-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis
Background:
Surveillance for hepatocellular carcinoma (HCC) has level I evidence among patients with hepatitis B but only level II evidence in patients with cirrhosis. This lack of randomized data has spurred questions regarding the utility of HCC surveillance in this patient population; however, lack of randomized data does not equate to a lack of data supporting the efficacy of surveillance. The aim of our study was to determine the effect of HCC surveillance on early stage tumor detection, receipt of curative therapy, and overall survival in patients with cirrhosis.Methods and Findings:
We performed a systematic literature review using Medline from January 1990 through January 2014 and a search of national meeting abstracts from 2009–2012. Two investigators identified studies that reported rates of early stage tumor detection, curative treatment receipt, or survival, stratified by HCC surveillance status, among patients with cirrhosis. Both investigators independently extracted data on patient populations, study methods, and results using standardized forms. Pooled odds ratios, according to HCC surveillance status, were calculated for each outcome using the DerSimonian and Laird method for a random effects model.
We identified 47 studies with 15,158 patients, of whom 6,284 (41.4%) had HCC detected by surveillance. HCC surveillance was associated with improved early stage detection (odds ratio [OR] 2.08, 95% CI 1.80–2.37) and curative treatment rates (OR 2.24, 95% CI 1.99–2.52). HCC surveillance was associated with significantly prolonged survival (OR 1.90, 95% CI 1.67–2.17), which remained significant in the subset of studies adjusting for lead-time bias. Limitations of current data included many studies having insufficient duration of follow-up to assess survival and the majority not adjusting for liver function or lead-time bias.Conclusions:
HCC surveillance is associated with significant improvements in early tumor detection, receipt of curative therapy, and overall survival in patients with cirrhosis.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(4): e32767. doi:10.1371/journal.pmed.1001624
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001624Summary
Background:
Surveillance for hepatocellular carcinoma (HCC) has level I evidence among patients with hepatitis B but only level II evidence in patients with cirrhosis. This lack of randomized data has spurred questions regarding the utility of HCC surveillance in this patient population; however, lack of randomized data does not equate to a lack of data supporting the efficacy of surveillance. The aim of our study was to determine the effect of HCC surveillance on early stage tumor detection, receipt of curative therapy, and overall survival in patients with cirrhosis.Methods and Findings:
We performed a systematic literature review using Medline from January 1990 through January 2014 and a search of national meeting abstracts from 2009–2012. Two investigators identified studies that reported rates of early stage tumor detection, curative treatment receipt, or survival, stratified by HCC surveillance status, among patients with cirrhosis. Both investigators independently extracted data on patient populations, study methods, and results using standardized forms. Pooled odds ratios, according to HCC surveillance status, were calculated for each outcome using the DerSimonian and Laird method for a random effects model.
We identified 47 studies with 15,158 patients, of whom 6,284 (41.4%) had HCC detected by surveillance. HCC surveillance was associated with improved early stage detection (odds ratio [OR] 2.08, 95% CI 1.80–2.37) and curative treatment rates (OR 2.24, 95% CI 1.99–2.52). HCC surveillance was associated with significantly prolonged survival (OR 1.90, 95% CI 1.67–2.17), which remained significant in the subset of studies adjusting for lead-time bias. Limitations of current data included many studies having insufficient duration of follow-up to assess survival and the majority not adjusting for liver function or lead-time bias.Conclusions:
HCC surveillance is associated with significant improvements in early tumor detection, receipt of curative therapy, and overall survival in patients with cirrhosis.
Please see later in the article for the Editors' SummaryIntroduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and one of the leading causes of death among patients with cirrhosis [1]. Its incidence in the United States and Europe is increasing due to the current epidemic of nonalcoholic steatohepatitis (NASH) and hepatitis C virus (HCV) cases [2]. Prognosis for patients with HCC depends on tumor stage, with curative therapies only available for patients detected at an early stage. Patients detected at an early stage can achieve 5-year survival rates of 70% with transplant or resection, whereas those with advanced HCC are only eligible for palliative treatments and have a median survival of less than one year [3],[4].
The American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) guidelines recommend surveillance with ultrasound every 6 months in high-risk patients, i.e., those with chronic hepatitis B virus (HBV) infection and/or cirrhosis [5],[6]. The goal of surveillance is to detect HCC at an early stage when it is amenable to curative therapy and to reduce all cause mortality. Although surveillance among HBV patients is supported by a large randomized controlled trial, there is no similar level I evidence supporting this practice among patients with cirrhosis [7]. Data from patients with HBV cannot be directly extrapolated to patients with cirrhosis for several reasons, including a higher competing risk of non-HCC mortality and lower sensitivity of surveillance tools for HCC with a nodular liver [8]. The lack of randomized data has spurred questions regarding the utility of HCC surveillance in this patient population [9].
Given the lack of a randomized trial of HCC surveillance among patients with cirrhosis, a meta-analysis of cohort and case-control studies can serve to better characterize any potential benefits of HCC surveillance. The aim of our study was to determine the association of HCC surveillance with (i) detection of tumors at an early stage, (ii) receipt of curative therapies, and (iii) overall survival in patients with cirrhosis.
Methods
Data Sources and Searches
We conducted a computer-assisted search with the Ovid interface to Medline to identify relevant published articles. We search the Medline database from January 1, 1989 through January 1, 2014 with the following keyword combinations: (liver ca$ OR hepatocellular ca$ OR hcc OR hepatoma) AND (screen$ OR surveillance OR ultrasound). We chose to include studies after January 1989 to accurately reflect the current performance of ultrasonography and the current availability of curative therapies (including liver transplantation and radiofrequency ablation [RFA]). Manual searches of reference lists from applicable studies were performed to identify any studies that may have been missed by the computer-assisted search. Additional searches of AASLD, EASL, Digestive Diseases Week (DDW), American College of Gastroenterology (ACG), and American Society of Clinical Oncology (ASCO) meeting abstracts from 2010–2012 were performed. Finally, consultation with expert hepatologists was performed to identify additional references or unpublished data. This study was conducted in accordance with PRISMA guidelines [10].
Study Selection
Two investigators (AGS and AP) reviewed citations identified by the search strategy to generate a list of potentially relevant articles. The abstract for each potentially relevant study was then reviewed by each of the two investigators. If the applicability of a study could not be determined by title or abstract alone, the full text was reviewed. Articles were independently checked for possible inclusion and disagreements were resolved through consensus with a third reviewer (JT).
Studies were included for analysis if they (i) utilized ultrasound, with or without concomitant alpha fetoprotein (AFP), for HCC surveillance; (ii) performed surveillance in a cohort of patients with cirrhosis; and (iii) reported the number of HCC detected at an early stage, number of HCC patients who received curative therapies, and/or overall survival in both patients undergoing surveillance and those not undergoing surveillance. If a study included both patients with cirrhosis and chronic hepatitis, only data regarding patients with cirrhosis were extracted if possible. We included articles published in English or Spanish. We excluded studies that (i) evaluated one-time screening instead of surveillance or (ii) only reported outcome measures for patients undergoing surveillance but not for those without surveillance. Additional exclusion criteria included non-human data, lack of original data and incomplete reports. If duplicate publications used the same cohort of patients, data from the most recent article were included.
Data Extraction and Quality Assessment
Two reviewers (AGS and AP) independently extracted required information from eligible studies using standardized forms. A third investigator (JT) was available to resolve discrepancies between the two sets of extracted data. The data extraction form included the following study design items: characteristics and size of study cohort, inclusion and exclusion criteria, surveillance tests, surveillance interval, and definition of early stage disease. In addition, we recorded the following primary data for patients who received and did not receive surveillance: number of patients with HCC, proportion of HCC discovered at an early stage, proportion of patients who received curative treatments, and overall survival. Two investigators (AGS and AP) assessed study quality by a modified checklist based upon the Ottawa-Newcastle scale (ONS), with discrepancies resolved by consensus. This instrument rates observational studies on a nine-point scale based on appropriateness of study sample, comparability of study groups, and adequacy of assessing exposure and outcomes [11].
