was evaluated for 96.9% (216/223) of male participants at the surgical visit immediately after MC and at 89.4% (1,196/1,338) of the scheduled weekly visits after MC through week 12. Individuals who missed weekly follow-up visits tended to be younger and Catholic and were less likely to have detectable penile HIV shedding at baseline. Neither self-reported ART status nor baseline CD4 was statistically significantly associated with there being missing outcome data (S2 Table).
The three ART/VL groups of men had similar distributions of age, marital status, and presence of GUD (Table 1). The men who reported ART use had fewer sexual partners, and higher condom and co-trimoxazole use than men not on ART. The median CD4 count was 466 cells/μl (interquartile range [IQR] = 314–655) in men not on ART, 358 cells/μl (IQR = 258–534) in men reporting ART use with an undetectable plasma VL, and 181 cells/μl (IQR = 81–249) for men reporting ART use but with a detectable plasma VL. The median plasma VLs were comparable in the men not on ART and the men on ART with detectable VL. Among men not on ART, 17 (9.3%) had undetectable VL at enrollment.
Tab. 1. Enrollment characteristics. 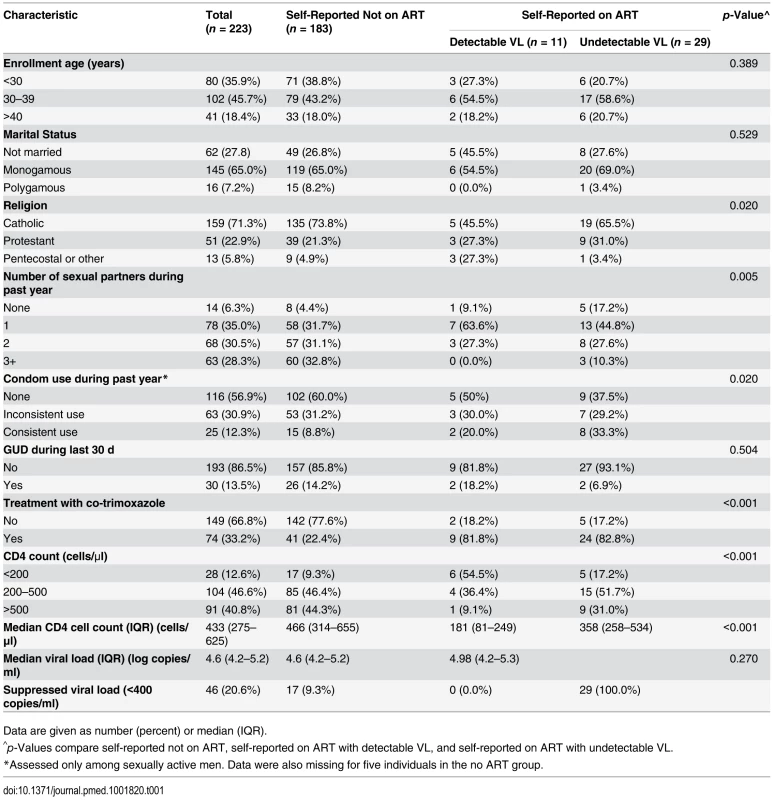
Data are given as number (percent) or median (IQR). HIV Shedding from Male Circumcision Wounds
Among all men, penile HIV shedding was detected among 1.9% (5/263) of the weekly visits in men with an undetectable plasma VL and among 14.0% (123/877) of the weekly visits in men with a detectable plasma VL (PRR = 0.13, 95% CI = 0.06–0.31). In multivariate analyses, HIV shedding from MC wounds occurred in more study visits among men with an HIV plasma VL >50,000 copies/ml than among those with an undetectable VL (adjusted PRR [adjPRR] = 10.3, 95% CI = 4.25–24.90, p<0.001) (Table 2). HIV shedding was less common in visits from men with healed MC wounds compared to those without fully healed wounds (adjPRR = 0.12, 95% CI = 0.07–0.23, p<001). Of the 14 visits at which shedding was detected at or after certified wound healing, 8 (57%) were at the visit when wound healing was certified. HIV shedding was also less common in visits from men who reported ART use and had an undetectable plasma VL compared to men not on ART (PRR = 0.15, 95% CI = 0.05–0.43, p = 0.001), and this difference remained statistically significant even after adjusting for treatment with co-trimoxazole, healed wounds, HIV shedding prior to MC, and baseline CD4 count (adjPRR = 0.13, 95% CI = 0.04–0.45, p = 0.001).
