-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Traditional and Emerging Lifestyle Risk Behaviors and All-Cause Mortality in Middle-Aged and Older Adults: Evidence from a Large Population-Based Australian Cohort
Analysis of large population cohort reveals short and long sleep duration and extended sitting for sedentary workers as lifestyle risks that can be added to other known mortality risk factors.
Published in the journal: . PLoS Med 12(12): e32767. doi:10.1371/journal.pmed.1001917
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001917Summary
Analysis of large population cohort reveals short and long sleep duration and extended sitting for sedentary workers as lifestyle risks that can be added to other known mortality risk factors.
Introduction
Noncommunicable disease is the leading cause of death worldwide [1]. Many noncommunicable diseases, such as cardiovascular disease, some cancers, and diabetes, can be largely attributed to modifiable lifestyle risk factors [2]. Hence, substantial disease, mortality, and economic burden could be prevented through modification of lifestyle behaviors [3–5].
Mounting evidence has implicated lifestyle risk behaviors, such as smoking [6], alcohol use [7], physical inactivity [8], and poor diet [9], in adverse health outcomes. As these common risk behaviors are often associated with multiple disease outcomes and tend to cluster within populations [10], understanding the combined effects of these risk factors on disease burden could be informative for policy making and resource allocation in the context of primary prevention [11].
A number of studies have examined combinations of lifestyle risk factors in relation to disease or mortality outcomes. These studies have mainly focused on the combined effects of smoking, alcohol consumption, physical activity, and diet, and some also included weight status. A recent meta-analysis based on 15 cohort studies found that having a combination of at least four healthy lifestyle factors was associated with a 66% reduction in all-cause mortality [12]. In the past few years, there has been emerging evidence on novel lifestyle risk factors. For example, sedentary behavior (i.e., prolonged sitting, as different from lack of physical activity) was found to be a risk factor for a range of cardiovascular and metabolic diseases and mortality, independent of moderate-to-vigorous-intensity physical activity [13,14]. Recent systematic reviews have also identified short and long sleep duration as predictors of type 2 diabetes [15], cardiovascular disease [16], and all-cause mortality [17]. As such research evidence continues to accumulate, incorporating these new risk factors into lifestyle risk indices will provide more clinically relevant information on combinations of lifestyle risk factors [18].
Using a large Australian cohort, the current study explores a broad range of lifestyle risk behaviors, including habitual sitting time and sleep duration. The objectives of this study are (1) to examine the association between a lifestyle risk index and all-cause mortality, and to quantify the population attributable risk associated with the risk score, and (2) to describe the most commonly occurring combinations of lifestyle risk behaviors, and to quantify the risk for all-cause mortality for each unique lifestyle combination.
Methods
Sampling and Procedures
The analyses are based on data from the 45 and Up Study [19], a large-scale (n = 267,079) population-based prospective cohort of men and women aged 45 y or older living in the state of New South Wales (NSW), Australia. Baseline data collection was conducted between February 1, 2006, and April 30, 2009. Eligible individuals were randomly sampled from the general population of NSW through the Medicare Australia database and were mailed an invitation to participate, an information leaflet, the study questionnaire, a consent form, and a prepaid reply envelope. Participants joined the study by completing the sex-specific questionnaire and the consent form and mailing them back to the study coordinating center. The final working sample size of the 45 and Up Study corresponded to 11% of the entire NSW population in the target age group. The estimated response rate to the mailed invitations was around 18%, though the exact participation rate could be higher as some individuals may not have received the invitations because of incorrect address or other reasons [19,20]. This study was approved by the NSW Population and Health Services Research Ethics Committee (reference No. 2010/05/234).
Measures
Outcome variable
All-cause mortality was ascertained from the NSW Registry of Births, Deaths and Marriages (RBDM) from February 1, 2006, to June 15, 2014. The mortality data were linked to the 45 and Up Study baseline data by the Centre for Health Record Linkage (NSW, Australia) using probabilistic record linkage methods and commercially available software (Choice-Maker, ChoiceMaker Technologies). We excluded participants with a missing date of recruitment or a self-reported date of recruitment that was outside the recruitment period. In the RBDM database, we removed any duplicated records of death and retained the earliest record. We then joined the two datasets (45 and Up Study baseline data and RBDM data) and excluded the following participants/records: (1) any records from the RBDM that did not match the 45 and Up Study data, (2) records in which the date of death occurred prior to the date of recruitment into the 45 and Up Study, (3) participants with missing area of residence, and (4) participants with missing lifestyle risk index score. Fig 1 presents the study flow and the sample size at each stage of exclusion. We summarized follow-up time using the median of the reverse Kaplan-Meier estimate of potential follow-up [21].
