-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Missing Men: HIV Treatment Scale-Up and Life Expectancy in Sub-Saharan Africa
In a Perspective accompanying Bor and colleagues, Alexander Tsai and Mark Siedner discuss the gender gap in ART uptake and HIV mortality in Africa.
Published in the journal: . PLoS Med 12(11): e32767. doi:10.1371/journal.pmed.1001906
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.1001906Summary
In a Perspective accompanying Bor and colleagues, Alexander Tsai and Mark Siedner discuss the gender gap in ART uptake and HIV mortality in Africa.
Delivery of effective HIV antiretroviral therapy (ART) to the more than 6 million persons with HIV in South Africa is well underway, with early data on the impact of this massive public health effort demonstrating a reversal of the previous decade’s precipitous decline in population life expectancy [1]. Although South Africa’s age and sex disparities in HIV acquisition have traditionally been described as disadvantaging young women [2], accumulating evidence now suggests a reverse disparity: although HIV care is available to both men and women and is nominally free of charge, women are more likely to be tested for HIV, engage in pre-treatment care, initiate treatment earlier, stay on treatment, and survive [3–6]. To adopt the classic Eisenberg and Power [7] analogy of health care as current flowing through an electric circuit, the voltage drops along the entire circuit of HIV care, from HIV infection to AIDS-free survival, are larger for men compared with women (Fig 1). There are simply too many missing men.
Fig. 1. The cascade of “voltage drops” from HIV infection to AIDS-free survival. 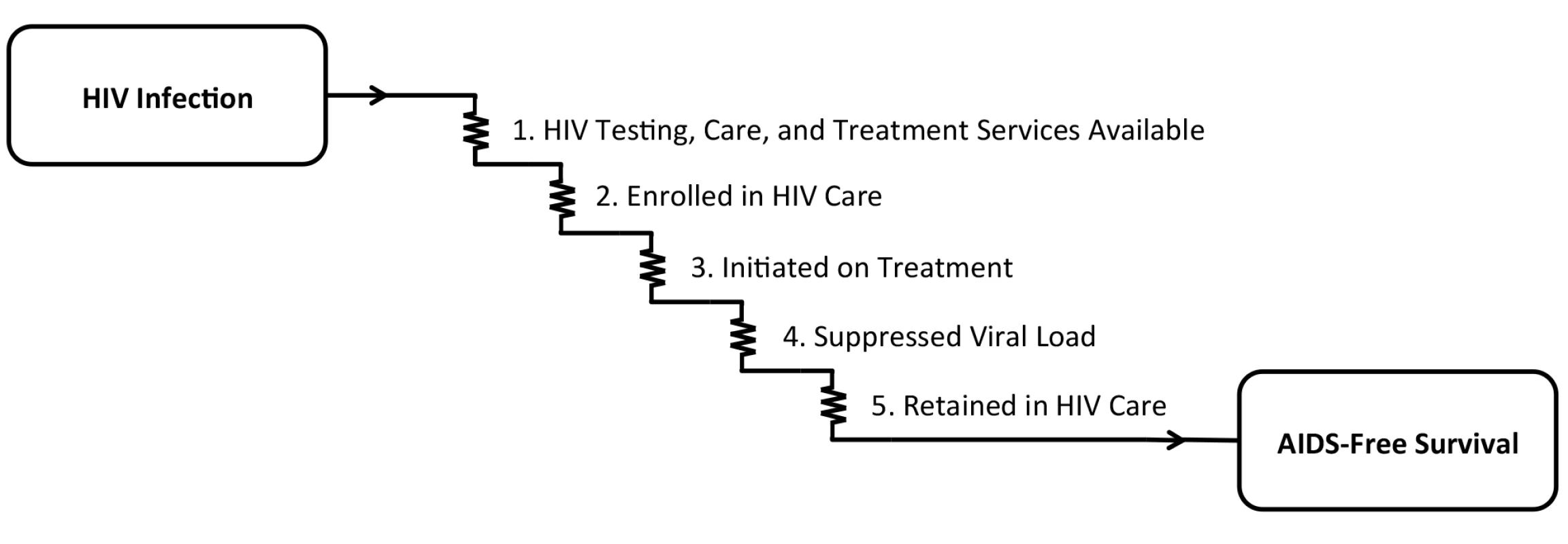
In order for the goal of AIDS-free survival to be achieved, (1) HIV testing, care, and treatment services must be available, and persons with HIV must (2) be enrolled in care, (3) initiate antiretroviral therapy, (4) achieve suppression of HIV-1 RNA viral load, and (5) be retained in care. In recent years, studies from South Africa [8], as well as Rwanda [9] and Uganda [10], have begun to demonstrate the cumulative impact of these voltage drops, which, in total, result in an approximately 10-year life expectancy gap between men and women initiating ART at 20 years of age (Fig 2). However, the findings of these studies should be interpreted in light of important limitations. First, they were based solely on data obtained from persons enrolled in HIV treatment programs. Poverty, food insecurity, HIV stigma, and geographic barriers still exert outsize influences on HIV testing, treatment, and retention in these settings [11–14], so it is unlikely that these enrollees are representative of the entire population of persons with HIV. Second, mortality had to be estimated among those lost to follow up [9,10]. Because persons in HIV treatment programs are much more likely to be lost to care than confirmed