Tab. 2. Associations with the detection of penile HIV shedding. 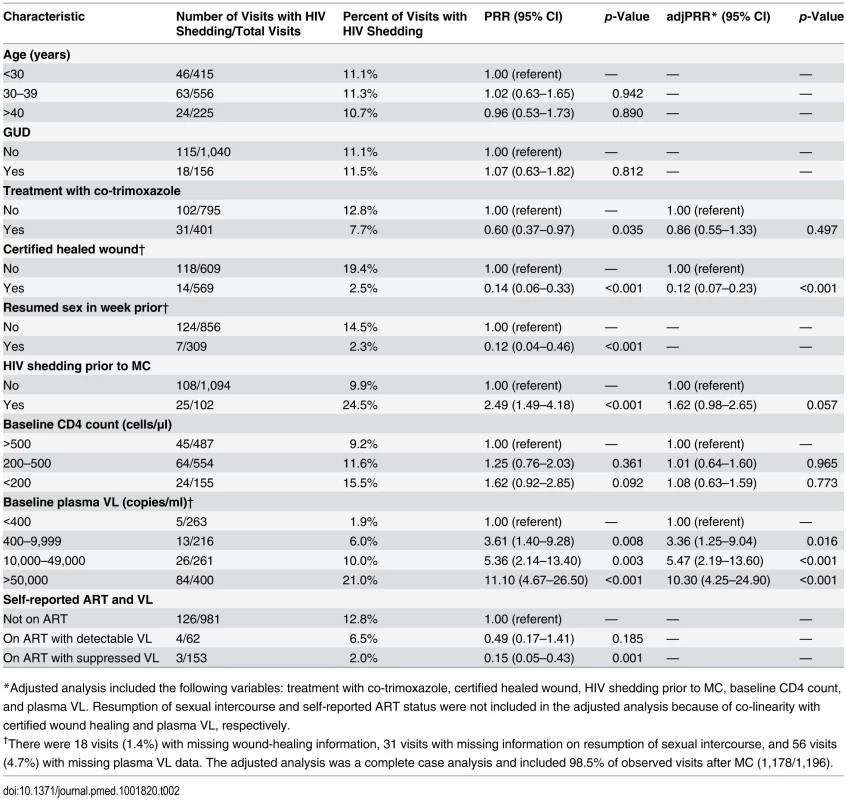
*Adjusted analysis included the following variables: treatment with co-trimoxazole, certified healed wound, HIV shedding prior to MC, baseline CD4 count, and plasma VL. Resumption of sexual intercourse and self-reported ART status were not included in the adjusted analysis because of co-linearity with certified wound healing and plasma VL, respectively. HIV Shedding from Male Circumcision Wounds Stratified by ART Status
Among men not on ART, penile HIV shedding was detected at 12.8% (126/981) of the weekly visits after MC and in 39.3% (72/183) of the men (Fig 2A). Prior to MC, HIV shedding was detected in 9.3% (17/183) of men not on ART, and during surgery HIV shedding was detected in 57.6% (102/177) of these men. Relative to baseline, the probability of detectable HIV shedding was significantly increased after MC at 1 wk (17.5% [31/177], PRR = 1.87, 95% CI = 1.12–3.14, p = 0.012), 2 wk (29.4% [48/163], PRR = 3.16, 95% CI = 1.94–5.13, p<0.001), and 3 wk (18.5% [30/162], PRR = 1.98, 95% CI = 1.19–3.28, p = 0.008). However, detectable HIV shedding was significantly lower than at baseline by 6 wk after MC (2.6% [4/153], PRR = 0.27, 95% CI = 0.09–0.83, p = 0.023). Twelve weeks after MC the proportion of men not on ART with detectable penile HIV shedding was only 1.9% (3/160, PRR = 0.19, 95% CI = 0.06–0.64, p = 0.008).