Fig. 1. Participant flow diagram. 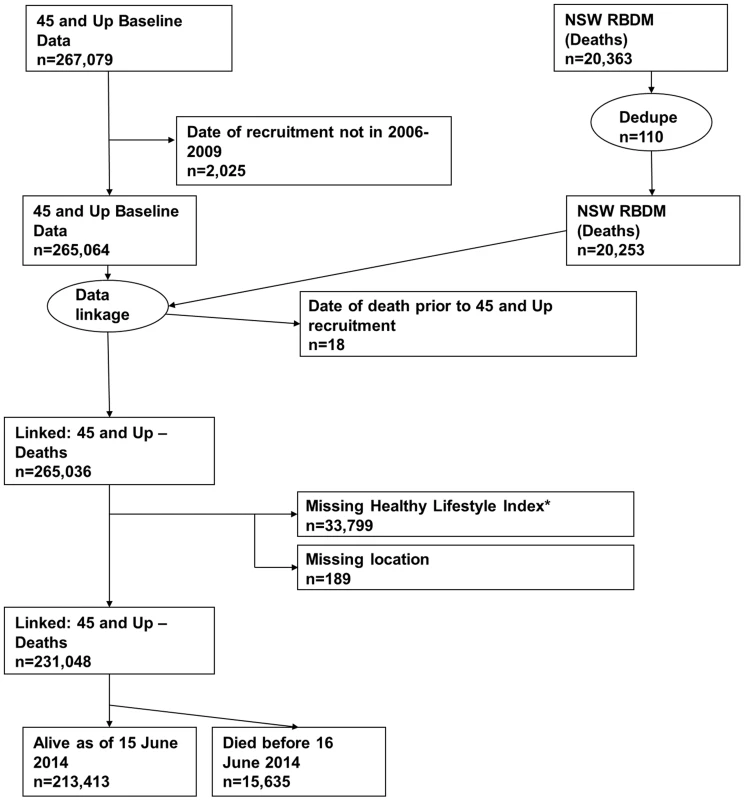
*A risk index including smoking, alcohol use, dietary behavior, physical activity, sedentary behavior, and sleep duration. Dedupe, deduplicated. Independent variables
Participants reported on a range of lifestyle risk behaviors in the questionnaire. Smoking status was derived from two questions: “Have you ever been a regular smoker?” and “Are you a regular smoker now?” Participants were asked, “About how many alcoholic drinks do you have each week?” with one drink defined as one glass of wine, one half pint of beer, or one shot of spirits. Dietary behavior was measured by a previously used index [18] of five food items (vegetable, fruit, fish, processed meat, and types of milk) based on the Dietary Guidelines for Australians [22], as an indicator for overall dietary behavior. Physical activity was measured using the Active Australia Survey [23], which has acceptable reliability (Spearman’s rho for test–retest reliability was 0.56–0.64, with 76% agreement on meeting the recommended physical activity level) and validity (Spearman’s rho for total minutes/week of moderate-to-vigorous physical activity was 0.52 against accelerometer measures) [24]. This instrument asked the total time one spent on walking, moderate-intensity, and vigorous-intensity physical activity (bouts of at least 10 min) in the previous week. Sedentary behavior was assessed using a single-item measure: hours spent sitting in a typical 24-h day. This question was adapted from the widely used International Physical Activity Questionnaire [25] and had acceptable reliability and validity [26]. A similar question was also asked about sleep duration in a 24-h day, and this question was comparable with single-item instruments of self-reported sleep duration used by previous studies [27,28]. The specific coding of these lifestyle risk behaviors is presented in Table 1.
Tab. 1. Scoring of risk factors in the lifestyle risk index based on the 45 and Up Study. 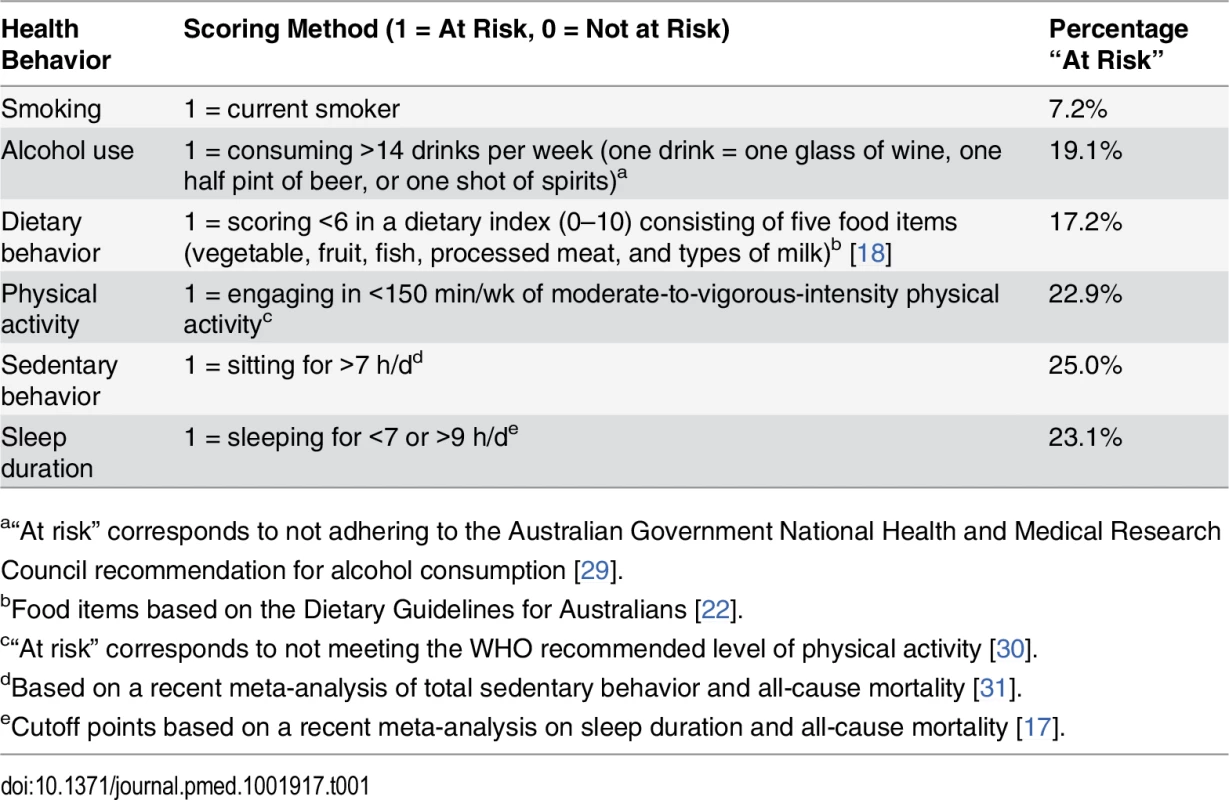
a“At risk” corresponds to not adhering to the Australian Government National Health and Medical Research Council recommendation for alcohol consumption [29]. Each behavior was coded as 1 (at risk) or 0 (not at risk) and was summed as an index (total score range 0–6). Obesity was not included in the index because it was not considered a behavior, but rather an intermediate health outcome influenced by several of the included lifestyle behaviors.