Data Synthesis and Statistical Analysis
For each individual study, an odds ratio for each outcome of interest was calculated according to receipt of surveillance (i.e., surveillance group versus non-surveillance group). Our first outcome of interest was the proportion of patients diagnosed with early stage HCC. Early stage HCC was defined by Milan criteria, i.e., one tumor less than 5 cm in maximum diameter or two to three lesions, each with a maximum diameter less than 3 cm [12]. Insufficient data on performance status and liver function in most studies precluded use of the Barcelona Clinic Liver Cancer (BCLC) staging system. The second outcome of interest was the proportion of patients with HCC who underwent curative therapy. Curative treatments included any of the following: liver transplantation, surgical resection, RFA, or percurateous ethanol injection (PEI). Although transarterial chemoembolization (TACE) has been demonstrated to improve survival, it is regarded as palliative and was not included in our treatment outcome. Finally, our third outcome of interest was overall survival.
For each outcome of interest, we calculated a pooled odds ratio estimate with corresponding 95% confidence intervals, using the DerSimonian and Laird method for a random effects model. Heterogeneity was evaluated graphically by examination of forest plots and then statistically by the chi-squared test of heterogeneity and the inconsistency index (I2). A chi-squared p-value<0.05 or I2 values >50% are consistent with possible substantial heterogeneity [13],[14]. Meta-influence analysis, in which one study is removed at a time, was performed to determine if there was possible undue influence of a single study. Publication bias was evaluated graphically by funnel plot analysis (Figures S1, S2, S3) and then statistically using Begg's test [15]. An asymmetric funnel plot would suggest the possibility of small studies not being published due to unfavorable results.
Subset analyses were planned for predefined variables, including (i) location of study (Asia versus Europe versus United States), (ii) study period (prior to 1990s versus 1990s versus 2000s), (iii) proportion of Child Pugh C cirrhosis (<10% versus ≥10%), (iv) type of surveillance tests (ultrasound versus ultrasound and AFP), and (v) length of surveillance interval (≤6 months versus >6 months). Study location and study period were evaluated given potential differences in available technology over time. Subset analyses were planned for type of surveillance tests and surveillance interval, as both have been previously demonstrated to affect surveillance efficacy [16]. Finally, we included population characteristics, such as Child Pugh class, given that liver function is a known determinant of treatment eligibility and survival [5]. All data analysis was conducted using Stata 11.
Results
Literature Search
The computer-assisted search yielded 5,999 potentially relevant titles published between January 1, 1989 and January 1, 2014. After initial review, 246 titles were potentially appropriate, and these abstracts were reviewed. Eighty-four publications underwent full-text review, and 45 were excluded. The remaining 39 met all inclusion criteria (Figure 1). Searches of annual meeting abstracts yielded seven relevant abstracts with sufficient data for inclusion. Finally, recursive literature searches identified one additional article that met inclusion criteria, producing a total of 47 studies for inclusion.
Fig. 1. Map of literature search and selection process. 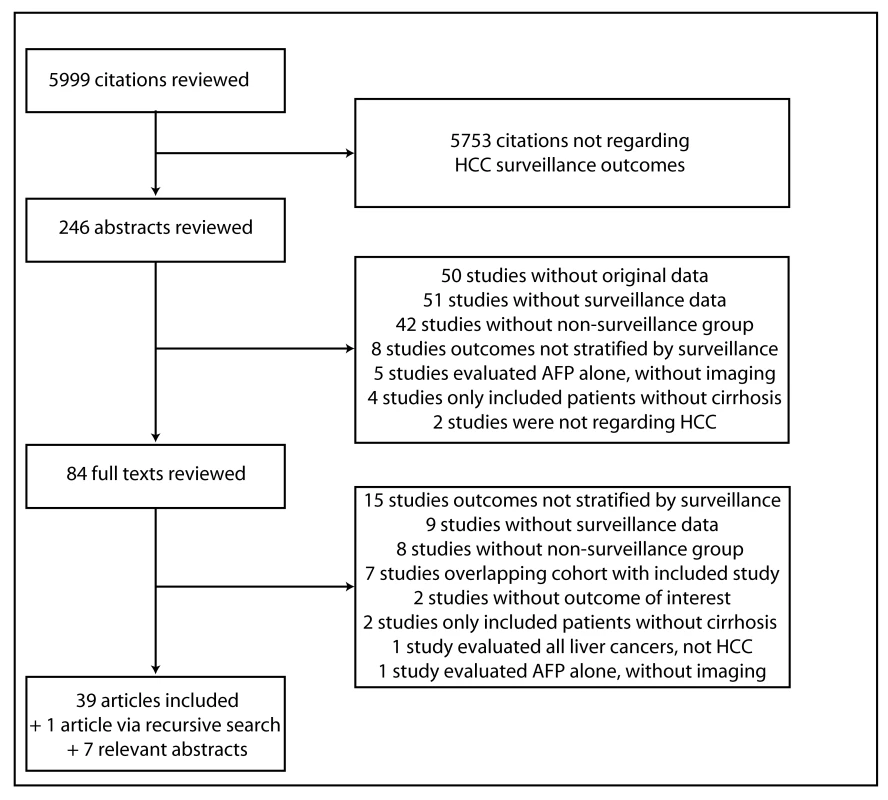
On the basis of evaluation of funnel plots (Figures S1, S2, S3), we could not exclude the possibility of publication bias. Most small studies produced larger positive effects than studies with large sample sizes, particularly for receipt of curative therapy and overall survival. There was a paucity of small “negative” studies, i.e., those failing to show a significant association between HCC surveillance and early detection, curative treatment, or overall survival. However, the association between HCC surveillance and each outcome remained statistically significant when only including large studies (i.e., those with at least 100 patients with HCC) (Table 4).
Study Characteristics
Characteristics of included studies are described in Table 1. We identified 47 studies, with a total of 15,158 patients, assessing the impact of HCC surveillance on at least one outcome of interest [17]–[63]. Of these patients, 6,284 (41.4%) HCC were detected by surveillance and 8,874 (58.6%) presented symptomatically or were found incidentally outside of a surveillance protocol. HCC was detected by surveillance in 51% (1,614 of 3,162) of patients among studies in the United States, 45% (1,182 of 2,611) of patients among studies in Europe, 37% (3,312 of 8,804) of patients among studies in Asia, and 30% (176 of 581) of patients among other studies. Most studies (n = 38) were retrospective in nature, although nine had collected data about HCC outcomes prospectively. Fifteen studies were conducted in the United States, 15 in Asia, 13 throughout Europe, and four studies were conducted in other countries. Of the 39 studies that specified surveillance tests used, only ten included ultrasound alone and most used a combination of ultrasound and/or AFP.
Tab. 1. Characteristics of included studies. 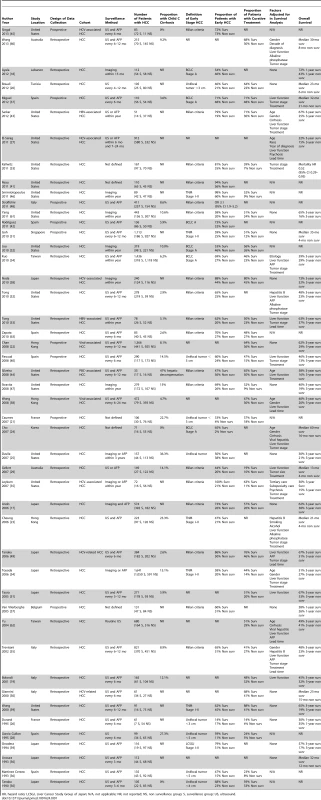
HR, hazard ratio; LCSGJ, Liver Cancer Study Group of Japan; N/A, not applicable; NR, not reported; NS, non surveillance group; S, surveillance group; US, ultrasound. Twenty-nine studies reported details regarding the proportion of patients with HCC and underlying cirrhosis. Overall, 90.9% (6,732 of 7,411) of patients had underlying cirrhosis, although rates ranged from 32.4% to 100% between studies. Twenty-seven studies reported information regarding liver function among included patients. The majority (55.5%) of patients (6,018 of 10,853) had Child Pugh A cirrhosis, with higher rates among those who received HCC surveillance (61.3% versus 51.4%, p<0.001) (2,607 of 4,255 for surveillance versus 3,213 of 6,247 for non-surveillance). Similarly, patients who received HCC surveillance had lower rates of Child Pugh class C cirrhosis (8.5% versus 11.7%, p<0.001) (310 of 3,647 for surveillance versus 592 of 5,062 for non-surveillance).