Fig. 2. Penile HIV shedding, wound healing, and resumption of sex stratified by plasma viral load and ART status. 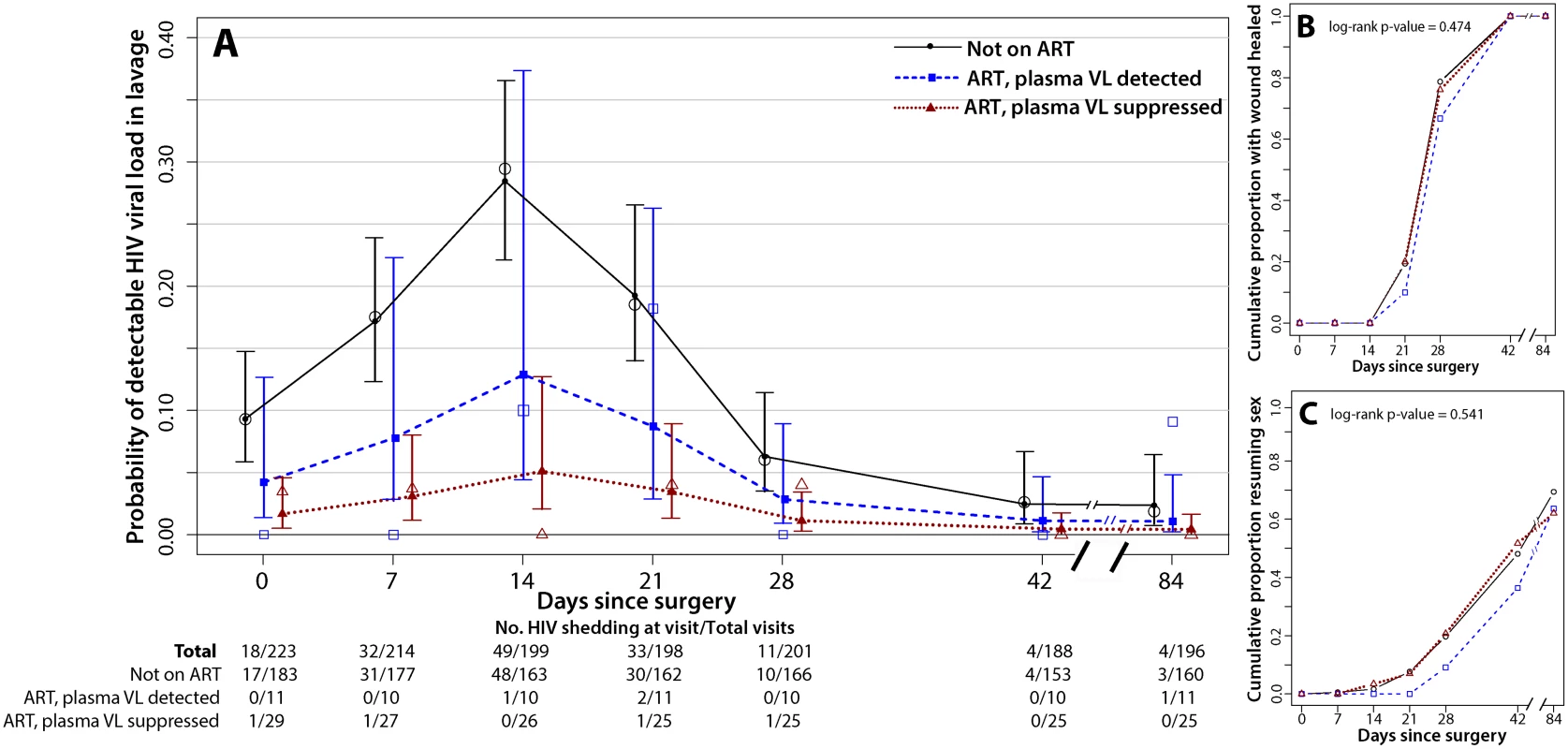
The day 0 visit is prior to surgery. (A) HIV shedding from MC wounds increased at 7, 14, and 21 d after MC. The open symbols are the empirical data, and the filled symbols and line are the fit estimates. The error bars represent the 95% CIs of the fitted values. (B) There was no difference in certified wound healing between the three ART/VL groups. (C) There was no difference in the proportion of men resuming sex between the three ART/VL groups. Men not on ART at baseline may have initiated treatment during the study. However, among the 165 men who self-reported not being on ART and who had a detectable plasma VL at enrollment, only nine (5.4%) had an undetectable plasma VL at their final follow-up visit. In a sensitivity analysis restricted to men who self-reported not being on ART and who had a detectable plasma VL both at enrollment and at the final follow-up visit, the probability of penile HIV shedding was still significantly reduced at 6 wk (PRR = 0.21, 95% CI = 0.06–0.75, p = 0.017) and 12 wk (PRR = 0.21, 95% CI = 0.06–0.70, p = 0.011).
Among the 11 men who reported ART use but had a detectable plasma VL at enrollment, three men (27.3%) had detectable penile HIV shedding, detected at four of the total of 62 weekly visits after MC (6.5%) (Fig 2A). Prior to MC, no HIV shedding was detected among these men, but during surgery, HIV shedding was detected among 45.5% (5/11) of these men. The probability of detectable shedding at any weekly visit after MC was not significantly lower than in men not on ART (PRR = 0.49, 95% CI = 0.17–1.41, p = 0.185) (Table 2). Two of the 11 men who reported ART use but had a detectable plasma VL at enrollment then had undetectable plasma VL by 6 wk. Both of these men also had undetectable plasma VL at 12 wk; however, one man had detectable penile HIV shedding at his final study visit.