Covariates
The following variables were examined as covariates: age group, sex, educational attainment (school certificate or lower; higher school, trade, or diploma; university degree or higher), marital status (married/cohabitating versus single/divorced/separated/widowed), country of birth (Australia versus other countries), and area of residence based on the Accessibility/Remoteness Index of Australia (major city versus regional/remote) [32].
Effect modifiers
Potential effect modifiers included age, sex, educational attainment (as an indicator for socioeconomic status), and body mass index (BMI) (categorized as normal weight/underweight, overweight, obese). In addition, we created a dichotomous variable for cardiovascular or metabolic disease, based on the self-report (at baseline) of (1) physician-diagnosed thrombosis, diabetes, heart disease, or stroke or (2) recent treatment (in the last month) for thrombosis, myocardial infarction, or any other type of heart disease. We created an additional dichotomous variable for recent cancer diagnosis (except for non-melanoma skin cancer), based on self-report for the 10 y prior to baseline data collection.
Statistical Analysis
All statistical analyses were performed using SAS 9.3 (SAS Institute). Cox proportional hazards analysis was used to examine the association between the lifestyle risk index and all-cause mortality. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. The outcome variable was survival time, which was measured as the time lapse (in weeks) from the date of baseline data collection to death or censoring (June 15, 2014). All Cox proportional hazards regression models were adjusted for sex, age, educational attainment, marital status, country of birth, and area of residence, with covariates classified categorically as per Table 2. Sensitivity analyses were conducted with further adjustment for (1) the presence of cardiovascular or metabolic disease, (2) total number of chronic diseases and/or conditions, and (3) BMI. Participants with a missing value on socio-demographic covariates were included in the analysis using a missing indicator approach. Prior to the analysis, we tested the proportional hazards assumption for the adjustment variables by inspecting plots of cumulative Martingale residuals and the results of a Kolmogorov-type supremum test, based on 1,000 simulations of the residuals [33]. We found no evidence that the proportional hazards assumption was violated (all tests had p > 0.30).
Tab. 2. Socio-demographic and health characteristics of adults by lifestyle risk index score in New South Wales, Australia (2006–2009, n = 231,048). 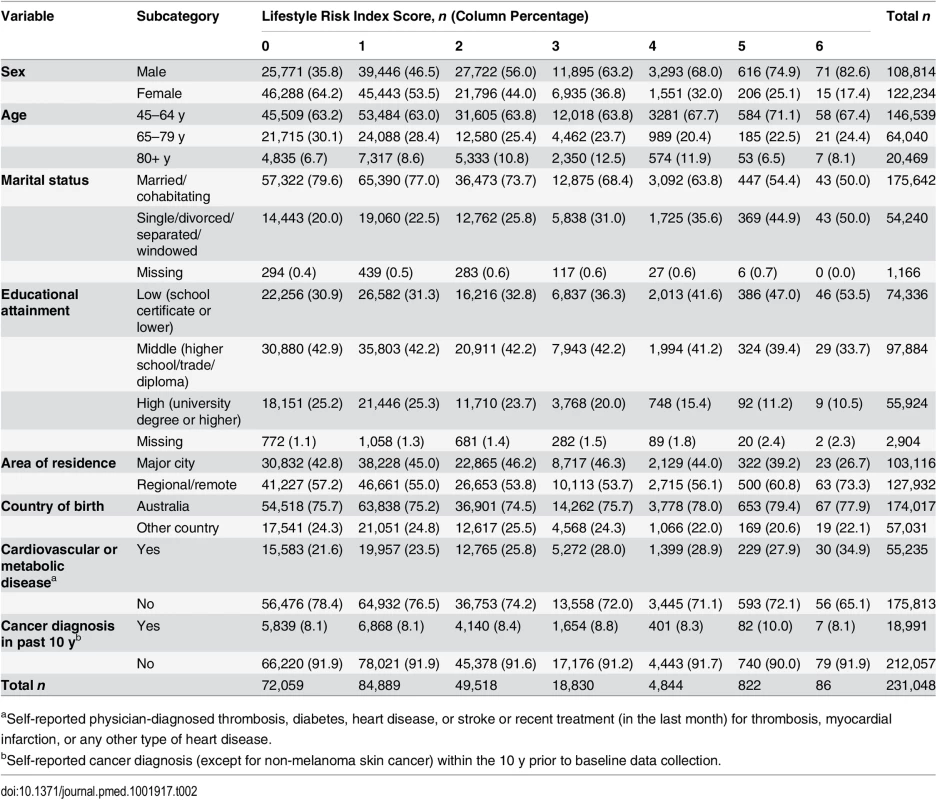
aSelf-reported physician-diagnosed thrombosis, diabetes, heart disease, or stroke or recent treatment (in the last month) for thrombosis, myocardial infarction, or any other type of heart disease. First, we tested independent associations between each individual risk behavior and survival time adjusted for other lifestyle risk behaviors and socio-demographic covariates, based on the log-likelihood test of including these in the model. Then, we tested the lifestyle risk index as the exposure variable, adjusted for all covariates, and presented the c index for risk discrimination/prediction. The c index is defined as the proportion of all possible pairs of participants whose predictions and outcomes are concordant. Therefore, the c index can be interpreted as the probability that the predicted risk is higher in those who die sooner; the c index score ranges from 0.5 (no discrimination) to 1.0 (perfect discrimination) [34].