as dead [15,16], and because the vast majority of HIV-related mortality events go unreported [17], the mortality estimates in these studies are likely to be biased. Third, and perhaps most notably, none of these studies directly observed non-HIV mortality. Thus, while they were able to document trends in mortality among persons with HIV, they were unable to assess the extent to which these changes were related to HIV care or to unrelated secular trends in health and health behavior.
Fig. 2. Gender gaps in life expectancy among men and women with HIV initiating antiretroviral therapy at 20 years of age. 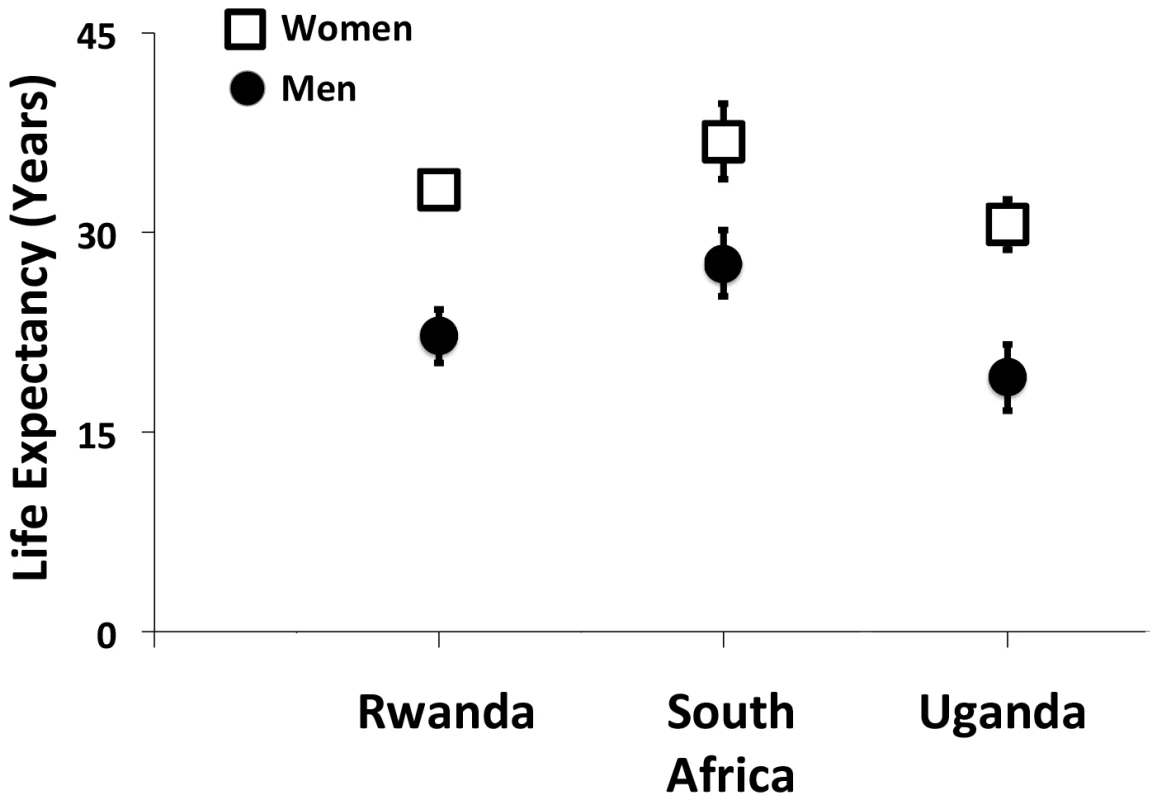
This figure summarizes the findings of studies from Rwanda [9], South Africa [8], and Uganda [10]. Estimates and associated 95% confidence intervals are shown as the number of additional years of life expected for men and women with HIV initiating antiretroviral therapy at 20 years of age. In this context, the research article by Jacob Bor and colleagues [18] that appears this week in PLOS Medicine provides new evidence of a widening gender gap in life expectancy, using data obtained from a general population sample in rural South Africa from 2001–2011, covering a period of coincident ART scale-up. By surveilling all persons in the region—whether HIV-negative, HIV-positive in care, or HIV-positive but not in care—and by using verbal autopsies to categorize mortality events that were recorded by the surveillance teams, this study addresses some of the limitations of previous work. Subject to the assumption that mortality events were comprehensively observed and accurately categorized—which would be generically limiting for any study conducted in a country with less than complete registration of vital events [19]—Bor and colleagues [18] were not obligated to account for non-uptake of HIV testing, nor were they required to estimate mortality among those lost to care or to estimate non-HIV mortality. Their findings are summarized by the stark observation that life expectancy gains among women far outstripped the life expectancy gains among men, and that these gains were independent of both age and first recorded CD4+ T-lymphocyte cell count. Perhaps more telling than the near-doubling of the gender gap in life expectancy during the observation period are the relative benefits women received throughout the entire HIV care circuit: HIV-related mortality rates were approximately 2-fold higher among men compared with women, whether prior to ART or during both early and long-term ART.
