-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Serotype-Specific Changes in Invasive Pneumococcal Disease after Pneumococcal Conjugate Vaccine Introduction: A Pooled Analysis of Multiple Surveillance Sites
Background:
Vaccine-serotype (VT) invasive pneumococcal disease (IPD) rates declined substantially following introduction of 7-valent pneumococcal conjugate vaccine (PCV7) into national immunization programs. Increases in non-vaccine-serotype (NVT) IPD rates occurred in some sites, presumably representing serotype replacement. We used a standardized approach to describe serotype-specific IPD changes among multiple sites after PCV7 introduction.Methods and Findings:
Of 32 IPD surveillance datasets received, we identified 21 eligible databases with rate data ≥2 years before and ≥1 year after PCV7 introduction. Expected annual rates of IPD absent PCV7 introduction were estimated by extrapolation using either Poisson regression modeling of pre-PCV7 rates or averaging pre-PCV7 rates. To estimate whether changes in rates had occurred following PCV7 introduction, we calculated site specific rate ratios by dividing observed by expected IPD rates for each post-PCV7 year. We calculated summary rate ratios (RRs) using random effects meta-analysis. For children <5 years old, overall IPD decreased by year 1 post-PCV7 (RR 0·55, 95% CI 0·46–0·65) and remained relatively stable through year 7 (RR 0·49, 95% CI 0·35–0·68). Point estimates for VT IPD decreased annually through year 7 (RR 0·03, 95% CI 0·01–0·10), while NVT IPD increased (year 7 RR 2·81, 95% CI 2·12–3·71). Among adults, decreases in overall IPD also occurred but were smaller and more variable by site than among children. At year 7 after introduction, significant reductions were observed (18–49 year-olds [RR 0·52, 95% CI 0·29–0·91], 50–64 year-olds [RR 0·84, 95% CI 0·77–0·93], and ≥65 year-olds [RR 0·74, 95% CI 0·58–0·95]).Conclusions:
Consistent and significant decreases in both overall and VT IPD in children occurred quickly and were sustained for 7 years after PCV7 introduction, supporting use of PCVs. Increases in NVT IPD occurred in most sites, with variable magnitude. These findings may not represent the experience in low-income countries or the effects after introduction of higher valency PCVs. High-quality, population-based surveillance of serotype-specific IPD rates is needed to monitor vaccine impact as more countries, including low-income countries, introduce PCVs and as higher valency PCVs are used.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(9): e32767. doi:10.1371/journal.pmed.1001517
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001517Summary
Background:
Vaccine-serotype (VT) invasive pneumococcal disease (IPD) rates declined substantially following introduction of 7-valent pneumococcal conjugate vaccine (PCV7) into national immunization programs. Increases in non-vaccine-serotype (NVT) IPD rates occurred in some sites, presumably representing serotype replacement. We used a standardized approach to describe serotype-specific IPD changes among multiple sites after PCV7 introduction.Methods and Findings:
Of 32 IPD surveillance datasets received, we identified 21 eligible databases with rate data ≥2 years before and ≥1 year after PCV7 introduction. Expected annual rates of IPD absent PCV7 introduction were estimated by extrapolation using either Poisson regression modeling of pre-PCV7 rates or averaging pre-PCV7 rates. To estimate whether changes in rates had occurred following PCV7 introduction, we calculated site specific rate ratios by dividing observed by expected IPD rates for each post-PCV7 year. We calculated summary rate ratios (RRs) using random effects meta-analysis. For children <5 years old, overall IPD decreased by year 1 post-PCV7 (RR 0·55, 95% CI 0·46–0·65) and remained relatively stable through year 7 (RR 0·49, 95% CI 0·35–0·68). Point estimates for VT IPD decreased annually through year 7 (RR 0·03, 95% CI 0·01–0·10), while NVT IPD increased (year 7 RR 2·81, 95% CI 2·12–3·71). Among adults, decreases in overall IPD also occurred but were smaller and more variable by site than among children. At year 7 after introduction, significant reductions were observed (18–49 year-olds [RR 0·52, 95% CI 0·29–0·91], 50–64 year-olds [RR 0·84, 95% CI 0·77–0·93], and ≥65 year-olds [RR 0·74, 95% CI 0·58–0·95]).Conclusions:
Consistent and significant decreases in both overall and VT IPD in children occurred quickly and were sustained for 7 years after PCV7 introduction, supporting use of PCVs. Increases in NVT IPD occurred in most sites, with variable magnitude. These findings may not represent the experience in low-income countries or the effects after introduction of higher valency PCVs. High-quality, population-based surveillance of serotype-specific IPD rates is needed to monitor vaccine impact as more countries, including low-income countries, introduce PCVs and as higher valency PCVs are used.
Please see later in the article for the Editors' SummaryIntroduction
In 2008, Streptococcus pneumoniae was estimated to have caused 540,000 deaths among children less than 5 years old worldwide [1]. Seven-valent pneumococcal conjugate vaccine (PCV7) was licensed and introduced in 2000 into the routine infant immunization schedule in the United States. Significant reductions in the incidence of invasive pneumococcal disease (IPD) were observed not only among children, but also among adults, reflecting reduced transmission and herd protection [2].
Several high - and middle-income countries introduced PCV7 in the several years after 2000. While IPD caused by vaccine serotypes (VTs) declined in virtually all settings, reported increases in IPD rates due to non-vaccine serotypes (NVTs) were negligible in some [3] and substantial in others [4]. Increases in NVT IPD following routine introduction of PCV7 were presumed to represent serotype replacement of VT by NVT, a phenomenon well-documented in pneumococcal nasopharyngeal colonization from randomized controlled trials [5] and observational studies [6],[7]. Direct comparison between settings, however, is complicated by variability in vaccine schedule and coverage and surveillance system characteristics.
Understanding serotype replacement is even more critical in low-income countries where most pneumococcal deaths occur [1],[8], a more diverse distribution of serotypes causes disease, and nasopharyngeal colonization occurs earlier in infancy [9]. At the request of its Strategic Advisory Group of Experts (SAGE) on Immunizations, the World Health Organization (WHO) convened an expert consultation on serotype replacement in July 2010. A key recommendation of the consultation was that a comprehensive analysis be undertaken to provide an estimate of the magnitude and variability of pneumococcal serotype replacement following PCV7 use to inform the expected experience of low-income countries currently introducing PCVs [10]. The key findings of that analysis are described here.
Methods
Search Strategy
We identified datasets from IPD surveillance systems that report rates through two approaches. First, we identified datasets gathered from a comprehensive systematic literature review on PCV dosing schedules [11]. In that systematic literature review, a search for English language publications on the immunogenicity, and direct and indirect effects of various PCV schedules on nasopharyngeal (NP) carriage, IPD, and pneumonia among children was performed using 14 databases (i.e., African Index Medicus; BioAbst/Reports, Reviews, Meetings; Biological Abstracts; Cochrane Library; EMBASE; Global Health; Index Medicus for Eastern Med. Region; Index Medicus for South-East Asia Region; IndiaMed; Latin America and Caribbean Health Sciences Information; Pan-American Health Organization; Pascal Biomed; PubMed; and Western Region Index Medicus) as well as meeting abstracts of the International Symposium on Pneumococci and Pneumococcal Disease (ISPPD) and the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). The search included studies published between 1994 and 2010. The complete list of database-specific and Medical Subject Headings (MeSH) search terms used in the literature search is detailed by the authors. We reviewed those publications with IPD as an outcome; these publications needed to include at least one “narrow vaccine” search term as well as an IPD related search term, i.e., (“Invasive disease” [all fields]), (“invasive pneumococcal disease” [all fields]), and/or (“invasive bacterial disease” [all fields]).