Based on the model with the lifestyle risk index as the exposure variable, we tested potential effect modification and presented stratified analyses by age group, sex, educational attainment, BMI, whether individuals were diagnosed with cardiovascular or metabolic disease, and whether individuals were diagnosed with cancer within the past 10 y. Finally, we calculated the partial population attributable risk (PARp) for the lifestyle risk index score, which can be interpreted as the proportion of survival time that could have been added if all participants had a risk index score of zero, adjusted for all covariates [35]. To account for “reverse causality” due to occult disease at baseline, the main statistical analysis was repeated excluding deaths that occurred within the first 2 y of follow-up.
Finally, to examine specific patterns of lifestyle risk behaviors, 96 variables representing all possible mutually exclusive combinations of smoking, high alcohol intake, physical inactivity, poor diet, prolonged sitting, and short/long sleep duration were created. Short and long sleep durations were separated as two different risk factors, as their associations with mortality may be explained by different mechanisms [17]. We present the prevalence and HR (95% CI) for each combination; we repeated the analysis excluding deaths within the first 2 y as an additional sensitivity analysis.
Results
Descriptive Statistics
We linked 20,253 records of death from the RBDM to 265,064 participant records from the 45 and Up Study (Fig 1). The final sample for analyses included 231,048 participants, of whom 15,635 died prior to June 15, 2014. S1 Table compares the characteristics and the mortality outcomes of the final analytical sample with those of individuals excluded because of missing lifestyle risk index score. Compared with the analytical sample, those who were excluded were older at baseline and more likely to die during the follow-up. The cohort had a median potential follow-up time of 5.9 y (mean recorded follow-up time 6.1 y), with a total of 1,409,591 person-years of follow-up before death or censoring. Overall, at baseline, 36.6% of the participants were aged 65 y or older, 52.9% were female, 76.0% were married/cohabitating, 24.2% had a university degree, 44.6% lived in a major city, and 75.3% were born in Australia (Table 2).
At baseline, 7.2% of study participants were smokers, 19.1% consumed more than 14 drinks of alcohol per week, 22.9% were not meeting physically activity recommendations, 17.2% were classified as having poor diet, 25.0% sat for more than 7 h per day, and 23.1% slept too little or too much (Table 1). Overall, 31.2% of participants reported no risk behavior (lifestyle risk index score = 0), 36.7% had one risk behavior, and 21.4%, 8.1%, 2.1%, 0.4%, and 0.04% had a lifestyle risk index score of 2, 3, 4, 5, and 6, respectively. Higher lifestyle risk index scores were more prevalent among males, those aged 80+ y, those who were not married/cohabitating, and those with lower educational attainment.
Individual Risk Behavior and All-Cause Mortality
When all six dichotomized individual risk behaviors were entered in the model with all covariates, five showed independent associations with all-cause mortality. Of them, smoking (HR = 1.94, 95% CI 1.82–2.06) and physical inactivity (HR = 1.72, 95% CI 1.66–1.77) had the strongest association with mortality, followed by prolonged sitting (HR = 1.33, 95% CI 1.29–1.38), short/long sleep (HR = 1.31, 95% CI 1.27–1.36), and poor diet (HR = 1.11, 95% CI 1.07–1.15). There was no significant association between high alcohol intake and all-cause mortality (HR = 0.98, 95% CI 0.94–1.02).
Lifestyle Risk Index and All-Cause Mortality
The results from the Cox proportional hazards regression analyses show the association between the lifestyle risk index score and all-cause mortality adjusted for age, sex, educational attainment, marital status, country of birth, and area of residence (Table 3). All-cause mortality HRs compared to individuals without lifestyle risk factors were 1.27 for those with one risk factor, and 1.73, 2.45, 3.06, 4.61, and 5.38 for those with 2, 3, 4, 5, and 6 risk factors, respectively. There was a positive relationship between lifestyle risk index score and all-cause mortality, though the HRs for those with a score of 5 or 6 had wide confidence intervals because of small sample sizes. Additional sensitivity analyses adjusted for BMI, physician-diagnosed cardiovascular and metabolic disease, diagnosis of cancer in the past 10 y, and the total number of chronic diseases/conditions yielded minimal (<2%) change in HRs. Overall, the lifestyle risk index showed good prediction of all-cause mortality (c index = 0.763, 95% CI 0.749–0.776). The PARp calculated based on the overall study sample was 31.3% (95% CI 27.6%–34.9%), which indicates that if other variables were held constant, 31.3% of survival time could have been added if all participants had a risk index score of zero.