Among the 29 men who reported ART use and had an undetectable plasma VL at baseline, there were three men (10.3%) with HIV shedding, detected at three of the total of 153 weekly visits after MC (2.0%) (Fig 2A). Prior to MC, HIV shedding was detected among 3.4% (1/29) of these men, and during surgery, HIV shedding was detected among 28.6% (8/28) of these men. The probability of detectable HIV shedding was significantly lower after MC for men reporting ART use who had an undetectable VL at baseline than for men not on ART (PRR = 0.15, 95% CI = 0.05–0.43, p = 0.001) (Table 2).
Wound Healing and Resumption of Sex
Time to wound healing and time to resumption of sexual intercourse did not vary between the three ART/VL groups (p = 0.474 and p = 0.541, respectively) (Fig 2B and 2C). All of the men in the study were fully healed by 6 wk post-MC (Fig 2B); 48% (n = 107) of the men in the study resumed sexual intercourse prior to 6 wk post-MC, and this did not vary by ART/VL group (Fig 2C).
HIV Viral Load from Male Circumcision Wounds
Among all men with detectable penile HIV shedding, the median log10 HIV shedding was significantly higher at 1 wk (2.99, IQR = 2.59–3.02, p = 0.008) and 2 wk (3.20, IQR = 2.83–3.56, p = 0.008) compared to prior to surgery (2.42, IQR = 2.11–2.86) or during surgery immediately following foreskin removal (2.49, IQR = 2.13–2.58) (Fig 3A). The median log10 penile HIV shedding increased significantly with higher plasma VL (Figs 3B and S2).
Fig. 3. Penile shedding HIV viral load stratified by ART status and plasma viral load. 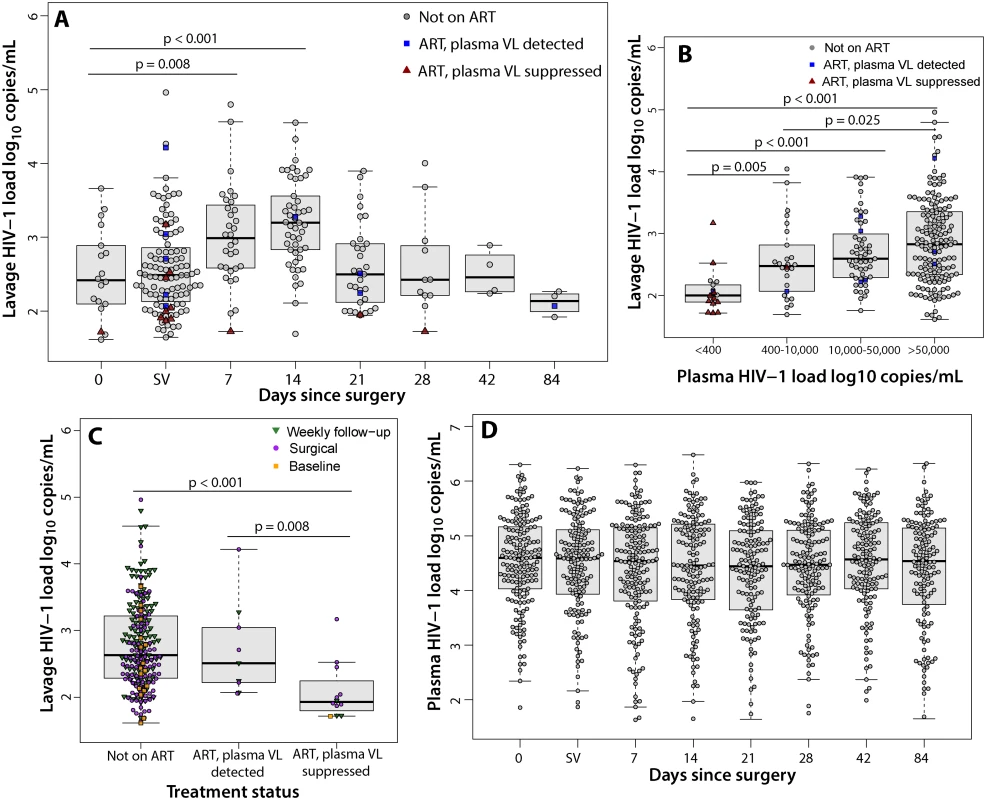
(A) The median log10 penile shedding VL increased at 7 and 14 d after surgery. Day 0 represents the visit prior to MC. SV represents the surgical visit. (B) The median log10 penile shedding VL is associated with increasing plasma VL. (C) The median log10 penile shedding VL is decreased among men on ART with an undetectable plasma VL. (D) The median log10 plasma VL does not increase after MC. The median log10 penile HIV shedding was significantly lower among men on ART who had an undetectable plasma VL (1.93, IQR = 1.83–2.14) compared to men not on ART (2.63, IQR = 2.28–3.22, p<0.001) or men with reported ART use but who had a detectable plasma VL (2.51, IQR = 2.22–3.04, p = 0.008) (Fig 3C). There were no significant increases in plasma VL after MC (Fig 3D). Among men who reported ART use at baseline and had an undetectable plasma VL, only 3.4% (1/29) had detectable plasma VL after MC.