Second, we solicited potential datasets from experts in pneumococcal disease, WHO headquarters and regional offices, and by reviewing references from publications.
Data Collection
We solicited datasets from investigators using a standardized format, requesting IPD case counts for up to 5 years before and 10 years after PCV7 introduction, stratified by age groups (0–1, 2–4, 5–17, 18–49, 50–64, and ≥65 years old), clinical syndrome (overall IPD and meningitis specifically), hospitalization status, and serotype (Text S1). Meningitis was defined as isolation of pneumococcus from cerebrospinal fluid by culture. We requested age - and year-specific catchment population denominators to estimate rates, and we solicited descriptions of the PCV7 vaccination program, IPD surveillance system, changes to surveillance methodology or clinical practices, and potential IPD outbreaks.
Data Quality Review
Two coordinators conducted a quality check of datasets included in the analysis using a checklist (Box 1). Any requests for data clarification were emailed to the contributing investigator and the data were updated as applicable.
Tab. 1. Box 1. Dataset quality checks performed 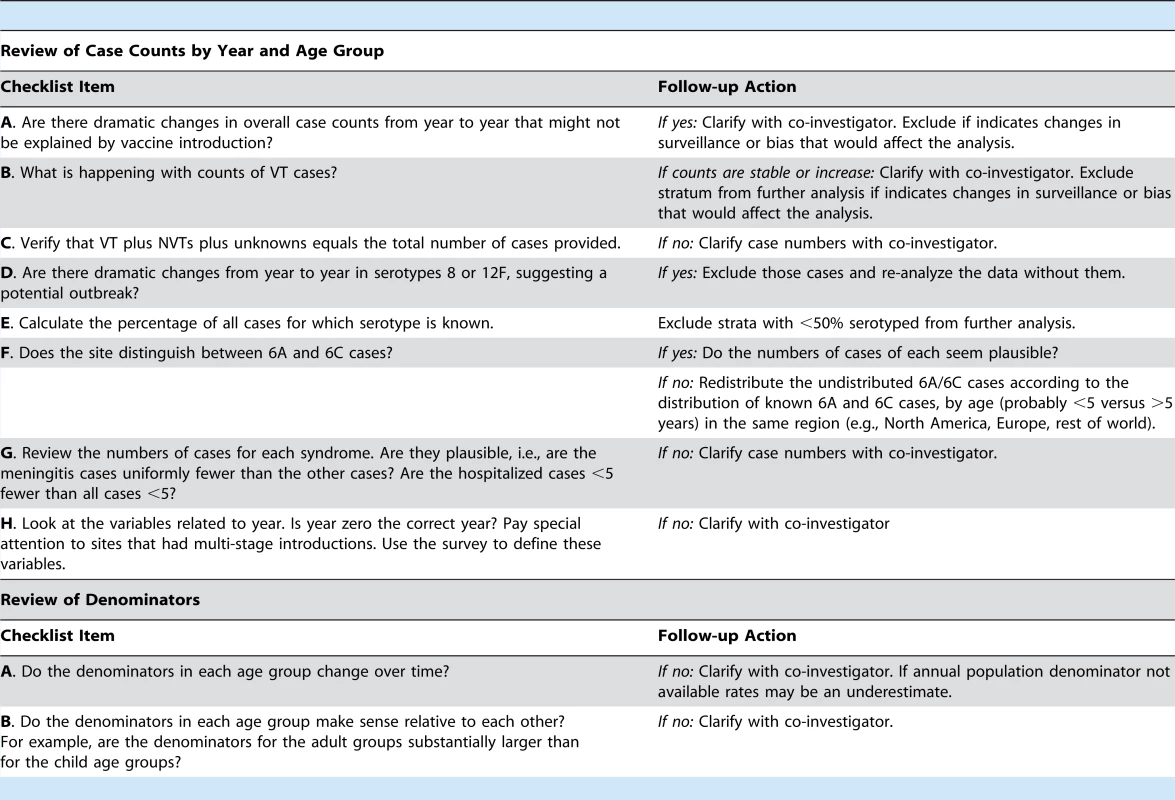
Data Analysis
The inclusion criteria of the datasets for collection and analysis are given in Figure 1.
Fig. 1. Flow diagram of datasets included in the analysis. 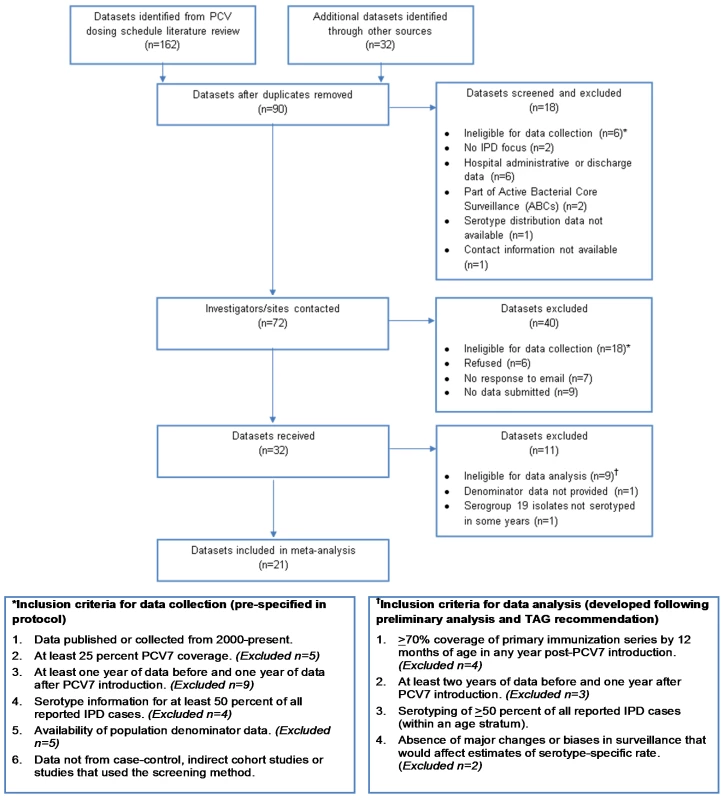
In datasets where serotypes 6A and 6C were not differentiated, we distributed these serotypes according to the known distribution of 6A and 6C in the same geographic region or globally in the pre - and post-PCV7 periods [12]. First, we calculated the percentage of 6A isolates out of all 6A and 6C isolates, using datasets where 6A and 6C isolates were distinguished. The percentage of true 6A isolates was calculated for all datasets, as well as by region for datasets from Europe and North America. Estimates of the percentage of true 6A isolates were weighted by the size of the site and calculated in four different time periods: pre-PCV introduction; 1–2 years post-; 3–4 years post-; and 5+ years post-PCV introduction. In sites that did not differentiate 6A and 6C serotypes, 6A/6C isolates were then redistributed according to the estimated regional (for North America and Europe datasets) or using all datasets’ (for datasets from sites outside of Europe or North America) distribution of differentiated 6A and 6C isolates.