Quality Assessment
The quality assessment for included studies is described in Table 2. Out of a maximum 9-point score, 27 studies had quality scores of 5 or 6, 12 studies had a score of 7, and eight had quality scores of 8 or 9. Most studies had appropriate cohort selection, including representativeness of the surveillance cohort and selection of the non-surveillance cohort. All studies ascertained surveillance exposure and HCC outcomes through medical records. However, only six of the 36 studies assessing the impact of surveillance on survival controlled for both lead-time bias and Child Pugh liver function. An additional 13 studies controlled for liver function alone but 17 studies did not control for either factor. Furthermore, 20 studies did not have sufficient follow-up length to assess survival and 27 studies failed to adequately account for patients lost to follow-up.
Tab. 2. Quality assessment of studies. 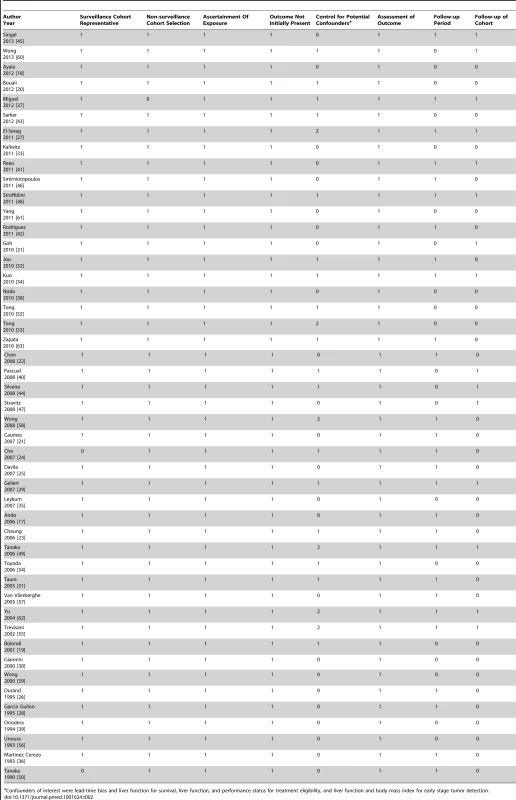
Confounders of interest were lead-time bias and liver function for survival, liver function, and performance status for treatment eligibility, and liver function and body mass index for early stage tumor detection. Association between HCC Surveillance and Detection of Tumors at an Early Stage
Thirty-eight studies, with a total of 10,904 patients, included data on tumor stage stratified by receipt of HCC surveillance [17],[18],[20],[21],[23]–[26],[28],[29],[31]–[50],[52]–[55],[57],[59],[61],[63]. Twenty-four studies defined early stage using BCLC or Milan criteria [17],[18],[24],[29],[32]–[35],[37],[38],[41]–[45],[47]–[49],[52],[53],[55],[57],[61],[63], whereas six studies used other staging systems (e.g., tumor node metastases [TNM]) [23],[31],[39],[46],[54],[59], and eight used operational definitions (e.g., unifocal lesion less than 3 cm) [20],[21],[25],[26],[28],[36],[40],[50] (Table 1). The 24 studies using BCLC or Milan criteria included a total of 6,573 patients, of whom 2,815 (44.1%) were diagnosed by surveillance.
When including all 38 studies, patients who underwent surveillance were significantly more likely to be found at an early stage (OR 2.11, 95% CI 1.88–2.33); however, there was significant heterogeneity (I2 = 73%, p<0.001) (Table 1). When only including studies using BCLC or Milan criteria, there was little change in effect size (OR 2.08, 95% CI 1.80–2.37) or heterogeneity (I2 = 77%, p<0.001). On subset analysis of the six studies using BCLC to define early stage, the pooled odds ratio was also stable at 1.96 (95% CI 1.41–2.73) [18],[24],[32],[34],[37],[42]. One notable outlier was a study by Cho and colleagues, which had a relative risk of 37.81 (95% CI 5.27–271.13) [24]. Only data on patients younger than 30 years old were reported for this study, so we excluded it from further analyses. Heterogeneity (I2 = 78%, p<0.001) could not be improved with removal of additional studies, and meta-influence analysis did not suggest undue influence of any single study. Among the 23 remaining studies, HCC surveillance was significantly associated with early stage tumor detection (OR 2.08, 95% CI 1.80–2.37) (Figure 2). The pooled rate of early stage HCC among patients undergoing surveillance was 70.9% (95% CI 69.3%–72.6%) (2,047 of 2,885 patients), compared to only 29.9% (95% CI 28.4–31.4%) (1,034 of 3,463 patients) among those who presented symptomatically and/or diagnosed incidentally.
Fig. 2. Association between HCC surveillance and early tumor detection rates. 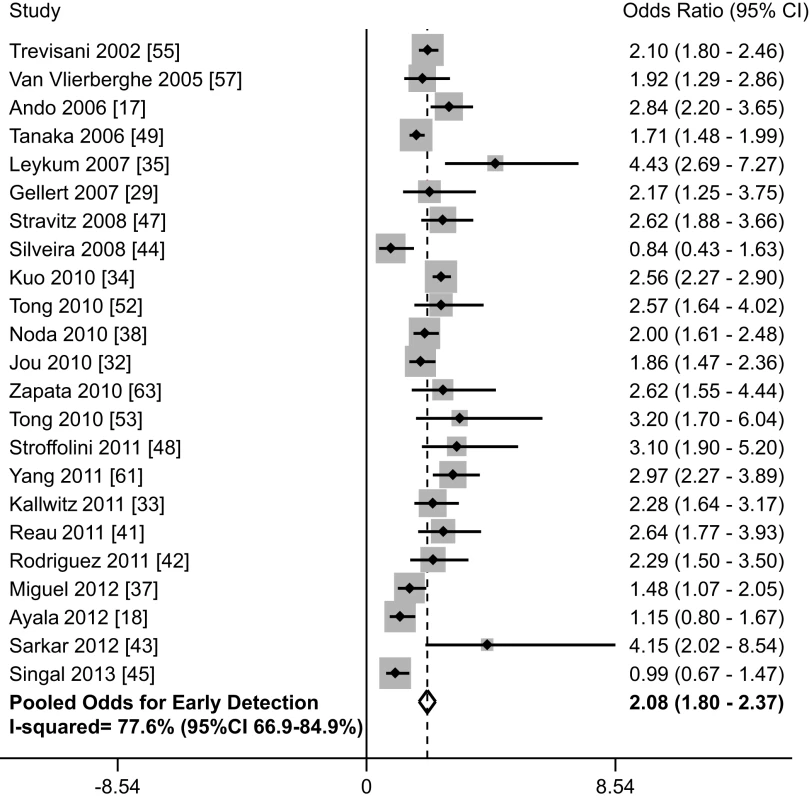
We performed pre-planned subset analyses according to study design, location of study, study period, and type of surveillance tests used (Table 4). Rates of early tumor detection were consistent across study location (OR 2.22 [95% CI 1.75–2.81] among studies conducted in Asia [17],[34],[38],[49] versus 2.00 [95% CI 1.70–2.35] among studies in Europe [37],[42],[55],[57],[63] versus 2.31 [95% CI 1.79–2.99] among studies in the United States [32],[33],[35],[41],[43],[44],[45],[47],[52],[53],[61]), study period (OR 2.22 [95% CI 1.77–2.79] among studies assessing surveillance in the 1990s [17],[29],[49],[53],[55] versus 2.18 [95% CI 1.86–2.56] among studies assessing surveillance after 2000 [18],[32]–[35],[37],[38],[41]–[43],[45],[47],[52],[57],[61],[63]), and type of surveillance tests (OR 2.04 [95% CI 1.55–2.68] with ultrasound alone [18],[32],[38],[47],[61] versus 2.16 [95% CI 1.80–2.60] with ultrasound and/or AFP [17],[29],[34],[35],[37],[42]–[45],[49],[52],[53],[55],[63]). There was no significant difference in the association between HCC surveillance and early stage tumor detection by study design (p = 0.10), with patients detected by surveillance being more likely to be found at an early stage in both subgroups. The pooled odds ratio was 2.30 (95% CI 1.98–2.67) among retrospective studies [17],[18],[29],[32]–[35],[38],[41],[43],[44],[47],[49],[52],[53],[61],[63], compared to 1.70 (95% CI 1.29–2.26) among studies in which data were prospectively collected [37],[42],[45],[55],[57]. Heterogeneity in early tumor detection may be related to several factors including variations in ultrasound operator experience and technique, patient body habitus, and liver nodularity, which we were unable to explore given the lack of patient-level data.