Discussion
HIV shedding from MC wounds was detected among 39% of HIV-infected men who reported not being on ART during the wound healing phase, but was detected among a significantly smaller number of individuals who reported ART use and had an undetectable plasma VL. HIV shedding was most common among men without complete wound healing. To our knowledge, this is the first study to suggest that ART decreases both the frequency of detected HIV shedding and the quantity of virus shed from MC wounds.
HIV shedding at 6 and 12 wk post-MC, when all MC wounds had healed, was significantly lower than prior to MC, as reported in one previous study [25]. These data support observational studies and mathematical models suggesting that MC performed prior to men’s sexual debut (i.e., before they become sexually active) may have long-term benefits for decreasing HIV transmission to women [18,19,23,31]. The decreased shedding 6 wk after MC may be due to the removal of the foreskin and formation of an intact scar.
While there was no change in plasma VL post-MC, penile HIV shedding VL increased at 1 and 2 wk after MC, even compared to samples obtained during surgery that were clearly contaminated with blood. This increase may be due to inflammation and recruitment of HIV target cells during wound healing [32,33].
This study has limitations. Only 73% of men self-reporting ART use had an undetectable plasma VL, similar to rates seen elsewhere in Africa and in developed countries [34,35]. Since the large majority of men received ART outside of our care program, we were unable to confirm actual receipt of ART. In addition, men self-reported whether they were currently taking ART; it is unknown whether men had previously taken ART or recently stopped ART. However, >90% of men who did not report ART use had a detectable plasma VL. The standard ART regimen in Uganda is efavirenz/lamivudine/tenofovir and has been reported to reduce plasma VL to <400 copies/ml in 55% of individuals within 2 wk of initiation, and in 65% of individuals by 4 wk [36,37]. However, only nine men not on ART (5.4%) in this study had an undetectable plasma VL after having a detectable plasma VL at enrollment. In a sensitivity analysis that evaluated men not on ART who had a detectable plasma VL both at enrollment and at the final follow-up visit, the probability of penile HIV shedding remained significantly lower at 6 and 12 wk compared to prior to MC. In addition, the proportion of men with an undetectable plasma VL does not affect the finding that men who were virally suppressed by ART had significantly fewer instances of HIV shedding and shed at a lower VL. Due to the observational nature of this study, the findings are associations and do not necessarily imply causality. Another limitation of this observational study is that there were significant differences in covariates at enrollment, which raises the possibility of residual uncontrolled confounding. The rates of HIV shedding from MC wounds presented here are lower than in one previous report [25]. This could be due to differences in sample collection, assay sensitivity, surgical procedure, or plasma VL.
Ideally, MC programs in sub-Saharan Africa should target HIV-negative men to prevent HIV acquisition, although it is inevitable that some HIV-infected men will request the procedure. WHO recommends that although MC should not be promoted for HIV-infected men, they should not be denied MC unless they have contraindications for surgery [21]. Programs designed to provide MC prior to sexual debut (such as in early adolescence or as a neonate, at which age only a very small proportion of African males are HIV-infected or sexually active) would avoid potential problems of HIV transmission during wound healing [38,39] and are cost-effective [40]. However, in reality, programs offering MC to adult men will remain an important HIV-prevention priority for the foreseeable future in order decrease HIV incidence as rapidly as possible. Approximately 6% of all men requesting MC are HIV-infected [23]. Because MC is becoming more normative in regions where it is promoted, it is likely that more HIV-infected men will request the procedure. A mathematical model evaluating MC in a general population of HIV-negative and HIV-infected men living in sub-Saharan Africa, with a proportion having sexual intercourse during the MC wound healing phase, predicted an overall 0.06% increase in HIV transmission to female partners [23]. With the WHO goal to circumcise 28.7 million men [41], this could lead to 17,000 new infections in female partners.