What could explain these findings? Certainly women’s differential access to HIV care during pregnancy might be partially responsible. South Africa’s successful program for preventing mother-to-child transmission largely requires HIV testing for all pregnant women and encourages ART initiation among those found to be HIV positive [20]. This institutional link to program entry could partially explain why more than half of HIV-related mortality events among men in 2007–2011 occurred during the pre-treatment period, compared with only one-third of HIV-related mortality events among women. However, because the lower HIV-related mortality rates among women persisted even after accounting for age, CD4 count, and ART initiation, clearly more data are needed to explain the widening gender gap in life expectancy. Other major contributions likely result from historically ingrained social forces (such as increased migratory needs resulting from apartheid) and differential patterns of health behavior [21–23]. Even in the absence of these crippling disadvantages, a gender gap in life expectancy may yet remain [24], but the data presented by Bor and colleagues [18] signal the urgent need to better understand this large and widening disparity in South Africa and elsewhere in sub-Saharan Africa.
What can be done to address this problem? Different types of interventions should be considered. Minimally, policies could be revised to “nudge” men into HIV care; for example, opt-out HIV testing among military service members could be mandated as part of annual examinations or after deployments, peacekeeping missions, or foreign trainings [25]. Home-based HIV counseling and testing can potentially provide a greater degree of privacy for men concerned about discrimination, or by providing convenience for men whose willingness to undergo testing is constrained by work obligations [26]. Similarly, workplace-based treatment programs [27] or alternative patient-centered care models [28] may help to retain men in care once treatment has been initiated. And finally, social marketing to emphasize collateral impacts—such as economic benefits for individuals and their households [29] or reduced risks of secondary transmission to domestic partners [30] and/or unborn children [31]—may provide additional impetus for testing and treatment. Of note, while these “gender sensitive” intervention strategies attempt to minimize the ways in which socially constructed gender roles in South Africa constrain men’s health behavior, they still leave intact a system of gender inequality that confers distinct health disadvantages for women while simultaneously marshaling other threats to the health of men. Truly “gender transformative” intervention strategies will need to understand men’s health behavior as being intimately tied to the same prevailing gender roles and norms of masculinity that produce violence against women, constraints on capital ownership, alcohol and substance abuse, and sexual risk taking [23]. Given the complexity of the problem, multipronged approaches will likely be needed. Certainly, the AIDS-free generation will remain a far-off mirage until men also receive the health benefits made possible through the mass provision of HIV treatment, which somehow remains out of reach for too many of them.