Association between HCC Surveillance and Receipt of Curative Treatment
Thirty-four studies, with a total of 12,187 patients, assessed the association of HCC surveillance with receipt of curative therapy [17],[19],[20]–[22],[26],[28]–[38],[40],[43],[44],[46],[47],[49],[50],[51],[53]–[55],[58]–[63]. Of the included patients, 4,655 (38.2%) were detected by surveillance and 7,532 (61.8%) presented symptomatically or were diagnosed incidentally. Patients diagnosed by surveillance were significantly more likely to undergo curative therapy, with a pooled odds ratio of 2.24 (95% CI 1.99–2.52) (Figure 3; Table 1). We found heterogeneity among studies (I2 = 75.3%, p<0.001). Meta-influence analysis did not suggest undue influence of any single study. Although four studies [33],[35],[46],[60] appeared to be outliers, we did not find clinical heterogeneity justifying their exclusion. The association between HCC surveillance and receipt of curative therapy did not substantially change if these four studies had been excluded (OR 2.11, 95% CI 1.89–2.37). The pooled rate of curative treatment receipt among patients undergoing surveillance was 51.6% (95% CI 50.2–53.0%) (2,402 of 4,655), compared to only 23.7% (95% CI 22.8%–24.7%) (1,790 of 7,532) among those who presented symptomatically or were diagnosed incidentally.
Fig. 3. Association between HCC surveillance and curative treatment rates. 
Among the 16 cohort studies that reported both early detection (using Milan or BCLC criteria) and curative treatment rates [17],[29],[32]–[35],[37],[38],[43],[44],[47],[49],[53],[55],[61],[63], we found a moderately strong positive correlation between early detection rates and curative treatment rates between studies (Pearson's correlation r = 0.54) (Figure S4). This finding suggests the association between surveillance and receipt of curative treatment is mediated by improved early tumor detection rates.
We performed pre-planned subset analyses, according to study design, location of study, study period, and type of surveillance tests used (Table 4). Rates of curative therapy receipt were consistent across study design (OR 2.18 [95% CI 1.94–2.45] among retrospective studies [17],[19],[20],[26],[28]–[30],[32]–[36],[38],[43],[44],[46],[47],[49],[50],[51],[53]–[55],[58]–[63] versus 2.37 [95% CI 1.51–3.72] among prospective studies [21],[22],[31],[37],[40]), study period (OR 2.12 [95% CI 1.25–3.61] among studies assessing surveillance prior to 1990 [26],[44],[50],[54] versus 2.23 [95% CI 1.87–2.67] among studies assessing surveillance in the 1990s [17],[19],[20], versus 2.13 [95% CI 1.85–2.44] among studies assessing surveillance after 2000 [21],[32],[34],[35],[37],[38],[43],[46],[47],[58],[61],[63]), and type of surveillance tests (OR 2.23 [95% CI 1.83–2.71] with ultrasound alone [20],[28],[32],[38],[46],[47],[61],[62] versus 2.19 [95% CI 1.89–2.53] with ultrasound and/or AFP [17],[19],[22],[26],[29]–[31],[34]–[37],[40],[43],[44],[49],[50],[51],[53]–[55],[58]–[60],[63]). Finally, there was no significant difference in the strength of association between HCC surveillance and curative therapy receipt by study location (p = 0.20); patients detected by surveillance were significantly more likely to receive curative therapy in both subgroups. The pooled odds of curative therapy were 1.87 (95% CI 1.51–2.31) for studies conducted in Europe [19],[21],[26],[28],[30],[36],[37],[40],[55],[63], 2.19 (95% CI 1.84–2.61) for studies conducted in Asia [17],[22],[31],[34],[38],[49],[50],[51],[54],[58],[62], and 2.52 (95% CI 1.99–3.20) for studies conducted in the United States [32],[33],[35],[43],[44],[46],[47],[53],[59],[61].
Association between HCC Surveillance and Overall Survival
Thirty-six studies, with a total of 13,361 patients (40.9% [n = 5,466] detected via surveillance), included data on survival stratified by receipt of HCC surveillance [17]–[20],[22]–[27],[29]–[31],[33]–[35],[37]–[40],[43],[44],[47],[49],[51]–[62]. There was substantial variability in reporting of survival data, with several studies reporting 1-year and/or 3-year survival rates, some reporting median survival without confidence intervals, and others showing a Kaplan Meier curve (Table 1). The most commonly reported survival outcome was 3-year survival, so this was used for further analysis. Three-year survival rates were estimated from Kaplan Meier curves if data were not otherwise presented. Among these 23 studies, HCC surveillance was significantly associated with improved survival, with a pooled odds ratio of 1.90 (95% CI 1.67–2.17) (Figure 4) [17],[19],[22],[25],[27],[34],[35],[38]–[40],[43],[44],[47],[49],[51]–[55],[58],[59],[61],[62]. The pooled 3-year survival rate was 50.8% among the 4,735 patients who underwent HCC surveillance, compared to only 27.9% among the 6,115 patients without prior surveillance (p<0.001).