We do not know the impact of penile HIV shedding from MC wounds on actual transmission to female partners, but higher plasma VL increases transmission risk [27], as does HIV shedding from genital ulcers [42,43]. Thus, it is plausible, and supported by the trial of MC in HIV-infected men [17], that viral shedding from a post-MC wound increases risk of transmission. A large randomized trial enrolling both men and female partners with long-term follow-up would be needed to confirm these results, but this type of trial may be logistically unfeasible. However, it is important to ensure that HIV transmission to female partners in the immediate post-surgical period is avoided. The findings from this study reinforce the need for MC programs to provide voluntary HIV counseling and testing prior to MC, promote sexual abstinence during wound healing and condom use thereafter, offer free condoms, and encourage counseling for HIV-infected men and their sexual partners on the risk of HIV transmission if sexual intercourse is resumed prior to complete wound healing.
While additional studies would need to be performed, we believe that consideration should be given to initiating ART prior to MC for HIV-infected men and encouraging adherence to suppress the plasma VL. This strategy would be similar to strategies employed for prevention of mother-to-child transmission. Initiating ART at time of MC or prior to MC could be difficult for MC programs, particularly those employing mobile MC camps. However, with recent WHO guidelines increasing the CD4 count treatment initiation threshold to ≤500 cells/μl [44], the majority of HIV-infected men seeking MC would already qualify for continued lifelong ART [45]. Men underutilize ART in sub-Saharan Africa [46], so incorporating ART treatment referral into MC programs may increase utilization, improve the health of these men, protect their female partners, and help integrate the health care delivery systems in sub-Saharan Africa. However, further research is needed to investigate the acceptability of, feasibility of, and time required for men initiating ART prior to MC to reduce HIV shedding from MC wounds.
Supporting Information
Zdroje
1. Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, et al. (2007) Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369 : 657–666. 17321311
2. Bailey RC, Moses S, Parker CB, Agot K, Maclean I, et al. (2007) Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369 : 643–656. 17321310
3. Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, et al. (2005) Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2: e298. 16231970
4. Gray RH, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, et al. (2009) The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 200 : 42 e1–7. doi: 10.1016/j.ajog.2008.07.069 18976733
5. Wawer MJ, Tobian AA, Kigozi G, Kong X, Gravitt PE, et al. (2011) Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet 277 : 209–218.
6. Sobngwi-Tambekou J, Taljaard D, Lissouba P, Zarca K, Puren A, et al. (2009) Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis 199 : 958–964. doi: 10.1086/597208 19220143
7. Auvert B, Sobngwi-Tambekou J, Cutler E, Nieuwoudt M, Lissouba P, et al. (2009) Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in orange farm, South Africa. J Infect Dis 199 : 14–19. doi: 10.1086/595566 19086814
8. Gray RH, Serwadda D, Kong X, Makumbi F, Kigozi G, et al. (2010) Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis 201 : 1455–1462. doi: 10.1086/652184 20370483
9. Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, et al. (2009) Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 360 : 1298–1309. doi: 10.1056/NEJMoa0802556 19321868
10. Tobian AA, Gray RH, Quinn TC (2010) Male circumcision for the prevention of acquisition and transmission of sexually transmitted infections: the case for neonatal circumcision. Arch Pediatr Adolesc Med 164 : 78–84. doi: 10.1001/archpediatrics.2009.232 20048246
11. Senkomago V, Backes DM, Hudgens MG, Poole C, Agot K, et al. (2015) Acquisition and persistence of human papillomavirus 16 (HPV-16) and HPV-18 among men with high-HPV viral load infections in a circumcision trial in Kisumu, Kenya. J Infect Dis 211 : 811–820. doi: 10.1093/infdis/jiu535 25261492
12. Gray RH, Serwadda D, Tobian AA, Chen MZ, Makumbi F, et al. (2009) Effects of genital ulcer disease and herpes simplex virus type 2 on the efficacy of male circumcision for HIV prevention: analyses from the Rakai trials. PLoS Med 6: e1000187. doi: 10.1371/journal.pmed.1000187 19936044