Tab. 3. Crude cumulative death rates and adjusted hazard ratios for all-cause mortality by lifestyle risk index score among a population-based Australian sample of adults from the 45 and Up Study (2006–2014, n = 231,048). 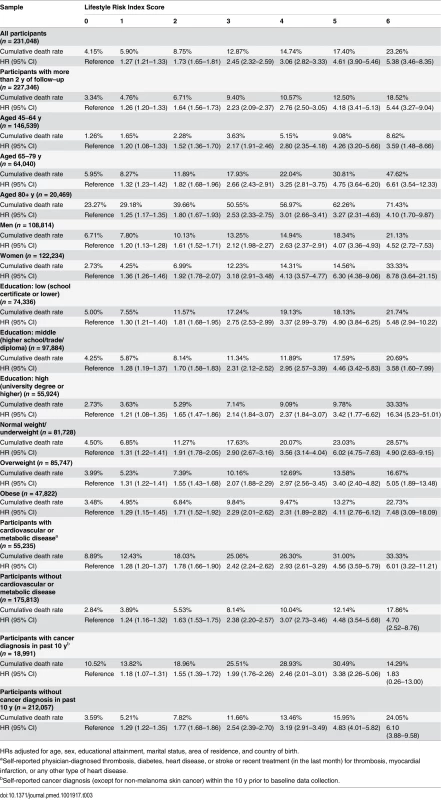
HRs adjusted for age, sex, educational attainment, marital status, area of residence, and country of birth. Analysis of effect modification showed that the association between lifestyle risk index score and all-cause mortality did not vary significantly by age category and did not differ among those with and without cardiovascular or metabolic disease. However, there were significant interactions between lifestyle risk index score and sex (χ2 = 65.9, p < 0.0001), educational attainment (χ2 = 42.4, p = 0.011), BMI (χ2 = 24.5, p < 0.001), and cancer diagnosis in the past 10 y (χ2 = 31.8, p < 0.0001). Stratified analysis suggested a stronger association between lifestyle risk index score and all-cause mortality among women, those with lower educational attainment, those with normal weight/underweight, and those without a cancer diagnosis in the past 10 y (Table 3). Finally, when the analysis was repeated among those with more than 2 y of follow-up, the magnitude of the association between lifestyle risk index score and all-cause mortality was similar, but slightly attenuated.
Combinations of Risk Behaviors
Table 4 presents all 96 mutually exclusive combinations of risk behaviors. Of these, the 30 most commonly occurring combinations accounted for more than 90% of all participants. Compared to being without any risk behavior, the majority of risk combinations were associated with a significantly elevated risk for all-cause mortality. Among those with one risk factor, the most common single risk behavior was prolonged sitting time (9.1%), followed by physical inactivity (7.1%), unhealthy diet (6.9%), short sleep duration (5.7%), high alcohol intake (4.2%), and long sleep duration only (2.4%). Among those with at least two risk factors, the most common combinations were physical inactivity plus prolonged sitting time (2.9%), followed by poor diet plus prolonged sitting time (2.2%). Out of all the single risk behaviors, smoking had the strongest association with all-cause mortality (HR = 1.90). Among the commonly occurring risk combinations, several showed a relatively strong association with all-cause mortality, such as physical inactivity plus prolonged sitting time (HR = 2.42), physical inactivity plus long sleep duration (HR = 2.68), high alcohol intake plus physical inactivity plus prolonged sitting time (HR = 2.51), physical inactivity plus prolonged sitting time plus short sleep duration (HR = 2.59), physical inactivity plus prolonged sitting time plus long sleep duration (HR = 4.23), smoking plus high alcohol intake (HR = 2.80), and smoking plus high alcohol intake plus short sleep duration (HR = 4.68). Sensitivity analysis excluding deaths within the first 2 y showed similar prevalence and HRs for all risk combinations (S2 Table).
Tab. 4. Prevalence of all 96 combinations of lifestyle risk behaviors and adjusted hazard ratios for their associations with all-cause mortality among middle-aged and older adults based on the 45 and Up Study, Australia (2006–2014, n = 231,048). 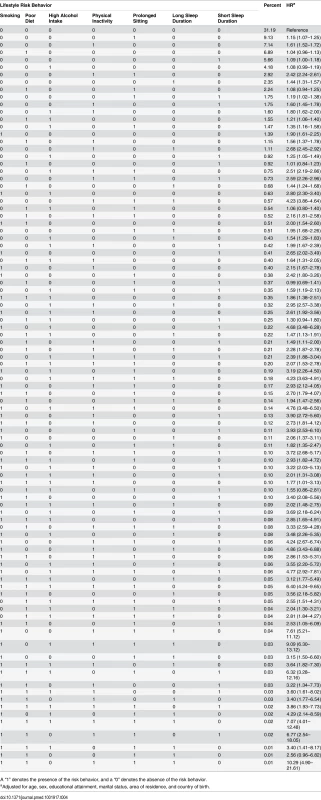
A “1” denotes the presence of the risk behavior, and a “0” denotes the absence of the risk behavior. Discussion
In this study, we found that multiple lifestyle risk factors among middle-aged and older Australian adults were associated with an increased risk for all-cause mortality over 6 y of follow-up. There was a clear association between the number of risk factors, as indicated by the lifestyle risk score, and all-cause mortality. Overall, all six risk factors accounted for a third of the person-year loss due to mortality when socioeconomic characteristics were held constant.
Evidence is accumulating on the health effects of the combined behavioral risk factors of smoking, high alcohol intake, poor diet, and physical inactivity. The associations found in the current study are similar to those from previous studies, which tended to have smaller sample sizes and a more limited range of lifestyle risk factors. For example, Ford and colleagues found a strong association between the number of lifestyle risk behaviors (smoking, non-moderate alcohol consumption, smoking, and poor diet based on the Healthy Eating Index) and all-cause mortality in a US-based sample [36]. Khaw et al. found a clear inverse relationship between adherence to four health behaviors (not smoking, being physically active, moderate alcohol intake, and fruit and vegetable intake indicated by plasma vitamin C level) and all-cause mortality in a UK-based sample [37]. These findings have been replicated by a number of epidemiological studies that assessed similar risk factors using various measures [38–43]. Despite the heterogeneous measures, risk classification, sample characteristics, and follow-up time of these studies, the additive nature of the association between risk indices and mortality has been consistent, suggesting the generalizability of these findings. Such evidence is furthered here by adding the new risk factors of prolonged sitting and unhealthy sleep duration.