Fig. 4. Association between HCC surveillance and survival. 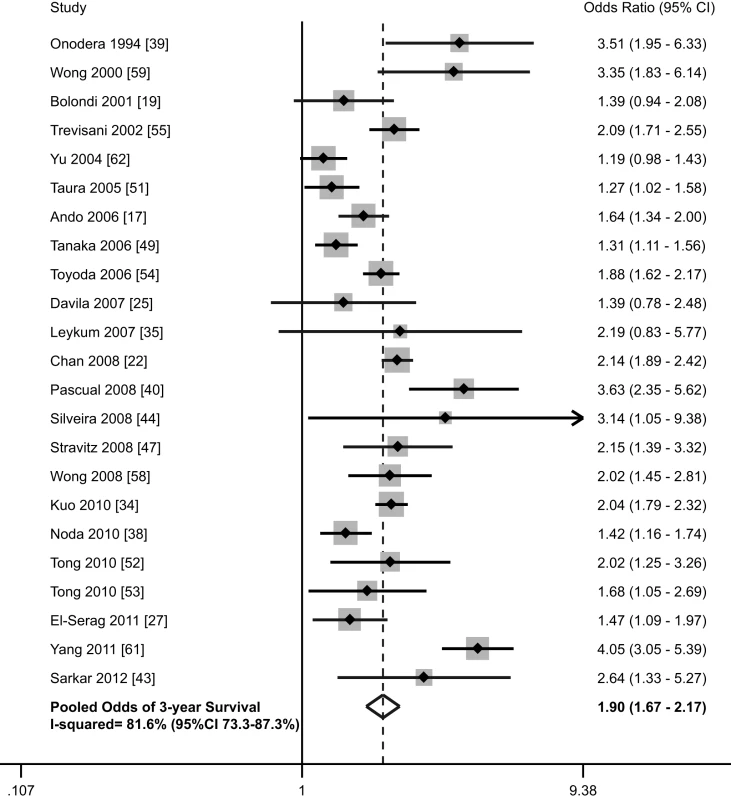
We performed pre-planned subset analyses, according to location of study, study period, proportion of Child Pugh C cirrhosis, and study quality (Table 4). The pooled 3-year survival rates for patients with and without surveillance were the highest among studies conducted in Asia [17],[22],[34],[38],[39],[49],[51],[54],[58],[62] (57.4% and 31.7%, respectively) (1,693 of 2,947 for surveillance versus 1,340 of 4,233 for non-surveillance), intermediate among studies from Europe [19],[40],[55] (47.3% and 21.8%, respectively) (259 of 548 for surveillance versus 159 of 728 for non-surveillance), and the lowest among studies conducted in the United States [25],[27],[35],[43],[44],[47],[52],[53],[59],[61] (36.5% and 18.2%, respectively) (453 of 1,240 for surveillance versus 210 of 1,154 for non-surveillance). Pooled 3-year survival rates were 51.1% (555/1086) and 25.4% (179/704) for surveillance and non-surveillance groups among studies assessing surveillance prior to 1990 [39],[44],[54], 57.6% (1,122/1,947) and 32.2% (893/2,773), respectively, among studies assessing surveillance during the 1990s [17],[19],[22],[40],[49],[51],[53],[55],[59], and 42.8% (728/1,702) and 24.1% (637/2,638) among those assessing surveillance after 2000 [25],[27],[34],[35],[38],[43],[47],[52],[58],[61]. There were 15 studies reporting the proportion of Child C patients and data regarding 3-year survival rates [19],[22],[25],[34],[40],[44],[47],[49],[51]–[55],[58],[61]. As anticipated, 3-year survival rates were inversely related to the proportion of patients with Child C cirrhosis. The pooled 3-year survival rates were 57.0% (1,033 of 1,813 patients) and 29.2% (960 of 3,293 patients) in patients with and without surveillance, respectively, among the eight studies with less than 10% Child Pugh C patients 22,[34],[49],[51]–[53],[55],[58]. In the seven studies with more than 10% Child Pugh C patients, the 3-year survival rates were only 49.8% (795 of 1,597 patients) and 22.0% (311 of 1,411 patients), respectively [19],[25],[40],[44],[47],[54],[61]. Finally, we evaluated survival according to study quality, with high-quality studies defined as those with a score of 7–9 [27],[34],[40],[44],[49],[51],[53],[55],[58],[62] and low-quality studies defined as those with scores less than 7 [17],[19],[22],[25],[35],[38],[39],[43],[47],[52],[54],[59],[61]. High-quality and low-quality studies had similar 3-year survival rates in the non-surveillance groups (28.8% versus 26.9%, respectively, p = 0.09) (965 of 3,346 patients for high-quality studies and 744 of 2,769 patients for low-quality studies). However, 3-year survival rates were significantly lower in the surveillance groups in high quality studies than low-quality studies (45.6% versus 54.7%, respectively, p<0.001) (927 of 2,031 patients for high-quality studies versus 1,478 of 2,704 patients for low-quality studies).
Six studies evaluated any potential benefit of surveillance on survival, after adjusting for lead-time bias (Table 3) [27],[49],[53],[55],[58],[62]. Among these studies, HCC surveillance was still associated with a significant improvement in survival (3-year survival rates 39.7% versus 29.1%, p<0.001) (556 of 1,401 patients for surveillance versus 567 of 1,946 for non-surveillance) (p<0.001). El-Serag and colleagues reported improved survival when assuming a tumor doubling time of 70 days (OR 0.81, 95% CI 0.70–0.94) [27]. Assuming a tumor doubling time of 90 days, surveillance was associated with improved survival in studies by Wong (p = 0.04) [58] and Tanaka (p = 0.02) [49]. Tong and colleagues found significantly improved 3-year survival (62.5% versus 36.6%, p = 0.007) after adjusting for a lead-time of 3.9 months, which was based on tumor doubling time among their patients [53]. Yu and colleagues also found significantly reduced mortality at 3 years among those with surveillance (OR 0.35, 95% CI 0.24–0.49) [62]. Adjusting for lead-time bias (239 days for 6-month surveillance and 98 days for annual surveillance), Trevisani and colleagues found patients undergoing surveillance had a median survival of 30 months, which was significantly better than the 20-month median survival among patients with incidentally discovered tumors (p<0.001) or the 9-month median survival among patients who presented symptomatically (p<0.001) [55].
Tab. 3. Studies assessing survival benefit of surveillance after adjusting for lead time bias. 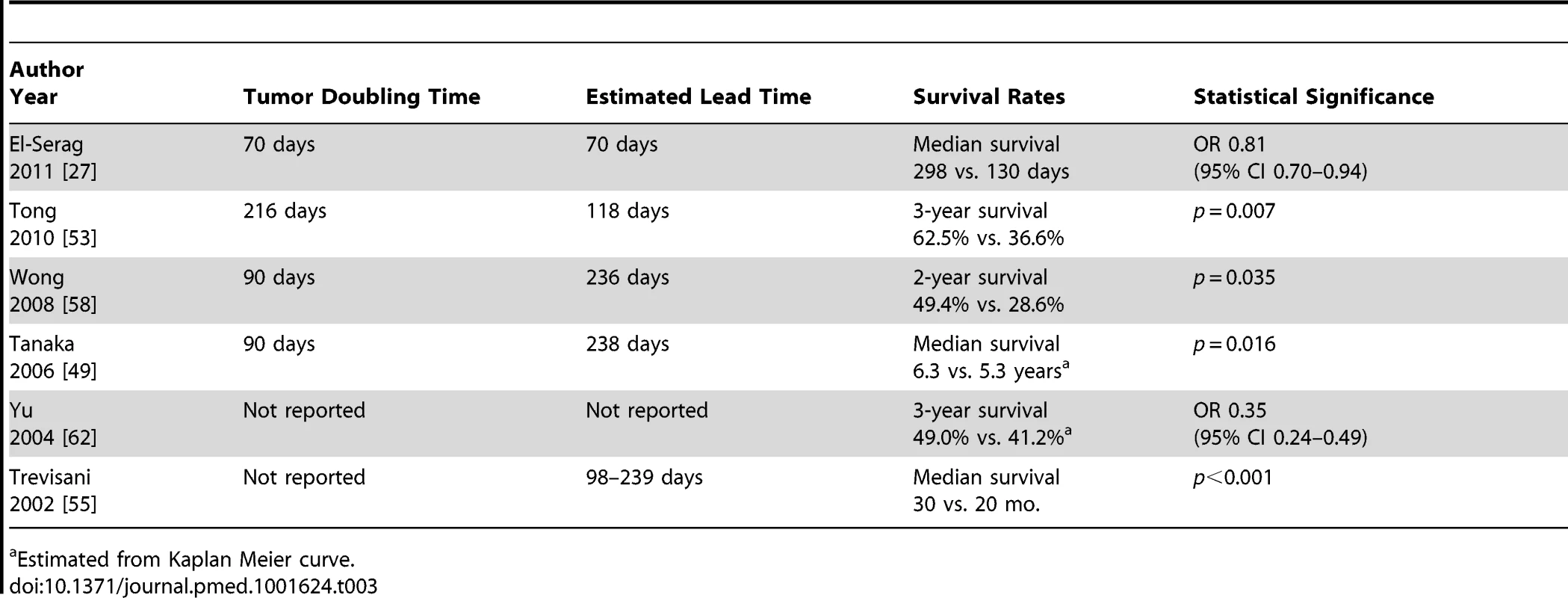
Estimated from Kaplan Meier curve. Tab. 4. Subgroup analyses for association between HCC surveillance and early detection, curative treatment rates, and survival. 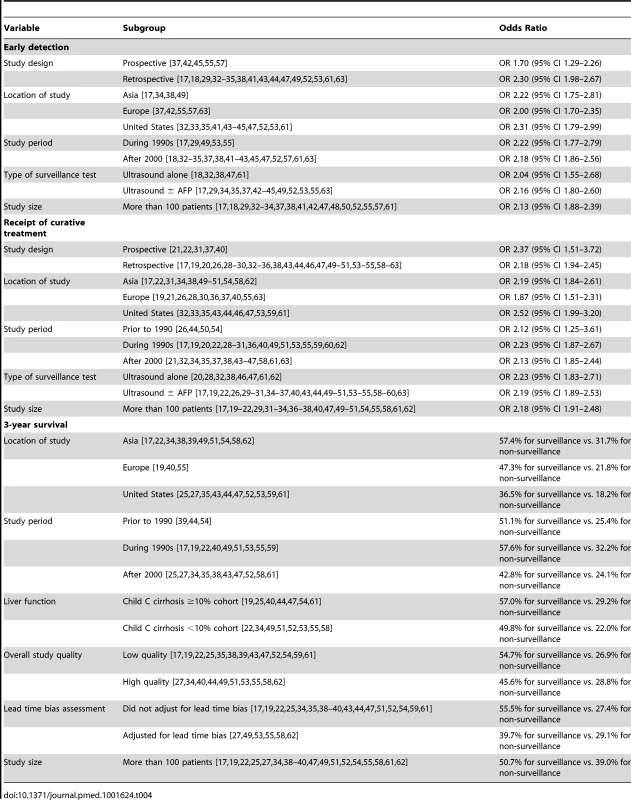
Discussion
To the best of our knowledge, our meta-analysis is the first to critically examine available literature and characterize the potential impact of HCC surveillance on outcomes in patients with cirrhosis. We demonstrated HCC surveillance was associated with significant improvement in early tumor detection and receipt of curative therapies. Most importantly, HCC surveillance was associated with a significant improvement in overall survival. However, there are limitations in current literature, including many studies having insufficient duration of follow-up to adequately assess survival and the majority not adjusting for liver function or lead-time bias. Overall, in the absence of randomized data of surveillance efficacy, our meta-analysis provides sufficient evidence to support guidelines that recommend HCC surveillance in patients with cirrhosis.