After redistributing serotype 6A with VT (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) and 6C with NVT (all other serotypes), remaining isolates with unknown serotype were redistributed. Specifically, isolates with known serotype were classified into four groups: VT serotypes (PCV7 serotypes and 6A); serotypes 1 and 5; serotypes 3, 7F, and 19A; and all other NVT serotypes. Serotypes 1 and 5 were grouped together to allow for modeling expected rates absent the potential influence of outbreaks of these two serotypes. The remaining additional serotypes included in higher valency PCVs–3, 7F, and 19A—were grouped together for analyses of changes over time as they, along with serotypes 1 and 5, are likely to be prevented by introduction of the higher valency PCVs. Non-typeable isolates were added to the category of all other NVT serotypes. We calculated the percentage of each of the four groups of known serotypes out of all known serotypes. Unknown isolates that were not serotyped were then redistributed into the four serotype groups per the calculated proportional distribution. Redistribution was performed by site, age group, year, and syndrome.
To minimize the effect of temporal and geographic differences in blood culturing practice among children in the outpatient setting, we restricted our analysis to hospitalized cases for children <5 years [13],[14]. Since IPD among adults is almost always a severe illness among inpatients, we assumed all cases among older persons were hospitalized. This assumption was confirmed in the few sites that did capture data on hospitalization status among adults with IPD [59],[60]. We excluded persons aged 5–17 years because case counts were too small for meaningful analysis.
Because IPD rates were changing before PCV7 introduction in some sites, we used the pre-PCV7 IPD trends (excluding the year of introduction) to predict future years’ IPD rates, absent PCV7 use [15],[16]. We used Poisson regression to model expected rates of VT, NVT, and overall IPD absent PCV7 introduction. We assumed that overall IPD was a more stable indicator of pre-PCV7 trends than either VT or NVT, which could be affected by outbreaks of a single serotype [16]. Therefore, we used the regression intercept and slope of the pre-PCV7 annual rates of overall IPD to estimate future rates, absent PCV7, for overall, VT, and NVT IPD. Because serotypes 1 and 5 rates can fluctuate annually owing to outbreaks, we excluded them from the regression estimation of pre-PCV7 trends, but included them in the actual rate estimates on the basis of the trends. Separately, we calculated the pre-PCV7 average proportions of IPD caused by VT and NVT and applied each to the expected overall IPD rate to generate the expected VT and NVT IPD rates. The annual surveillance population denominator was included as an offset variable, and the slope of the modeled expected rates was assigned a value of zero from 4 years post-PCV7 onwards, assuming stabilization of any pre-PCV7 IPD surveillance trends by then.
For children aged <5 years, expected rates for 11 of 19 sites (58%) were modeled. Among the 15 sites included in the IPD analysis for adults aged 18–49 years, 50–64 years, and ≥65 years, expected rates were generated using modeling for 10 (67%), 5 (33%), and 7(47%) sites, respectively. For age strata with an annual pre-PCV7 average of <20 IPD cases or <3 years of pre-PCV7 data, we felt that pre-PCV7 rates were unreliable to define surveillance trends because of small sample size or too few years. For these strata, expected IPD rates absent PCV7 introduction were estimated by averaging annual IPD rates before PCV7 introduction.
We estimated the change in IPD rates following PCV7 introduction by calculating rate ratios (RRs), dividing the observed IPD rate by the expected IPD rate for each post-PCV7 year. We calculated 95% confidence intervals around RRs through simulation of observed and expected case counts and the delta method [17]. The delta method can be used to approximate the variance of a ratio and has previously been applied to estimate the variance of the log RR [17],[18]. To estimate the variance of the log RR, we simulated 200 observed and expected case counts using the Poisson distribution with the actual observed and calculated expected number of cases as the mean. We converted these simulated observed and expected case counts to rates. From these simulated rates we calculated the variance of the observed and expected rate, as well as the covariance between these rates using STATA Version 12.1 (StataCorp.).
Using the delta method formula below, we combined the variance of the observed and expected rate to estimate the variance of the log RR.
Where σ2 is the variance; Y is the observed rate; X is the expected rate; and COV (X,Y) is the covariance between the observed and expected rate.
We included the covariance in the calculation of the variance of the log RR because for a few strata the covariance was greater than zero and so we were unable to assume independence between the observed and expected rates. The square-root of the variance of the log RR was used to estimate the standard error of the log RR. The standard error of the log RR was calculated separately for each site, age group, serotype combination, and post-PCV7 year.
A value of 0·5 cases was assigned as a continuity correction to each stratum (i.e., site-age group-serotype group) with zero cases reported [19] so as to avoid undefined RRs (when zero cases of IPD were expected in a year) or undefined variances (as Poisson simulation would generate missing values for zero cells).
Because the impact of PCV7 was expected to be heterogeneous across sites, we used random effects meta-analysis to pool the site-specific RRs [20]. Meta-analysis was performed for each age and serotype group for each of the 7 years after PCV7 introduction, generating a summary RR with 95% confidence intervals. Meta-analysis of RRs was performed both including all datasets available for each year post-PCV7, as well as including only those datasets with at least 7 years of post-PCV7 data, which was the last year with enough datasets for robust meta-analysis (i.e., five datasets). The same analysis comparing observed and expected rates was performed limited to meningitis cases.
We performed several sensitivity analyses for IPD. First, we used a continuity correction of 0·1. Second, we performed an analysis completely excluding serotypes 1 and 5 from both pre - and post-PCV7 IPD rates. Third, we performed the analyses with the expected IPD rate as the observed average pre-PCV7 introduction IPD rate for all site-age group-serotype group strata (i.e., no modeling of expected IPD rates).
Additionally, we performed an analysis comparing observed and expected IPD rates for two separate NVT serotype groups: NVT serotypes in the higher valency pneumococcal conjugate vaccines that are not in PCV7 (i.e., serotypes 1, 3, 5, 7F, and 19A) and NVT serotypes not in the higher valency vaccines. The RR of the observed over the expected rates in the years after PCV7 introduction and 95% CI were calculated for each site, age, and year stratum for both of these categories of NVT. A summary RR for both NVT categories was obtained for each age group in each post-PCV7 year using random-effects meta-analysis.
To compare the contribution of these two NVT categories to the overall IPD incidence post-PCV7 introduction, we performed a separate analysis restricted to the post-PCV7 period where we defined the RR as the observed rate of IPD due to the NVT included in the higher valency vaccines over the observed rate of all other NVT not included in those vaccines. The 95% CI for this RR was also calculated using the delta method for each site, age, and post-PCV7 year. A summary RR for each age group and post-PCV7 year was calculated using random-effects meta-analysis.
The analysis dataset was generated using SAS Version 9·2 (SAS Institute Inc.). Meta-analyses were conducted using STATA Version 12·1 (StataCorp).
Results
Description of Sites
We identified 72 potentially eligible datasets and requested information from the investigators (Figure 1). Of 32 datasets received, 21 from four geographic regions (six North America, 11 Europe, three Australasia, one South America) met the inclusion criteria for analysis (Figure 1). For children, 19 datasets were included in the IPD and meningitis analyses, although two sites were included only for IPD and two different sites were included only for meningitis. For adults, 15 and 11 datasets were included in the analyses of IPD and meningitis, respectively. At least 19 datasets included in the analysis have previously published IPD surveillance data, though not necessarily including the same data used for this analysis (i.e., age group, case population, syndrome, and years of surveillance) [3],[4],[16],[21]–[36].