The validity of our findings was also enhanced through comprehensive sensitivity analyses, where we conducted subgroup analyses, excluded deaths within the first 2 y, and further adjusted for chronic disease and BMI as additional covariates. Despite statistically significant effect modification by sex, educational attainment, BMI, and cancer diagnosis in the past 10 y, the overall difference in effect sizes across subgroups or when adjusting for additional covariates was small, and the patterns of associations were consistent. This reinforces an important message for public health and clinical practice that adherence to low-risk lifestyles is likely to be protective for all.
It is important to acknowledge that not all risk behaviors contribute to mortality similarly and that their combined effects may not be additive. We therefore supplemented risk index analysis with risk combination analysis. This allowed in-depth exploration of interactions among behaviors in relation to all-cause mortality. One compelling observation was that some risk behaviors tend to cluster, particularly in certain patterns, and that the joint risk could be much higher than the sum of the individual risks. For example, smoking was the least common single risk factor (only 1.39% of participants reported only smoking risk), and it was more than four times more likely to occur with other risk factors than on its own. Smoking was also the most “deadly” single risk factor (HR = 1.90). The risk behavior that co-occurred with smoking the most was high alcohol consumption. Though high alcohol intake on its own was not significantly associated with higher mortality risk (HR = 1.08), it augmented the risk noticeably when paired with smoking (HR = 2.80). Furthermore, when these two risk factors co-occurred with short sleep duration, which was marginally associated with all-cause mortality on its own (HR = 1.09), the combined risk was increased dramatically (HR = 4.68). These findings suggest that there is a “synergistic effect” among risk factors and that future epidemiological research and behavioral interventions should take into account the patterns of risk factor co-occurrence and their interactive effects on health outcomes.
A unique contribution of the current study is the inclusion of prolonged sitting and short/long sleep duration as additional risk indicators, which were not reported in previous cohort studies [12]. Growing research evidence on the health effects of sedentary behavior and sleep [13,16,44,45] suggests that both may be important behaviors that together constitute a large proportion of one’s daily life and contribute to chronic disease risk. However, few studies have examined the interactions between these behaviors and other lifestyle risk factors in relation to health outcomes. A key finding that emerged from the current study is that prolonged sitting time alone, as the most common single risk factor, had a small effect on all-cause mortality (HR = 1.15). However, the combination of prolonged sitting time and physical inactivity had a much stronger association with mortality (HR = 2.42). This might indicate that prolonged sitting tends to be particularly harmful among those who are physically inactive. Such interactive effects were noted in a recent meta-analysis, which found that the association between sedentary behavior and health outcomes was more pronounced among those with lower physical activity [46]. When sleep was present as a lone risk factor, short sleep duration was only marginally associated with mortality (HR = 1.09), while long sleep duration was associated with much higher risk (HR = 1.44). Such a pattern of associations was noted in recent meta-analyses [17,47]; one meta-analysis also found that the effect of long sleep duration was stronger in older than younger cohorts [17]. It is biologically plausible that short sleep duration may increase mortality risk through adverse endocrinologic, immunologic, and metabolic effects [48,49,50] or through chronic inflammation [47,51,52]. The mechanism for the association between long sleep duration and mortality is not well understood [17,47]. Most studies suggest that long sleep duration tends to be associated with sleep fragmentation, fatigue, depression, and underlying disease and poor health [53]. Therefore, the observed association between long sleep duration and all-cause mortality could be due to “reverse causality” or residual confounding [17,54]. An interesting observation from the current study is that risk combinations involving long sleep duration, prolonged sitting, and/or physical inactivity tended to be among those with the strongest associations with mortality, with HRs ranging from 2 to above 4. These associations remained significant and of similar magnitude after excluding deaths within the first 2 y of follow-up (S2 Table). This may suggest that the underlying characteristics associated with such behavioral patterns involving long sleep, sedentariness, and inactivity, perhaps not limited to major occult disease or failing health, may have contributed to the elevated risk for morality.
Strengths and Limitations
The current study is the first to our knowledge to test a lifestyle risk index and multiple behavioral risk combinations incorporating sedentary and sleep behaviors as additional risk factors for all-cause mortality. In rigorous sensitivity analyses, subgroup analyses, and tests of effect modification, the association between the lifestyle risk index and all-cause mortality remained robust, implying internal validity for our findings. Using a large population-based sample allowed us to test all lifestyle risk combinations, which provided unique insights into understanding the complex interacting relationships among lifestyle risk factors, particularly for sedentary behavior and sleep.