The lack of randomized data supporting HCC surveillance in cirrhotic patients has caused some providers to question its benefit, which may contribute to low utilization rates. Prior studies have reported HCC surveillance rates below 20% in the United States, with lower rates among primary care physicians than gastroenterologists/hepatologists [64]–[68]. However, a lack of randomized data does not necessarily equate to a lack of efficacy. For example, colonoscopy is widely embraced for colorectal cancer screening, without randomized data, based on cohort and case-control studies as well as extrapolation of fecal occult blood test data [69]–[71]. HCC surveillance fulfills all criteria established by the World Health Organization for a surveillance program [72]: the disease burden of HCC is an important health problem, there is an identifiable target population, surveillance is accepted by patients and providers, surveillance achieves an acceptable level of accuracy, there are standardized recall procedures, surveillance is affordable, there is an advantage of treating occult HCC, and surveillance reduces mortality. Our meta-analysis highlights consistent improvements in early tumor detection, receipt of curative therapy, and overall survival with HCC surveillance among patients with cirrhosis. In light of these data, a randomized controlled trial of HCC surveillance could be deemed unethical. In fact, prior attempts at a randomized trial were unsuccessful, as patients refused participation and desired surveillance after the benefits and harms were discussed [73].
We found substantial statistical heterogeneity between studies, suggesting benefits of surveillance may not be uniform among all patients. Several studies included patients with Child C cirrhosis, which may explain some heterogeneity with regard to treatment eligibility and survival. Trevisani and colleagues demonstrated the survival benefit of HCC surveillance was most marked in patients with Child A cirrhosis [55]. Those with Child C cirrhosis failed to achieve a significant benefit, given lower treatment eligibility rates and higher competing risk of liver-related mortality. Surveillance is not recommended in patients with Child C cirrhosis unless they are transplant candidates [5], so their inclusion in several studies may have mitigated reported benefits of surveillance on treatment eligibility and overall survival.
Furthermore, the risk of HCC may not be uniform across patients and etiologies of liver disease [74]. For example, patients with HCV cirrhosis have a higher risk of HCC than those with alcohol-induced cirrhosis or NASH [75],[76]. Predictive models have been created using several risk factors but are limited by moderate accuracy to date [77],[78]. Similarly, surveillance is performed with ultrasound and AFP in all patients despite variations in accuracy among patients. Ultrasound is less sensitive in obese patients and those with advanced fibrosis, whereas AFP may be less accurate among HCV positive patients [8],[79]. Accurate assessment of HCC risk and surveillance performance characteristics may allow personalized surveillance programs, which could optimize benefits and cost-effectiveness of HCC surveillance. Surveillance may be avoided in low-risk patients, whereas high-risk patients could benefit from a more intensive surveillance regimen.
On subgroup analysis for the association between HCC surveillance and overall survival, we found substantial differences according to study location. We did not find any significant variation in study quality (p = 0.37) or size (p = 0.07) by study location that might help explain the differences. Further studies are needed to explore this heterogeneity, as there are several potential explanations. There are differences in patient populations, such as higher rates of obesity and NASH-related cirrhosis in the United States than Europe and Asia [80], which may affect treatment response and recurrence rates. There are also differential rates and choice of curative treatment among patients found at an early stage, which can influence response rates, recurrence rates, and overall survival [81].
Results from our study must be interpreted within the limitations of included studies. Many studies failed to adequately account for patients lost to follow-up and did not have sufficient follow-up to adequately assess survival. Furthermore, several studies used operational definitions of surveillance, such as ultrasound or AFP in a two-year period, which were not consistent with guideline recommendations. Guidelines recommend ultrasound every 6 months to optimize sensitivity, and AFP should not be used alone without imaging [5]. Clear definitions and measures should be used in future studies to better interpret and quantify any benefits of HCC surveillance.
All studies in this meta-analysis were non-randomized cohort studies, with potential for lead-time and length-time biases. However, several studies demonstrated a significant improvement in survival after statistically adjusting for lead-time bias [82]. Furthermore, lead-time bias may be less problematic for patients diagnosed at an early stage by surveillance, given the selective availability of curative options at that stage. Liver transplantation, surgical resection, and RFA have been associated with 5-year survival rates approaching 70% but are only available for patients with early stage tumors [5]. The survival benefit of HCC surveillance is contingent on subsequent receipt of curative therapy [83]. This relationship is further highlighted by the strong positive correlation between early tumor detection and curative treatment rates among studies in our meta-analysis.
Study results were also potentially limited by selection bias, with a differential distribution of liver function and/or performance status among surveillance and non-surveillance groups. Surveillance group patients were less likely to have Child Pugh C liver disease, although liver function was not reported in all studies. Other studies have suggested that patients with hepatic decompensation are more likely to have recognized cirrhosis and therefore receive surveillance [68]. We did not find information regarding functional status in any of the included studies. Detailed reporting of performance status and liver function is important given both are key factors in determining treatment eligibility. Patients with poor functional status or Child C cirrhosis, if not transplant candidates, should be excluded given HCC surveillance is not recommended in these subgroups. Finally, a comprehensive assessment of surveillance should weigh benefits and harms; however, no study in our meta-analysis assessed downstream harms. Although ultrasound and AFP have minimal direct harms, there are potential downstream harms from recall policies (e.g., complications of liver biopsy or cross-sectional imaging) that should be considered in future studies.
In summary, current data suggest that HCC surveillance is associated with significant improvement in early tumor detection. By facilitating receipt of curative therapy in a higher proportion of patients, HCC surveillance is associated with a significant improvement in overall survival. There are notable limitations in current literature, including many studies failing to adequately adjust for lead-time bias. However, the preponderance of data that consistently demonstrate benefits should provide sufficient rationale to recommend HCC surveillance, even in the absence of a randomized controlled trial among patients with cirrhosis.
Supporting Information
Zdroje
1. El-SeragHB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142 : 1264–1273.
2. El-SeragHB, DavilaJA, PetersenNJ, McGlynnKA (2003) The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 139 : 817–823.
3. LlovetJM, BustamanteJ, CastellsA, VilanaR, Ayuso MdelC, et al. (1999) Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 29 : 62–67.
4. PadhyaKT, MarreroJA, SingalAG (2013) Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol 29 : 285–292.
5. BruixJ, ShermanM (2010) Management of hepatocellular carcinoma: an update. Hepatology 53 : 1–35.
6. BruixJ, ShermanM, LlovetJM, BeaugrandM, LencioniR, et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35 : 421–430.