Specific reasons for exclusion from analysis for 11 datasets received were the following: no denominator provided (one); serogroup 19 not serotyped (one), <70% coverage of the primary PCV7 series by 12 months of age (four) [37]; <2 years of pre-PCV7 data (three); inability to define a proper denominator population (one); and substantial changes over time in case ascertainment of the surveillance system (one). The average annual number of IPD cases pre-PCV7 introduction for the 11 datasets excluded (six Europe, three North America, one Africa, and one Western Pacific) ranged from 8–1,490. Furthermore, among datasets included, two and six site-age group strata were excluded from the IPD and meningitis analyses, respectively, because <50% of isolates in those strata were serotyped (Table 1). In one site, adult strata were excluded from the analysis due to an increase in VT cases in the post-PCV7 introduction period, indicating changes in surveillance or bias that would affect the analysis (Table 1). No sites were excluded due to implausible distributions of serotype 6A/6C isolates.
Tab. 2. Datasets included. 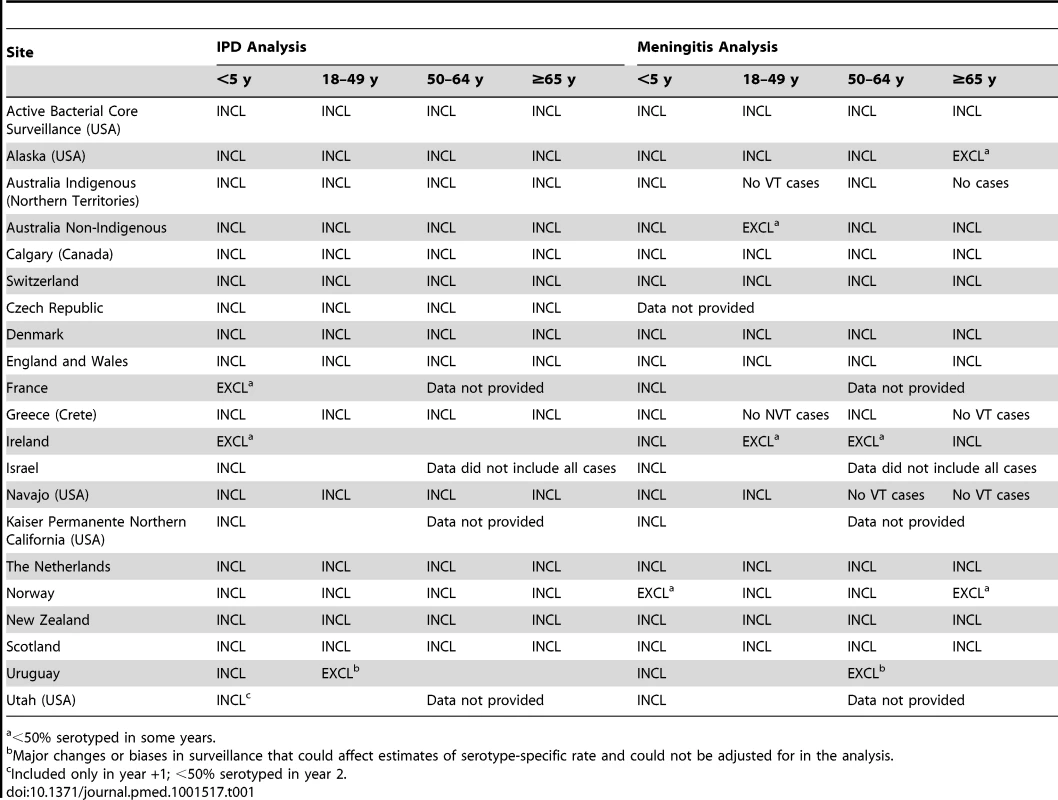
<50% serotyped in some years. The PCV7 schedules used included two primary doses plus a booster (nine sites), three primary doses without a booster (one site), and three primary doses with a booster (11 sites); 16 sites had catch-up campaigns (Table 2). All sites achieved ≥70% immunization coverage during the surveillance period and the range of average immunization coverage estimates for all post-PCV7 years was 55%–97% (Table S1).
Tab. 3. Characteristics of surveillance sites included in meta-analysis (n = 21). 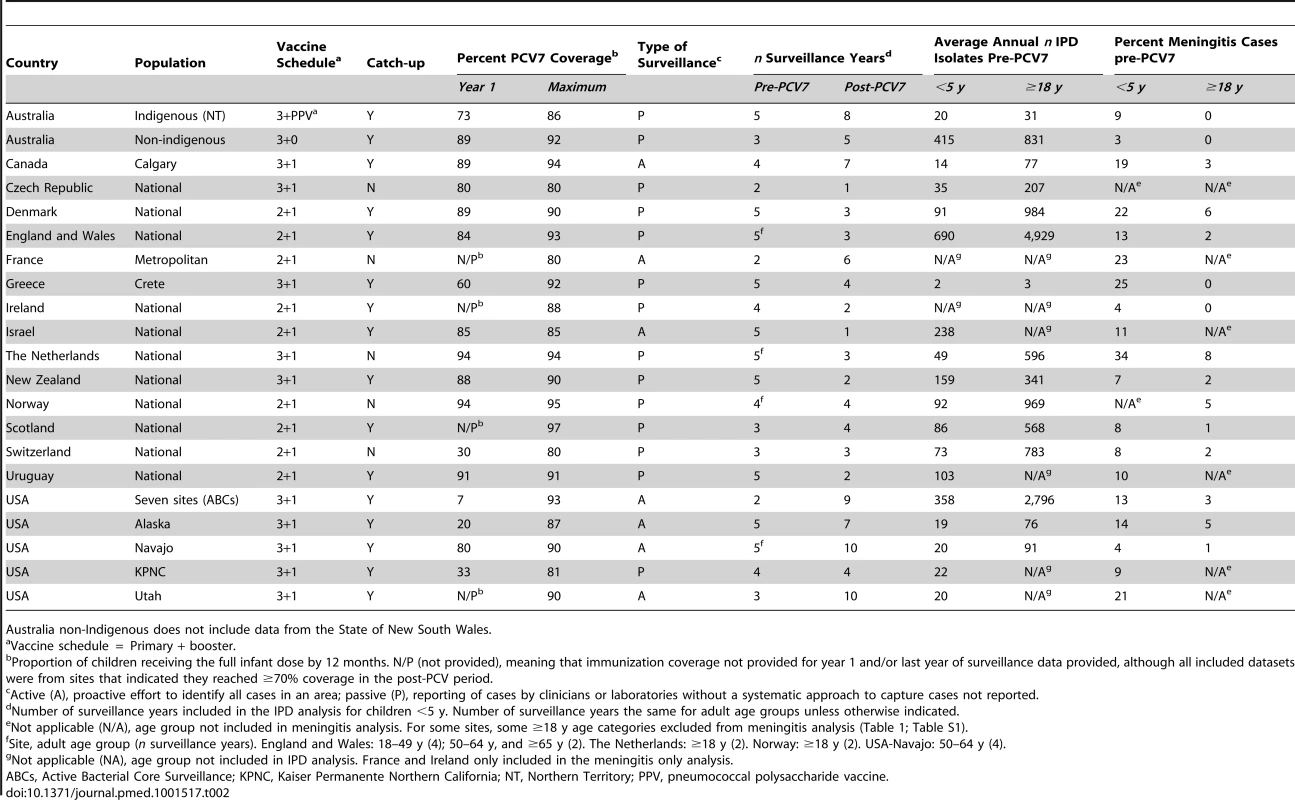
Australia non-Indigenous does not include data from the State of New South Wales. Children <5 Years Old
The annual number of IPD isolates at baseline for children <5 years ranged from 2 to 690 and the median baseline rate was 31·4 cases per 100,000 (range 4·7–280·3) (Figure 2; Table 2). Our meta-analysis showed that the rate of overall IPD decreased significantly by 1 year after introduction (summary RR 0·55, 95% CI 0·46–0·65), which was then maintained out to 7 years post-introduction (RR 0·49, 95% CI 0·35–0·68) (Figure 3; Table 3). Although there was heterogeneity in the effect across sites, as expressed by the I2 statistic, the point estimates tended in the same direction with all 19 sites showing a decrease (in 15, these reductions were statistically significant) compared to baseline in overall IPD in at least one post-introduction year (Figure 4). The rate of VT IPD declined significantly by 1 year after introduction (summary RR 0·34, 95% CI 0·28–0·41) and continued to decrease through 7 years (summary RR 0·03, 95% CI 0·01–0·10) (Figures 3 and 5; Table 3). The rate of NVT IPD increased significantly by 2 years after introduction (summary RR 1·34, 95% CI 1·02–1·77) and increased through 5 years, with little change thereafter through year 7 (summary RR 2·81, 95% CI 2·12–3·71) (Figure 3; Table 3). Most sites (seven statistically significant) showed an increase in NVT IPD rate in at least one post-introduction year (Figure 6). To account for the possible confounder of varying numbers of datasets included by year after PCV introduction, we repeated the meta-analysis including only the five sites with 7 years of post-PCV7 data. For VT, NVT, and all serotypes, the summary RRs were similar to those when all sites were included (Tables 3 and S2). The results were also similar when using a continuity correction of 0·1 instead of 0·5 (Table S3) and when excluding serotypes 1 and 5 (Table S4). In the analysis in which all expected rates used the average pre-PCV7 rates (i.e., no modeling of expected rates), the trends of post-PCV7 IPD changes were similar to those from the modeling approach, although NVT summary RRs tended to be slightly higher, as would be expected with no adjustment for increasing surveillance sensitivity over time (Table S5).