13. Liu CM, Hungate BA, Tobian AA, Serwadda D, Ravel J, et al. (2013) Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. mBio 4: e00076. doi: 10.1128/mBio.00076-13 23592260
14. Tobian AA, Kacker S, Quinn TC (2014) Male circumcision: a globally relevant but under-utilized method for the prevention of HIV and other sexually transmitted infections. Annu Rev Med 65 : 293–306. doi: 10.1146/annurev-med-092412-090539 24111891
15. Pintye J, Baeten JM, Manhart LE, Celum C, Ronald A, et al. (2014) Association between male circumcision and incidence of syphilis in men and women: a prospective study in HIV-1 serodiscordant heterosexual African couples. Lancet Global health 2: e664–71. doi: 10.1016/S2214-109X(14)70315-8 25442691
16. Serwadda D, Wawer MJ, Makumbi F, Kong X, Kigozi G, et al. (2010) Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. J Infect Dis 201 : 1463–1469. doi: 10.1086/652185 20370481
17. Wawer MJ, Makumbi F, Kigozi G, Serwadda D, Watya S, et al. (2009) Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet 374 : 229–237. doi: 10.1016/S0140-6736(09)60998-3 19616720
18. Gray RH, Kiwanuka N, Quinn TC, Sewankambo NK, Serwadda D, et al. (2000) Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. AIDS 14 : 2371–2381. 11089626
19. Baeten JM, Donnell D, Kapiga SH, Ronald A, John-Stewart G, et al. (2010) Male circumcision and risk of male-to-female HIV-1 transmission: a multinational prospective study in African HIV-1-serodiscordant couples. AIDS 24 : 737–744. doi: 10.1097/QAD.0b013e32833616e0 20042848
20. Tobian AA, Kong X, Wawer MJ, Kigozi G, Gravitt PE, et al. (2011) Circumcision of HIV-infected men and transmission of human papillomavirus to female partners: analyses of data from a randomised trial in Rakai, Uganda. Lancet Infect Dis 11 : 604–612. doi: 10.1016/S1473-3099(11)70038-X 21489882
21. Joint United Nations Programme on HIV/AIDS (2007) New data on male circumcision and HIV prevention: policy and programme implications. Montreux: Joint United Nations Programme on HIV/AIDS.
22. Kigozi G, Gray RH, Wawer MJ, Serwadda D, Makumbi F, et al. (2008) The safety of adult male circumcision in HIV-infected and uninfected men in Rakai, Uganda. PLoS Med 5: e116. doi: 10.1371/journal.pmed.0050116 18532873
23. Hewett PC, Hallett TB, Mensch BS, Dzekedzeke K, Zimba-Tembo S, et al. (2012) Sex with stitches: assessing the resumption of sexual activity during the postcircumcision wound-healing period. AIDS 26 : 749–756. doi: 10.1097/QAD.0b013e32835097ff 22269970
24. Herman-Roloff A, Bailey RC, Agot K (2012) Factors associated with the early resumption of sexual activity following medical male circumcision in Nyanza province, Kenya. AIDS Behav 16 : 1173–1181. doi: 10.1007/s10461-011-0073-1 22052231
25. Odoyo-June E, Rogers JH, Jaoko W, Bailey RC (2013) Changes in plasma viral load and penile viral shedding after circumcision among HIV-positive men in Kisumu, Kenya. J Acquir Immune Defic Syndr 64 : 511–517. doi: 10.1097/QAI.0b013e3182a7ef05 23933768
26. Paz-Bailey G, Sternberg M, Lewis DA, Cutler E, Vos M, et al. (2009) Comparison of lavage and swabs for the collection of genital ulcer specimens to measure HIV RNA shedding. J Clin Virol 46 : 165–168. doi: 10.1016/j.jcv.2009.06.025 19628428
27. Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, et al. (2000) Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 342 : 921–929. 10738050
28. Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159 : 702–706. 15033648
29. Barros AJ, Hirakata VN (2003) Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 3 : 21. 14567763
30. McNutt LA, Wu C, Xue X, Hafner JP (2003) Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 157 : 940–943. 12746247
31. Hallett TB, Alsallaq RA, Baeten JM, Weiss H, Celum C, et al. (2011) Will circumcision provide even more protection from HIV to women and men? New estimates of the population impact of circumcision interventions. Sex Transm Infect 87 : 88–93. doi: 10.1136/sti.2010.043372 20966458
32. Johnson KE, Redd AD, Quinn TC, Collinson-Streng AN, Cornish T, et al. (2011) Effects of HIV-1 and HSV-2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. J Infect Dis 203 : 602–609. doi: 10.1093/infdis/jiq091 21220779
33. Johnson KE, Sherman ME, Ssempiija V, Tobian AA, Zenilman JM, et al. (2009) Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. AIDS 23 : 1807–1815. doi: 10.1097/QAD.0b013e32832efdf1 19584700