7. ZhangBH, YangBH, TangZY (2004) Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 130 : 417–422.
8. SingalAG, ConjeevaramHS, VolkML, FuS, FontanaRJ, et al. (2012) Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev 21 : 793–799.
9. LederleFA, PochaC (2012) Screening for liver cancer: the rush to judgment. Ann Intern Med 156 : 387–389.
10. MoherD, LiberatiA, TetzlaffJ, AltmanDG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151 : 264–269, W264.
11. Wells GA, Shea B, O'Connell D, Peterson J (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
12. MazzaferroV, RegaliaE, DociR, AndreolaS, PulvirentiA, et al. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334 : 693–699.
13. HigginsJP, ThompsonSG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21 : 1539–1558.
14. HigginsJP, ThompsonSG, DeeksJJ, AltmanDG (2003) Measuring inconsistency in meta-analyses. BMJ 327 : 557–560.
15. CopasJB, ShiJQ (2001) A sensitivity analysis for publication bias in systematic reviews. Stat Methods Med Res 10 : 251–265.
16. SingalA, VolkML, WaljeeA, SalgiaR, HigginsP, et al. (2009) Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 30 : 37–47.
17. AndoE, KuromatsuR, TanakaM, TakadaA, FukushimaN, et al. (2006) Surveillance program for early detection of hepatocellular carcinoma in Japan: results of specialized department of liver disease. J Clin Gastroenterol 40 : 942–948.
18. AyalaG, DicksonR, ToorA (2012) Does surveillance affect the outcome of patients with HCC? Hepatology 56 : 486A.
19. BolondiL, SofiaS, SiringoS, GaianiS, CasaliA, et al. (2001) Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 48 : 251–259.
20. Bouali R, Bizid S, Jeddi H (2012) Screening for hepatocellular carcinoma in cirrhotics: 20 years' experience. Berlin: International Liver Cancer Association.
21. CaumesJL, NousbaumJB, BessaguetC, FaycalJ, RobaszkiewiczM, et al. (2007) Epidemiology of hepatocellular carcinoma in Finistere. Prospective study from June 2002 to May 2003. Gastroenterol Clin Biol 31 : 259–264.
22. ChanAC, PoonRT, NgKK, LoCM, FanST, et al. (2008) Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg 247 : 666–673.
23. CheungTK, LaiCL, WongBC, FungJ, YuenMF (2006) Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther 24 : 573–583.
24. ChoSJ, YoonJH, HwangSS, LeeHS (2007) Do young hepatocellular carcinoma patients with relatively good liver function have poorer outcomes than elderly patients? J Gastroenterol Hepatol 22 : 1226–1231.
25. DavilaJA, WestonA, SmalleyW, El-SeragHB (2007) Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol 41 : 777–782.
26. DurandF, BuffetC, PelletierG, HagegeH, InkO, et al. (1995) Screening of hepatocellular carcinoma in French patients. Dig Dis Sci 40 : 706–707.
27. El-SeragHB, KramerJR, ChenGJ, DuanZ, RichardsonPA, et al. (2011) Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut 60 : 992–997.
28. Garcia GullonC, Rendon UncetaP, Martin HerreraL, Soria de la CruzM, MaciasM, et al. (1995) Utilidad de la ecografia en el diagnostico precoz del hepatocarcinoma en pacientes con cirrosis hepatica. Restiva espanola de enfermedades digestivas 87 : 798–801.
29. GellertL, JalaludinB, LevyM (2007) Hepatocellular carcinoma in Sydney South West: late symptomatic presentation and poor outcome for most. Intern Med J 37 : 516–522.
30. GianniniE, ArzaniL, BorroP, BottaF, FasoliA, et al. (2000) Does surveillance for hepatocellular carcinoma in HCV cirrhotic patients improve treatment outcome mainly due to better clinical status at diagnosis? Hepatogastroenterology 47 : 1395–1398.
31. GohB, ChangJ, OngW (2010) Surveillance for hepatocellular carcinoma in chronic liver disease is associated with increased diagnosis of early HCC and a genuine improvement in survival - a study of 1,113 cases. Hepatology 52 : 1136A.
32. JouJH, ChenPH, JazwinskiA, BounevaI, SmithAD, et al. (2010) Rates of surveillance and management of hepatocellular carcinoma in patients evaluated at a liver transplant center. Dig Dis Sci 55 : 3591–3596.
33. KallwitzE, GabaRC, HuangY (2011) Survival with hepatocellular carcinoma is associated with surveillance, lesion size, ablation and transplant in an area with limited organ availability. Hepatology 54 : 1381A.
34. KuoYH, LuSN, ChenCL, ChengYF, LinCY, et al. (2010) Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer 46 : 744–751.
35. LeykumLK, El-SeragHB, CornellJ, PapadopoulosKP (2007) Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin Gastroenterol Hepatol 5 : 508–512.
36. Martinez CerezoF, PuigM, Martinez NoguerasA, TomasA, PamplonaJ, et al. (1993) Valor de la ecografia en el diagnostico precoz del carcinoma hepatocelular. Revista espanola de enfermedades digestivas 84 : 311–314.
37. MiguelM, SopenaJ, VergaraM, GilM (2012) Factors related to survival in hepatocellular carcinoma in the geographic area of Sabadell (Catalonia, Spain). Rev Esp Enferm Dig 104 : 242–247.
38. NodaI, KitamotoM, NakaharaH, HayashiR, OkimotoT, et al. (2010) Regular surveillance by imaging for early detection and better prognosis of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol 45 : 105–112.
39. OnoderaH, UkaiK, NakanoN, TakedaT, SuzukiH, et al. (1994) Outcomes of 116 patients with hepatocellular carcinoma. Cancer Chemother Pharmacol 33 (Suppl) S103–108.
40. PascualS, IrurzunJ, ZapaterP, SuchJ, SempereL, et al. (2008) Usefulness of surveillance programmes for early diagnosis of hepatocellular carcinoma in clinical practice. Liver Int 28 : 682–689.
41. ReauN, AronsohnA, TeHS (2011) Managing physician, not patient factors, predict hepatocellular carcinoma (HCC) screening outcomes. Hepatology 54 : 1410A.
42. RodriguezR, GutierrezM, Gonzalez de FrutosC, de Artaza VarasaT, de la Cruz PerezG, et al. (2011) Caracteristicas clinicas, estadiicacion y tratamiento de los pacientes con carcinoma hepatocelular en la practica clinica. Estudio propsectivo de una serie de 136 pacientes. Gastroenterologia y Hepatologia 34 : 524–531.
43. SarkarM, StewartS, YuA, ChenMS, NguyenTT, et al. (2012) Hepatocellular carcinoma screening practices and impact on survival among hepatitis B-infected Asian Americans. J Viral Hepat 19 : 594–600.
44. SilveiraMG, SuzukiA, LindorKD (2008) Surveillance for hepatocellular carcinoma in patients with primary biliary cirrhosis. Hepatology 48 : 1149–1156.
45. SingalAG, NehraM, Adams-HuetB, YoppAC, TiroJA, et al. (2013) Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol 108 : 425–432.
46. SmirniotopoulosJ, WrightH, NusbaumJ (2011) Surveillance for hepatocelluar carcinoma (HCC) and impact on management at a liver transplant center. Hepatology 54 : 1414A.
47. StravitzRT, HeumanDM, ChandN, SterlingRK, ShiffmanML, et al. (2008) Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med 121 : 119–126.
48. StroffoliniT, TrevisaniF, PinzelloG, BrunelloF, TommasiniMA, et al. (2011) Changing aetiological factors of hepatocellular carcinoma and their potential impact on the effectiveness of surveillance. Dig Liver Dis 43 : 875–880.
49. TanakaH, NousoK, KobashiH, KobayashiY, NakamuraS, et al. (2006) Surveillance of hepatocellular carcinoma in patients with hepatitis C virus infection may improve patient survival. Liver Int 26 : 543–551.
50. TanakaS, KitamuraT, NakanishiK, OkudaS, YamazakiH, et al. (1990) Effectiveness of periodic checkup by ultrasonography for the early diagnosis of hepatocellular carcinoma. Cancer 66 : 2210–2214.