Fig. 2. Pre-PCV7 introduction average annual invasive pneumococcal disease rates and percent vaccine serotype isolates. 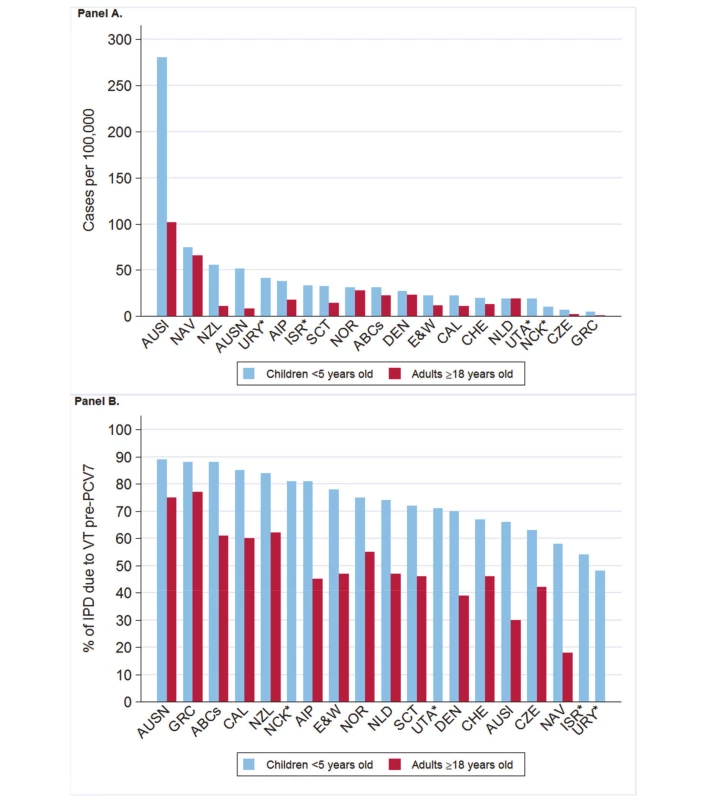
(A) IPD rates as cases per 100,000. (B) Percent VT isolates as a proportion of all pre-PCV7 introduction isolates. *Only children aged <5 years included. Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 3. Post-PCV7 introduction invasive pneumococcal disease summary rate ratios. 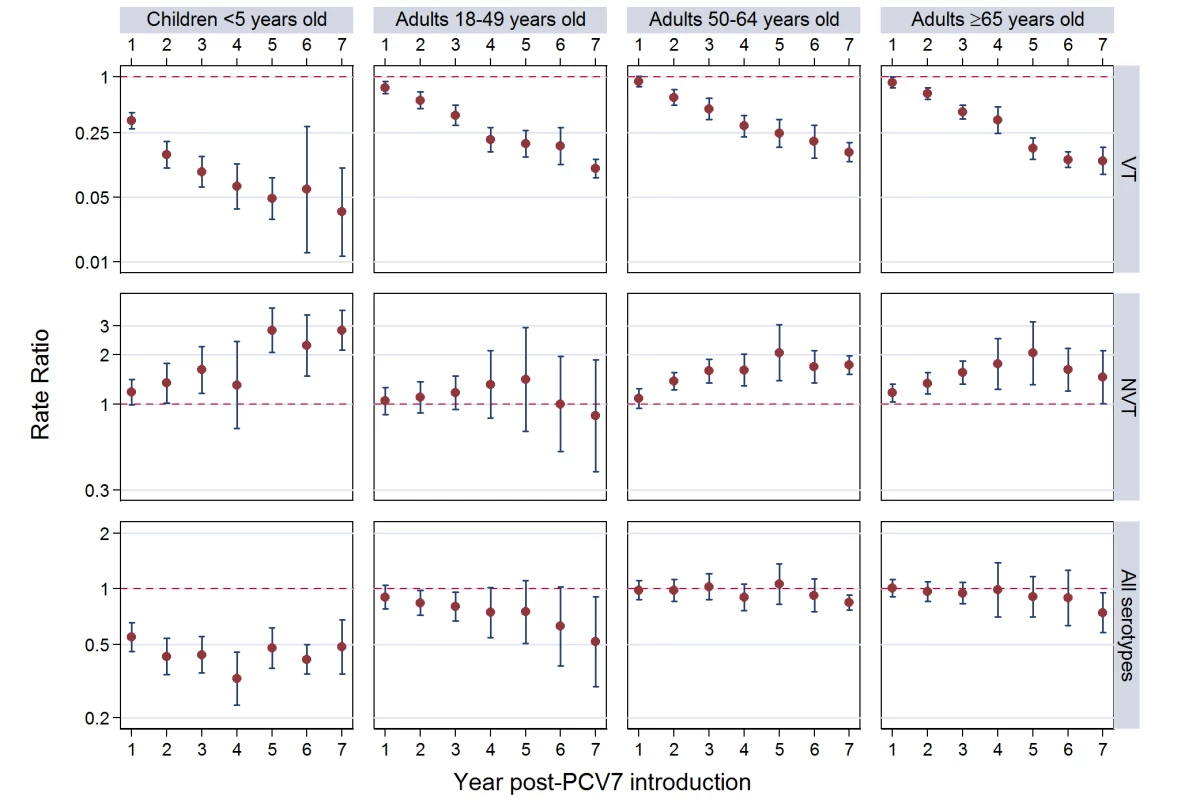
Summary RRs from random effects meta-analysis. Summary RRs estimated by dividing observed over expected rates and calculated for each age-serotype group. 