However, despite the novelty and methodologic rigor, the findings from this study should be interpreted in the light of the study’s limitations. First, all lifestyle risk behaviors were self-reported, although using established and validated questions. Given that misclassification due to self-report is potentially non-random (i.e., if people tended to report desirable behaviors because of social desirability bias), the results are most likely biased toward the null [36]. Therefore, the potential risk reduction related to the six lifestyle behaviors, as indicated by PARp, is likely to be underestimated. Second, the measures of several risk behaviors are under-specified; for example, the alcohol measure did not capture short-term alcohol risk, such as binge drinking, and could not distinguish non-drinkers from ex-drinkers who might have quit drinking due to prior alcohol-related problems. The dietary measure was limited to a small number of food items. The sleep measure was limited to quantity only, without taking into account other aspects of sleep hygiene or sleep quality. The smoking measure did not take into account past smoking. However, a recent study from the same cohort found a much lower risk for all-cause mortality among past smokers than current smokers, and the mortality risk among those who quit before 45 y of age did not differ significantly from that of never smokers [55]. Furthermore, when we adjusted for past smoking in the main analysis, the results did not change substantially. On the other hand, a strength of this analysis is that it focused on six lifestyle behaviors and did not conflate behaviors with their outcomes, as some lifestyle risk indices have done before by including weight status or other metabolic health indicators in the index [11,56]. Overall, despite limitations in measurement, the use of indices such as ours supports policy-relevant public health recommendations by using categorical thresholds for risk and allowing lifestyle risk to be easily captured and assessed across settings. The third limitation of our study is that it focused on participants’ reports of lifestyle risk behaviors at one time point; therefore, we could not determine the habitual or changing patterns of participants’ behaviors over time. For example, the risk classification for smoking was based on current smoking status only, equating past and never smokers, which could lead to underestimation of the health risk associated with smoking. The same applies to other risk behaviors. Therefore, the current analyses could be further improved by incorporating past behavioral patterns and future waves of follow-up data. Fourth, this study could be further strengthened by including cause-specific mortality outcomes, such as cardiovascular disease mortality, but these data are not yet available for the time period studied. Fifth, although the cohort sample was not representative of the population (participants in the 45 and Up Study were healthier than the general population because of selection bias), a recent study comparing the current cohort with a population representative sample in NSW found similar estimates for the associations between risk factors and health outcomes, despite the difference in the prevalence of risk factors [57]. Furthermore, most prior epidemiological studies have found little evidence for considerable bias attributable to nonparticipation [20]. This reinforces the epidemiological axiom that associations, compared to prevalence, are less dependent on the representativeness of the sample.
Conclusion
This large study reaffirms the importance of healthy lifestyles, here evidenced for adults aged 45 y and older. This analysis investigated four established and two novel risk factors, namely, prolonged sitting and unhealthy sleep duration, which may be added to behavioral indices or risk combinations to quantify health risk. The prevalent combinations of risk factors suggest new strategic targeting for chronic disease prevention.
Supporting Information
Zdroje
1. World Health Organization. World health statistics 2014. Geneva: World Health Organization; 2014.
2. Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058 19399161
3. Scarborough P, Bhatnagar P, Wickramasinghe KK, Allender S, Foster C, Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006–07 NHS costs. J Public Health (Oxf). 2011;33 : 527–535. doi: 10.1093/pubmed/fdr033
4. Cadilhac DA, Magnus A, Sheppard L, Cumming TB, Pearce DC, Carter R. The societal benefits of reducing six behavioural risk factors: an economic modelling study from Australia. BMC Public Health. 2011;11 : 483. doi: 10.1186/1471-2458-11-483 21689461
5. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380 : 2224–2260. doi: http://dx.doi.org/10.1016/S0140-6736(12)61766-8 23245609
6. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The health consequences of smoking: a report of the Surgeon General. Washington (District of Columbia): US Government Printing Office; 2004.
7. Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38 : 613–619. doi: 10.1016/j.ypmed.2003.11.027 15066364
8. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380 : 219–229. doi: 10.1016/S0140-6736(12)61031-9 22818936
9. Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Stroke. 2004;35 : 1999–2010. 15272139
10. Shankar A, McMunn A, Steptoe A. Health-related behaviors in older adults relationships with socioeconomic status. Am J Prev Med. 2010;38 : 39–46. doi: 10.1016/j.amepre.2009.08.026 20117555
11. Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam Study. Arch Intern Med. 2009;169 : 1355–1362. doi: 10.1001/archinternmed.2009.237 19667296
12. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55 : 163–170. doi: 10.1016/j.ypmed.2012.06.017 22735042
13. Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55 : 2895–2905. doi: 10.1007/s00125-012-2677-z 22890825
14. Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS ONE. 2012;7:e34916. doi: 10.1371/journal.pone.0034916 22514690
15. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33 : 414–420. doi: 10.2337/dc09-1124 19910503
16. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32 : 1484–1492. doi: 10.1093/eurheartj/ehr007 21300732
17. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33 : 585–592. 20469800
18. Ding D, Rogers K, Macniven R, Kamalesh V, Kritharides L, Chalmers J, et al. Revisiting lifestyle risk index assessment in a large Australian sample: should sedentary behavior and sleep be included as additional risk factors? Prev Med. 2014;60 : 102–106. doi: 10.1016/j.ypmed.2013.12.021 24380793
19. Banks E, Redman S, Jorm L, Armstrong B, Bauman A, Beard J, et al. Cohort profile: the 45 and Up Study. Int J Epidemiol. 2008;37 : 941–947. doi: 10.1093/ije/dym184 17881411
20. Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17 : 643–653. doi: 10.1016/j.annepidem.2007.03.013 17553702
21. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17 : 343–346. doi: 10.1016/0197-2456(96)00075-X 8889347
22. Australian Government National Health and Medical Research Council. Eat for health: Australian dietary guidelines. Canberra: National Health and Medical Research Council; 2013.
23. Australian Institute of Health and Welfare. The Active Australia Survey: a guide and manual for implementation, analysis and reporting. Canberra: Australian Institute of Health and Welfare; 2003.
24. Brown WJ, Burton NW, Marshall AL, Miller YD. Reliability and validity of a modified self-administered version of the Active Australia physical activity survey in a sample of mid-age women. Aust N Z J Public Health. 2008;32 : 535–541. doi: 10.1111/j.1753-6405.2008.00305.x 19076744
25. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35 : 1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB 12900694
26. Rosenberg DE, Bull FC, Marshall AL, Sallis JF, Bauman AE. Assessment of sedentary behavior with the International Physical Activity Questionnaire. J Phys Act Health. 2008;5 (Suppl 1):S30–S44. 18364524
27. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19 : 838–845. doi: 10.1097/EDE.0b013e318187a7b0 18854708
28. Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27 : 440–444. 15164896
29. Australian Government National Health and Medical Research Council. Australian guidelines to reduce health risks from drinking alcohol. Canberra: Australian Government National Health and Medical Research Council; 2009.