51. TauraN, HamasakiK, NakaoK, IchikawaT, NishimuraD, et al. (2005) Clinical benefits of hepatocellular carcinoma surveillance: a single-center, hospital-based study. Oncol Rep 14 : 999–1003.
52. TongMJ, ChavalitdhamrongD, LuDS, RamanSS, GomesA, et al. (2010) Survival in Asian Americans after treatments for hepatocellular carcinoma: a seven-year experience at UCLA. J Clin Gastroenterol 44: e63–70.
53. TongMJ, SunHE, HsienC, LuDS (2010) Surveillance for hepatocellular carcinoma improves survival in Asian-American patients with hepatitis B: results from a community-based clinic. Dig Dis Sci 55 : 826–835.
54. ToyodaH, KumadaT, KiriyamaS, SoneY, TanikawaM, et al. (2006) Impact of surveillance on survival of patients with initial hepatocellular carcinoma: a study from Japan. Clin Gastroenterol Hepatol 4 : 1170–1176.
55. TrevisaniF, DeNS, RapacciniG, FarinatiF, BenvegnuL, et al. (2002) Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol 97 : 734–744.
56. UnouraM, KanekoS, MatsushitaE, ShimodaA, TakeuchiM, et al. (1993) High-risk groups and screening strategies for early detection of hepatocellular carcinoma in patients with chronic liver disease. Hepatogastroenterology 40 : 305–310.
57. Van VlierbergheH, ColleI, HenrionJ, MichielsenP, DelwaideJ, et al. (2005) The HepCar registry: report on a one-year registration program of hepatocellular carcinoma (HCC) in Belgium. What is daily practice in HCC? Acta Gastroenterol Belg 68 : 403–411.
58. WongGL, WongVW, TanGM, IpKI, LaiWK, et al. (2008) Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. Liver Int 28 : 79–87.
59. WongLL, LimmWM, SeverinoR, WongLM (2000) Improved survival with screening for hepatocellular carcinoma. Liver Transpl 6 : 320–325.
60. WongN, HaydonA, KempW, WijeratneP, RobertsS (2013) Improved survival trend of patients with hepatocellular carcinoma at an Australian tertiary hospital between 1995–2009. Intern Med J 43 : 197–203.
61. YangJD, HarmsenWS, SlettedahlSW, ChaiteerakijR, EndersFT, et al. (2011) Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 9 : 617–623 e611.
62. YuEW, ChieWC, ChenTH (2004) Does screening or surveillance for primary hepatocellular carcinoma with ultrasonography improve the prognosis of patients? Cancer J 10 : 317–325.
63. ZapataE, ZubiaurreL, CastiellaA, SalvadorP, Garcia-BengoecheaM, et al. (2010) Are hepatocellular carcinoma surveillance programs effective at improving the therapeutic options. Rev Esp Enferm Dig 102 : 484–488.
64. DavilaJA, MorganRO, RichardsonPA, DuXL, McGlynnKA, et al. (2010) Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 52 : 132–141.
65. SingalA, VolkM, RakoskiM, FuS, SuG, et al. (2011) Patient Involvement is Correlated with Higher HCC Surveillance in Patients with Cirrhosis. J Clin Gastroenterol 45 : 727–732.
66. SingalAG, TiroJA, GuptaS (2013) Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastroenterol Hepatol 11 : 472–477.
67. SingalAG, YoppA, CSS, PackerM, LeeWM, et al. (2012) Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 27 : 861–867.
68. SingalAG, YoppAC, GuptaS, SkinnerCS, HalmEA, et al. (2012) Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev Res (Phila) 5 : 1124–1130.
69. LiebermanDA (2009) Clinical practice. Screening for colorectal cancer. N Engl J Med 361 : 1179–1187.
70. RexDK, JohnsonDA, LiebermanDA, BurtRW, SonnenbergA (2000) Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am J Gastroenterol 95 : 868–877.
71. VijanS, HwangEW, HoferTP, HaywardRA (2001) Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med 111 : 593–601.
72. SingalA, MarreroJ (2008) Screening for hepatocellular carcinoma. Gastroenterology and Hepatology 4 : 1–8.
73. PoustchiH, FarrellGC, StrasserSI, LeeAU, McCaughanGW, et al. (2011) Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 54 : 1998–2004.
74. FattovichG, StroffoliniT, ZagniI, DonatoF (2004) Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 127: S35–50.
75. JepsenP, OttP, AndersenPK, SorensenHT, VilstrupH (2012) Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: a danish nationwide cohort study. Ann Intern Med 156 : 841–847.
76. WhiteDL, KanwalF, El-SeragHB (2012) Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 10 : 1342–1359 e1342.
77. LeeE, EdwardS, SingalAG, LavieriMS, VolkM (2013) Improving screening for hepatocellular carcinoma by incorporating data on levels of alpha-fetoprotein, over time. Clin Gastroenterol Hepatol 11 : 437–440.
78. SingalAG, MukherjeeA, Joseph ElmunzerB, HigginsPD, LokAS, et al. (2013) Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol 108 : 1723–1730.
79. GopalP, YoppAC, WaljeeAK, ChiangJ, NehraM, et al. (2013) Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol In press.
80. LoombaR, SanyalAJ (2013) The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10 : 686–690.
81. TanD, YoppA, BegMS, GopalP, SingalAG (2013) Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther 38 : 703–712.
82. DuffySW, NagtegaalID, WallisM, CaffertyFH, HoussamiN, et al. (2008) Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol 168 : 98–104.
83. ChenJG, ParkinDM, ChenQG, LuJH, ShenQJ, et al. (2003) Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen 10 : 204–209.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 4- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- What We Know and What We Don't Know About Preventing Stroke
- Safety of Pediatric HIV Elimination: The Growing Population of HIV- and Antiretroviral-Exposed but Uninfected Infants
- The Use of Preliminary Scientific Evidence in Public Health: A Case Study of XMRV
- A Physicians' Wish List for the Clinical Application of Intestinal Metagenomics
- HIV Monoclonal Antibodies: A New Opportunity to Further Reduce Mother-to-Child HIV Transmission
- Fialuridine Induces Acute Liver Failure in Chimeric TK-NOG Mice: A Model for Detecting Hepatic Drug Toxicity Prior to Human Testing
- Regional Changes in Charcoal-Burning Suicide Rates in East/Southeast Asia from 1995 to 2011: A Time Trend Analysis
- Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis
- Changes in HIV Incidence among People Who Inject Drugs in Taiwan following Introduction of a Harm Reduction Program: A Study of Two Cohorts
- Burden of Total and Cause-Specific Mortality Related to Tobacco Smoking among Adults Aged ≥45 Years in Asia: A Pooled Analysis of 21 Cohorts
- Fetal Growth and Risk of Stillbirth: A Population-Based Case–Control Study
- Indoor Residual Spraying in Combination with Insecticide-Treated Nets Compared to Insecticide-Treated Nets Alone for Protection against Malaria: A Cluster Randomised Trial in Tanzania
- Association between Prenatal Exposure to Antiretroviral Therapy and Birth Defects: An Analysis of the French Perinatal Cohort Study (ANRS CO1/CO11)
- Optimal Evidence in Difficult Settings: Improving Health Interventions and Decision Making in Disasters
- Modifiable Etiological Factors and the Burden of Stroke from the Rotterdam Study: A Population-Based Cohort Study
- Geographical Inequalities in Use of Improved Drinking Water Supply and Sanitation across Sub-Saharan Africa: Mapping and Spatial Analysis of Cross-sectional Survey Data
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Burden of Total and Cause-Specific Mortality Related to Tobacco Smoking among Adults Aged ≥45 Years in Asia: A Pooled Analysis of 21 Cohorts
- What We Know and What We Don't Know About Preventing Stroke
- Safety of Pediatric HIV Elimination: The Growing Population of HIV- and Antiretroviral-Exposed but Uninfected Infants
- Regional Changes in Charcoal-Burning Suicide Rates in East/Southeast Asia from 1995 to 2011: A Time Trend Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