95% confidence interval indicated by error bars. Y-Axis on log scale. Tab. 4. Invasive pneumococcal disease summary rate ratios from random effects meta-analysis, comparing observed over expected rates, by age, serotype group, and post-PCV7 introduction year for all sites. 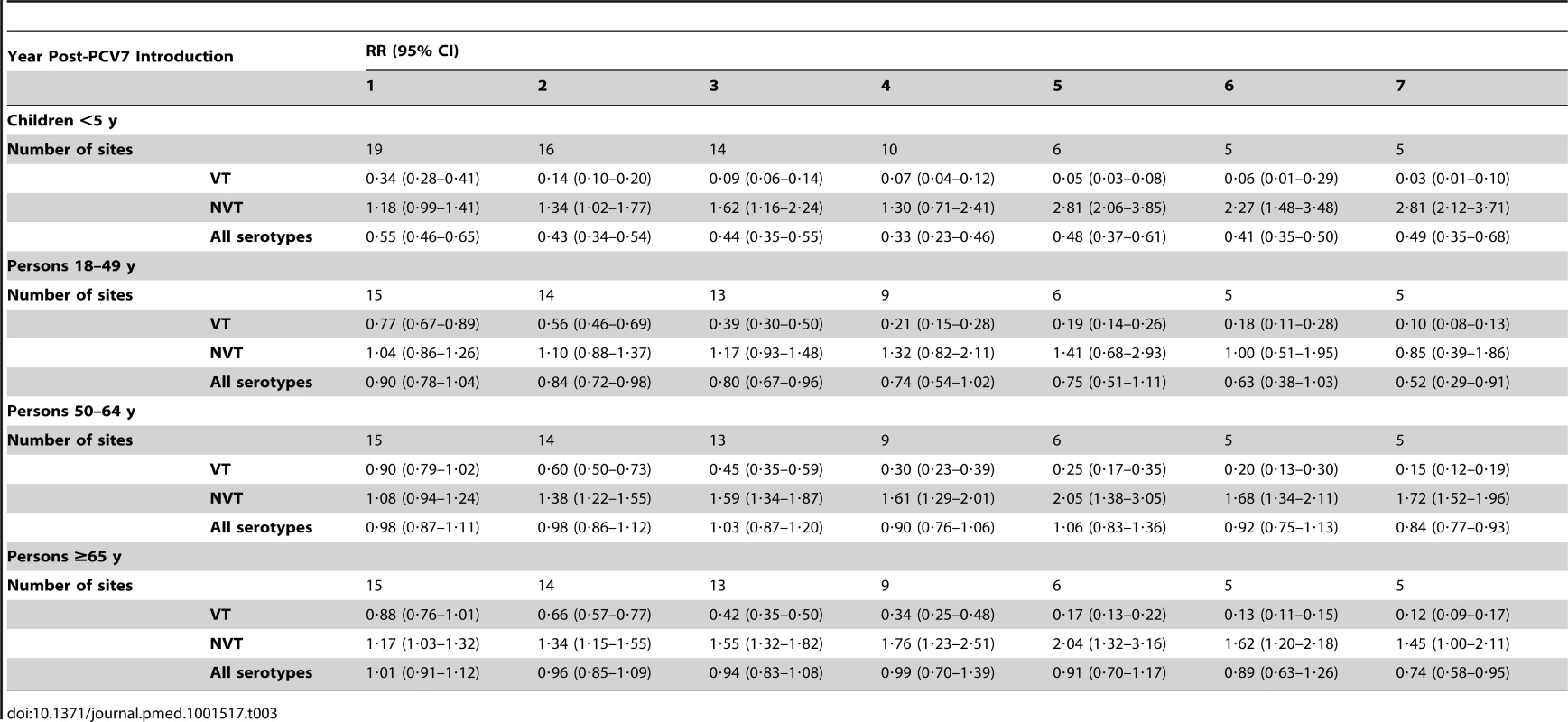
Fig. 4. All serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for children aged <5 years. 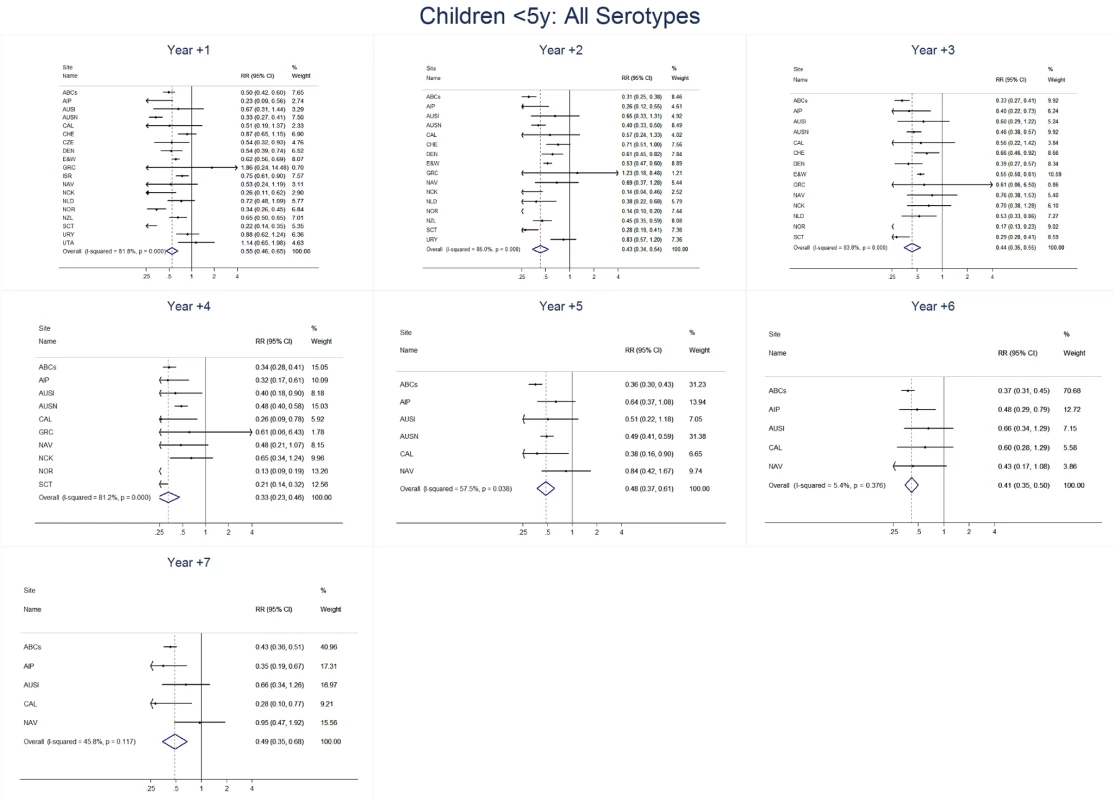
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 5. Vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for children aged <5 years. 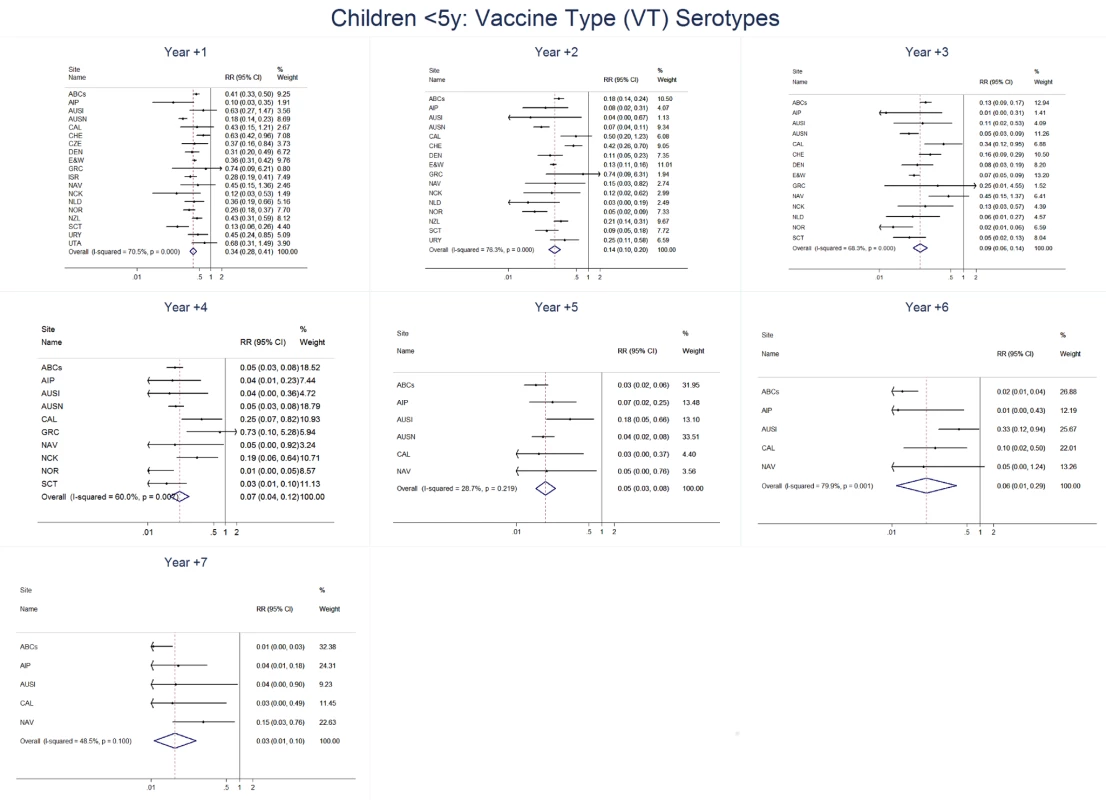
Site abbreviations: ABCs (USA Active Bacterial Core Surveillance); AIP (USA Alaska); Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 6. Non-vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for children aged <5 years. 