34. Yehia BR, Fleishman JA, Metlay JP, Moore RD, Gebo KA (2012) Sustained viral suppression in HIV-infected patients receiving antiretroviral therapy. JAMA 308 : 339–342. doi: 10.1001/jama.2012.5927 22820781
35. Elul B, Basinga P, Nuwagaba-Biribonwoha H, Saito S, Horowitz D, et al. (2013) High levels of adherence and viral suppression in a nationally representative sample of HIV-infected adults on antiretroviral therapy for 6, 12 and 18 months in Rwanda. PLoS ONE 8: e53586. doi: 10.1371/journal.pone.0053586 23326462
36. Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, et al. (2004) Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292 : 191–201. 15249568
37. Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, et al. (2007) Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr 46 : 125–133. 17721395
38. Tobian AA, Gray RH (2011) The medical benefits of male circumcision. JAMA 306 : 1479–1480. doi: 10.1001/jama.2011.1431 21972310
39. Morris BJ, Waskett JH, Banerjee J, Wamai RG, Tobian AA, et al. (2012) A ‘snip’ in time: what is the best age to circumcise? BMC Pediatr 12 : 20. doi: 10.1186/1471-2431-12-20 22373281
40. Kacker S, Frick KD, Quinn TC, Gray RH, Tobian AA (2013) Financial implications of male circumcision scale-up for the prevention of HIV and other sexually transmitted infections in a sub-Saharan African community. Sex Transm Dis 40 : 559–568. doi: 10.1097/OLQ.0b013e3182945e56 23965771
41. Joint United Nations Programme on HIV/AIDS (2011) Joint strategic action framework to accelerate the scale-up of voluntary medical male circumcision for HIV prevention in eastern and southern Africa: 2012–2016. Geneva: Joint United Nations Programme on HIV/AIDS. doi: 10.1371/journal.pmed.1001641 24800840
42. Gray RH, Li X, Wawer MJ, Serwadda D, Sewankambo NK, et al. (2004) Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis 189 : 1209–1215. 15031789
43. Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, et al. (2011) Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 3 : 77ra29. doi: 10.1126/scitranslmed.3001888 21471433
44. World Health Organization (2013) Global update on HIV treatment 2013: results, impact and opportunities. Geneva: World Health Organization.
45. Akinbami A, Dosunmu A, Adediran A, Ajibola S, Oshinaike O, et al. (2012) CD4 count pattern and demographic distribution of treatment-naive HIV patients in Lagos, Nigeria. AIDS Res Treat 2012 : 352753. doi: 10.1155/2012/352753 23056931
46. Mills EJ, Beyrer C, Birungi J, Dybul MR (2012) Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med 9: e1001167. doi: 10.1371/journal.pmed.1001167 22346735
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 4- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Bringing Access to the Full Spectrum of Cancer Research: A Call for Papers
- The Guyana Diabetes and Foot Care Project: A Complex Quality Improvement Intervention to Decrease Diabetes-Related Major Lower Extremity Amputations and Improve Diabetes Care in a Lower-Middle-Income Country
- How to Get All Trials Reported: Audit, Better Data, and Individual Accountability
- Evaluating Clinical Trial Designs for Investigational Treatments of Ebola Virus Disease
- Improving Men’s Participation in Preventing Mother-to-Child Transmission of HIV as a Maternal, Neonatal, and Child Health Priority in South Africa
- Geographic and Temporal Trends in the Molecular Epidemiology and Genetic Mechanisms of Transmitted HIV-1 Drug Resistance: An Individual-Patient- and Sequence-Level Meta-Analysis
- Research Priorities to Improve the Management of Acute Malnutrition in Infants Aged Less Than Six Months (MAMI)
- A New Synthesis for Dual Use Research of Concern
- Rationale for WHO's New Position Calling for Prompt Reporting and Public Disclosure of Interventional Clinical Trial Results
- Defining the Phenotype of Artemisinin-Resistant Malaria: A Modelling Approach
- HIV Shedding from Male Circumcision Wounds in HIV-Infected Men: A Prospective Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Shedding from Male Circumcision Wounds in HIV-Infected Men: A Prospective Cohort Study
- A New Synthesis for Dual Use Research of Concern
- Research Priorities to Improve the Management of Acute Malnutrition in Infants Aged Less Than Six Months (MAMI)
- Defining the Phenotype of Artemisinin-Resistant Malaria: A Modelling Approach
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