Zdroje
1. Bor J, Herbst AJ, Newell ML, Barnighausen T (2013) Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 339 : 961–965. doi: 10.1126/science.1230413 23430655
2. Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S (1992) Seroprevalence of HIV infection in rural South Africa. AIDS 6 : 1535–1539. 1492937
3. Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, Holst H, et al. (2010) Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS 24 Suppl 1: S37–44. doi: 10.1097/01.aids.0000366081.91192.1c 20023438
4. Geng EH, Bwana MB, Muyindike W, Glidden DV, Bangsberg DR, Neilands TB, et al. (2013) Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr 63: e64–71. doi: 10.1097/QAI.0b013e31828af5a6 23429504
5. Geng EH, Nash D, Kambugu A, Zhang Y, Braitstein P, Christopoulos KA, et al. (2010) Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep 7 : 234–244. doi: 10.1007/s11904-010-0061-5 20820972
6. Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. (2013) Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS 27 : 417–425. doi: 10.1097/QAD.0b013e328359b89b 22948271
7. Eisenberg JM, Power EJ (2000) Transforming insurance coverage into quality health care: voltage drops from potential to delivered quality. JAMA 284 : 2100–2107. 11042759
8. Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. (2013) Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 10: e1001418. doi: 10.1371/journal.pmed.1001418 23585736
9. Nsanzimana S, Remera E, Kanters S, Chan K, Forrest JI, Ford N, et al. (2015) Life expectancy among HIV-positive patients in Rwanda: a retrospective observational cohort study. Lancet Glob Health 3: e169–e177. doi: 10.1016/S2214-109X(14)70364-X 25701995
10. Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. (2011) Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med 155 : 209–216. doi: 10.7326/0003-4819-155-4-201108160-00358 21768555
11. Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. (2013) Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 16 : 18640. doi: 10.7448/IAS.16.3.18640 24242258
12. Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC (2014) Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav 18 : 1199–1223. doi: 10.1007/s10461-014-0729-8 24563115
13. Chimbindi N, Bor J, Newell ML, Tanser F, Baltussen R, Hontelez J, et al. (2015) Time and money: the true costs of health care utilization for patients receiving "free" HIV/tuberculosis care and treatment in rural KwaZulu-Natal. J Acquir Immune Defic Syndr 70: e52–60. doi: 10.1097/QAI.0000000000000728 26371611
14. Weiser SD, Palar K, Frongillo EA, Tsai AC, Kumbakumba E, dePee S, et al. (2014) Longitudinal assessment of associations between food insecurity, antiretroviral adherence and HIV treatment outcomes in rural Uganda. AIDS 28 : 115–120. doi: 10.1097/01.aids.0000433238.93986.35 23939234
15. Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN (2008) Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. J Am Med Assoc 300 : 506–507.
16. Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. (2010) Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr 53 : 405–411. doi: 10.1097/QAI.0b013e3181b843f0 19745753
17. Geng EH, Odeny TA, Lyamuya RE, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. (2015) Estimation of mortality among HIV-infected people on antiretroviral treatment in east Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV 2: e107–116. doi: 10.1016/S2352-3018(15)00002-8 26424542
18. Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, et al. (2015) Mass HIV treatment and sex disparities in life expectancy: demographic surveillance in rural South Africa. PLoS Med 12: e1001905. doi: 10.1371/journal.pmed.1001905
19. Mahapatra P, Shibuya K, Lopez AD, Coullare F, Notzon FC, Rao C, et al. (2007) Civil registration systems and vital statistics: successes and missed opportunities. Lancet 370 : 1653–1663. 18029006
20. Goga AE, Dinh TH, Jackson DJ, Lombard C, Delaney KP, Puren A, et al. (2015) First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health 69 : 240–248. doi: 10.1136/jech-2014-204535 25371480
21. Courtenay WH (2000) Constructions of masculinity and their influence on men's well-being: a theory of gender and health. Soc Sci Med 50 : 1385–1401. 10741575