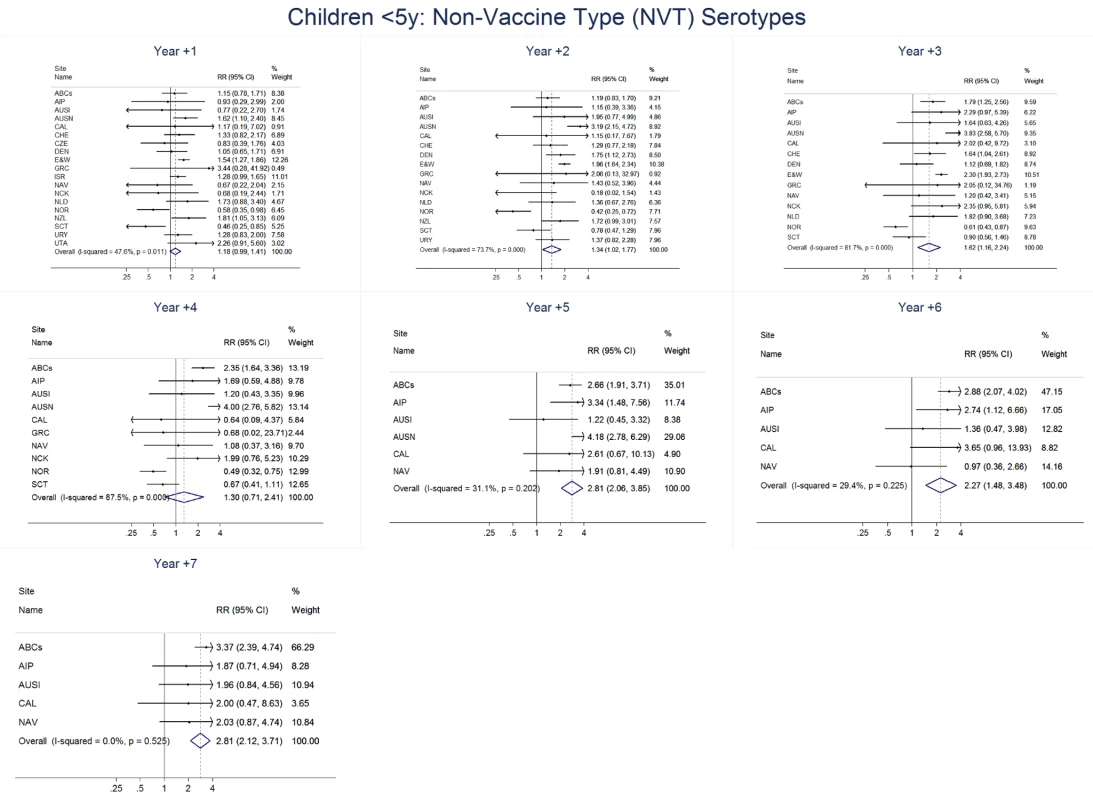
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. In the pre-PCV7 period, the percentage of IPD due to meningitis ranged from 3%–34% by site (Table 2). The meta-analysis results for meningitis were similar to overall IPD, with sustained reductions in meningitis due to all serotypes through 7 years post-PCV7 introduction (RR 0·40, 95% CI 0·25–0·64) (Figure 7; Tables 4 and S6). Due to smaller numbers of meningitis cases, there was more variability by year and wider confidence intervals for the RR point estimates (Figures and 7; Tables 3 and 4).