30. World Health Organization. Global recommendations on physical activity for health. Geneva: World Health Organization; 2010.
31. Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS ONE. 2013;8:e80000. doi: 10.1371/journal.pone.0080000 24236168
32. Department of Health and Aged Care Information and Research Branch. Measuring remoteness. Accessibility/Remoteness Index of Australia (ARIA). Canberra: Department of Health and Aged Care; 2001.
33. Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80 : 557–572. doi: 10.1093/biomet/80.3.557
34. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15 : 361–387. 8668867
35. Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18 : 571–579. doi: 10.1007/s10552-006-0090-y 17387622
36. Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality: findings from the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health. 2011;101 : 1922–1929. doi: 10.2105//AJPH.2011.300167 21852630
37. Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5:e12. doi: 10.1371/journal.pmed.0050012 18184033
38. van den Brandt PA. The impact of a Mediterranean diet and healthy lifestyle on premature mortality in men and women. Am J Clin Nutr. 2011;94 : 913–920. doi: 10.3945/ajcn.110.008250 21795445
39. Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292 : 1433–1439. doi: 10.1001/jama.292.12.1433 15383513
40. Nechuta SJ, Shu XO, Li HL, Yang G, Xiang YB, Cai H, et al. Combined impact of lifestyle-related factors on total and cause-specific mortality among Chinese women: prospective cohort study. PLoS Med. 2010;7:e1000339. doi: 10.1371/journal.pmed.1000339 20856900
41. Gopinath B, Flood VM, Burlutsky G, Mitchell P. Combined influence of health behaviors on total and cause-specific mortality. Arch Intern Med. 2010;170 : 1605–1607. doi: 10.1001/archinternmed.2010.303 20876415
42. Hamer M, Bates CJ, Mishra GD. Multiple health behaviors and mortality risk in older adults. J Am Geriatr Soc. 2011;59 : 370–372. doi: 10.1111/j.1532-5415.2011.03258.x 21314658
43. van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a144042 18796495
44. Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. Am J Prev Med. 2011;41 : 207–215. doi: 10.1016/j.amepre.2011.05.004 21767729
45. Cappuccio FP, Miller MA. Is prolonged lack of sleep associated with obesity? BMJ. 2011;342:d3306. doi: 10.1136/bmj.d3306 21622519
46. Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162 : 123–132. doi: 10.7326/M14-1651 25599350
47. Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18 : 148–158. doi: 10.1111/j.1365-2869.2008.00732.x 19645960
48. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11 : 163–178. 17442599
49. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. 15602591
50. Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89 : 5762–5771. 15531540
51. Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24 : 775–784. doi: 10.1016/j.beem.2010.08.014 21112025
52. Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43 : 678–683. 14975482
53. Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11 : 341–360. 17625932
54. Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol 2013;23 : 361–370. doi: 10.1016/j.annepidem.2013.03.015 23622956
55. Banks E, Joshy G, Weber MF, Liu B, Grenfell R, Egger S, et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 2015;13 : 38. doi: 10.1186/s12916-015-0281-z 25857449
56. Lee C - D, Sui X, Hooker SP, Hébert JR, Blair SN. Combined impact of lifestyle factors on cancer mortality in men. Ann Epidemiol. 2011;21 : 749–754. doi: 10.1016/j.annepidem.2011.04.010 21683616
57. Mealing N, Banks E, Jorm L, Steel D, Clements M, Rogers K. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol. 2010;10 : 26. doi: 10.1186/1471-2288-10-26 20356408
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 12- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials
- Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled
- Police Killings and Police Deaths Are Public Health Data and Can Be Counted
- A Successful Failure: Missing the MDG4 Target for Under-Five Mortality in South Africa
- The Ebola Vaccine, Iatrogenic Injuries, and Legal Liability
- Progress in Medicine: Experts Take Stock
- Use of Viremia to Evaluate the Baseline Case Fatality Ratio of Ebola Virus Disease and Inform Treatment Studies: A Retrospective Cohort Study
- Acute Cardiovascular Events after Herpes Zoster: A Self-Controlled Case Series Analysis in Vaccinated and Unvaccinated Older Residents of the United States
- Moving Beyond “Food Deserts”: Reorienting United States Policies to Reduce Disparities in Diet Quality
- Public Health and International Partnerships in the Democratic People’s Republic of Korea
- Self-Administered Outpatient Antimicrobial Infusion by Uninsured Patients Discharged from a Safety-Net Hospital: A Propensity-Score-Balanced Retrospective Cohort Study
- Association between Regimen Composition and Treatment Response in Patients with Multidrug-Resistant Tuberculosis: A Prospective Cohort Study
- 10-y Risks of Death and Emergency Re-admission in Adolescents Hospitalised with Violent, Drug- or Alcohol-Related, or Self-Inflicted Injury: A Population-Based Cohort Study
- World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010
- Bariatric Surgery in the United Kingdom: A Cohort Study of Weight Loss and Clinical Outcomes in Routine Clinical Care
- Traditional and Emerging Lifestyle Risk Behaviors and All-Cause Mortality in Middle-Aged and Older Adults: Evidence from a Large Population-Based Australian Cohort
- Inequalities in Alcohol-Related Mortality in 17 European Countries: A Retrospective Analysis of Mortality Registers
- World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis
- A Molecular Host Response Assay to Discriminate Between Sepsis and Infection-Negative Systemic Inflammation in Critically Ill Patients: Discovery and Validation in Independent Cohorts
- World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Self-Administered Outpatient Antimicrobial Infusion by Uninsured Patients Discharged from a Safety-Net Hospital: A Propensity-Score-Balanced Retrospective Cohort Study
- Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials
- World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010
- Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