22. Mills EJ, Beyrer C, Birungi J, Dybul MR (2012) Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med 9: e1001167. doi: 10.1371/journal.pmed.1001167 22346735
23. Jewkes R, Morrell R (2010) Gender and sexuality: emerging perspectives from the heterosexual epidemic in South Africa and implications for HIV risk and prevention. J Int AIDS Soc 13 : 6. doi: 10.1186/1758-2652-13-6 20181124
24. Oeppen J, Vaupel JW (2002) Demography. Broken limits to life expectancy. Science 296 : 1029–1031. 12004104
25. Thomas AG, Grillo MP, Djibo DA, Hale B, Shaffer RA (2014) Military HIV policy assessment in sub-Saharan Africa. Mil Med 179 : 773–777. doi: 10.7205/MILMED-D-13-00495 25003863
26. Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N (2012) Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med 9: e1001351. doi: 10.1371/journal.pmed.1001351 23226107
27. Meyer-Rath G, Pienaar J, Brink B, van Zyl A, Muirhead D, Grant A, et al. (2015) The impact of company-level ART provision to a mining workforce in South Africa: a cost-benefit analysis. PLoS Med 12: e1001869. doi: 10.1371/journal.pmed.1001869 26327271
28. Decroo T, Telfer B, Biot M, Maikere J, Dezembro S, Cumba LI, et al. (2011) Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic Syndr 56: e39–44. doi: 10.1097/QAI.0b013e3182055138 21084990
29. Tsai AC, Bangsberg DR, Weiser SD (2013) Harnessing poverty alleviation to reduce the stigma of HIV in sub-Saharan Africa. PLoS Med 10: e1001557. doi: 10.1371/journal.pmed.1001557 24319400
30. King R, Lifshay J, Nakayiwa S, Katuntu D, Lindkvist P, Bunnell R (2009) The virus stops with me: HIV-infected Ugandans' motivations in preventing HIV transmission. Soc Sci Med 68 : 749–757. doi: 10.1016/j.socscimed.2008.11.008 19101063
31. Matthews LT, Crankshaw T, Giddy J, Kaida A, Smit JA, Ware NC, et al. (2013) Reproductive decision-making and periconception practices among HIV-positive men and women attending HIV services in Durban, South Africa. AIDS Behav 17 : 461–470. doi: 10.1007/s10461-011-0068-y 22038045
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Dispersion of the HIV-1 Epidemic in Men Who Have Sex with Men in the Netherlands: A Combined Mathematical Model and Phylogenetic Analysis
- Venous Thrombosis Risk after Cast Immobilization of the Lower Extremity: Derivation and Validation of a Clinical Prediction Score, L-TRiP(cast), in Three Population-Based Case–Control Studies
- From Checklists to Tools: Lowering the Barrier to Better Research Reporting
- Care that Matters: Quality Measurement and Health Care
- The Missing Men: HIV Treatment Scale-Up and Life Expectancy in Sub-Saharan Africa
- The First Use of the Global Oral Cholera Vaccine Emergency Stockpile: Lessons from South Sudan
- The PneuCarriage Project: A Multi-Centre Comparative Study to Identify the Best Serotyping Methods for Examining Pneumococcal Carriage in Vaccine Evaluation Studies
- Mass HIV Treatment and Sex Disparities in Life Expectancy: Demographic Surveillance in Rural South Africa
- The HIV Treatment Gap: Estimates of the Financial Resources Needed versus Available for Scale-Up of Antiretroviral Therapy in 97 Countries from 2015 to 2020
- Selection of an HLA-C*03:04-Restricted HIV-1 p24 Gag Sequence Variant Is Associated with Viral Escape from KIR2DL3+ Natural Killer Cells: Data from an Observational Cohort in South Africa
- Shortening Turnaround Times for Newborn HIV Testing in Rural Tanzania: A Report from the Field
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venous Thrombosis Risk after Cast Immobilization of the Lower Extremity: Derivation and Validation of a Clinical Prediction Score, L-TRiP(cast), in Three Population-Based Case–Control Studies
- The First Use of the Global Oral Cholera Vaccine Emergency Stockpile: Lessons from South Sudan
- The HIV Treatment Gap: Estimates of the Financial Resources Needed versus Available for Scale-Up of Antiretroviral Therapy in 97 Countries from 2015 to 2020
- Selection of an HLA-C*03:04-Restricted HIV-1 p24 Gag Sequence Variant Is Associated with Viral Escape from KIR2DL3+ Natural Killer Cells: Data from an Observational Cohort in South Africa
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