Fig. 7. Post-PCV7 introduction pneumococcal meningitis summary rate ratios. 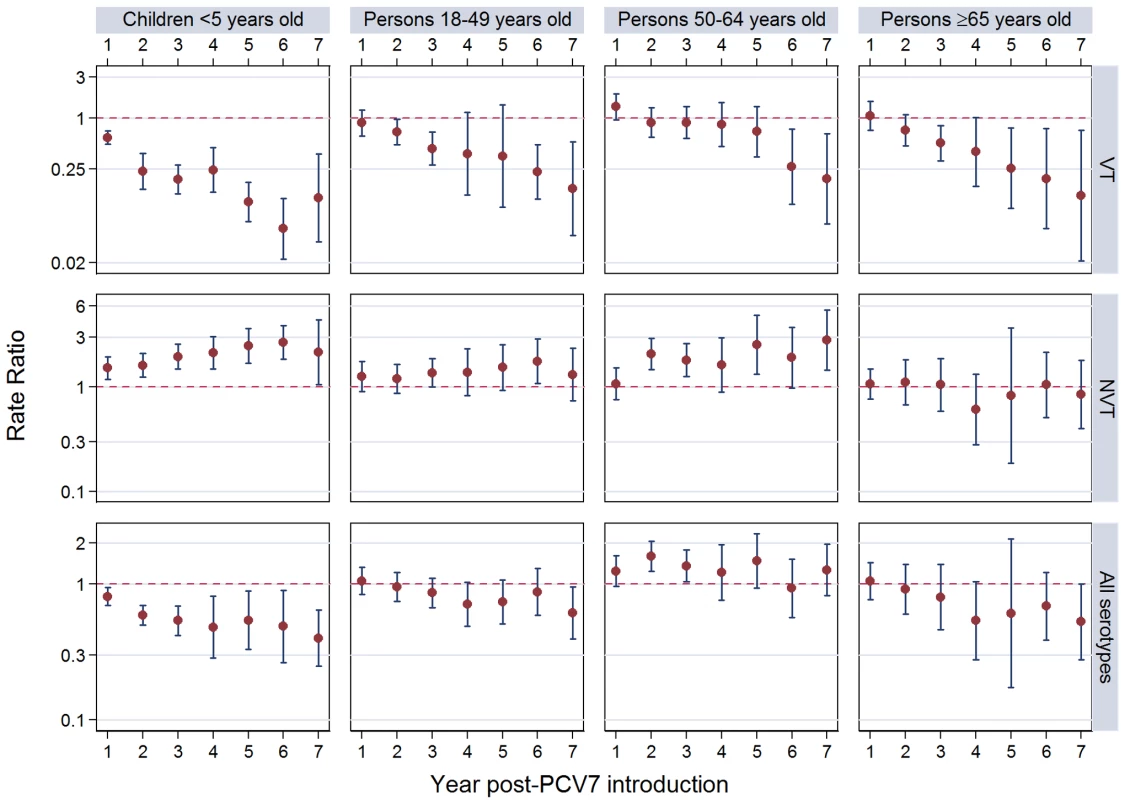
Summary RRs from random effects meta-analysis. Summary RRs esimated by dividing observed by expected rates and calculated for each age-serotype group. 95% confidence interval indicated by error bars. Y-Axis on log scale. Tab. 5. Meningitis summary rate ratios from random effects meta-analysis, comparing observed over expected rates, by age, serotype group and post-PCV7 introduction year for all sites. 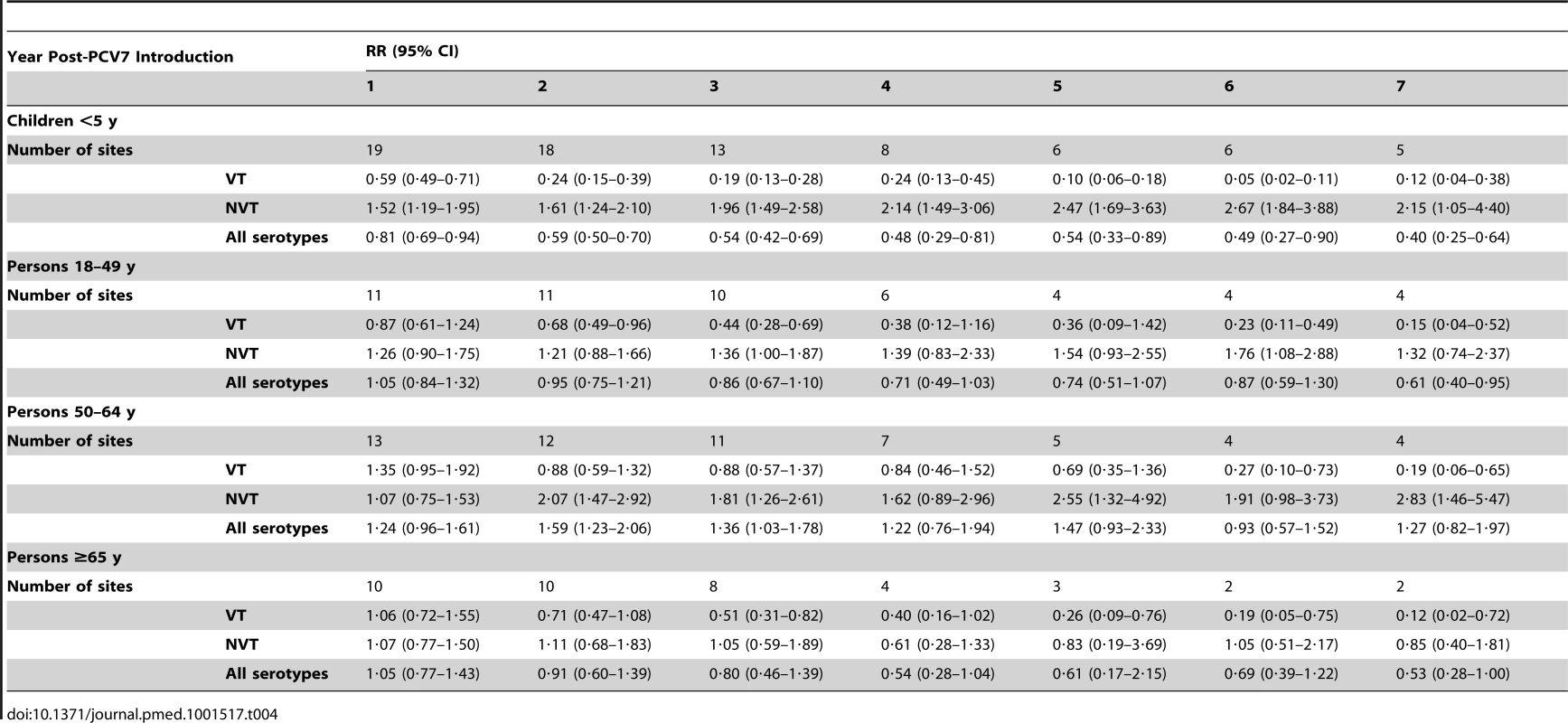
Adults
For adults, the annual number of IPD isolates at baseline ranged from 3 to 4,929 with a median IPD baseline rate of 14·2 cases per 100,000 (range 0·6–101·7) (Figure 2; Table 2). The summary RR point estimates from the meta-analysis showed reductions in overall IPD for most years, though not statistically significant in years 1–6 post-introduction (Figures 3, 8, 11, and 14; Table 3). Among the five sites with data 7 years post-introduction, statistically significant reductions were seen in year 7 for persons 18–49 years (summary RR 0·52, 95% CI 0·29–0·91), for persons 50–64 years old (summary RR 0·84, 95% CI 0·77–0·93), and for persons ≥65 years old (summary RR 0·74, 95% CI 0·58–0·95) (Figures 8, 11, and 14; Table S2). VT IPD decreased significantly for all adult age groups by the second year after PCV7 introduction (Figures 3, 9, 12, and 15; Table 3). In contrast to children, this decrease in VT IPD rates occurred more gradually; not until the fourth year after PCV7 introduction did adults have decreases in VT IPD similar in magnitude to those seen among children in the first post-PCV7 year (Figure 3; Table 3). In adults aged 18–49 years old, there was no significant increase in NVT IPD rates compared to baseline for any year, while for adults aged 50–64 years and ≥65 years, significant increases in NVT IPD were observed from year 2 and year 1 post-introduction, respectively (Figures 3, 10, 13, and 16; Table 3). There was substantial variability in the magnitude of NVT IPD increase by site (Figures 10, 13, and 16). For adults, the meta-analyses using a 0·1 continuity correction, excluding serotypes 1 and 5, limited to the five sites with 7 years of data, and using only averaged pre-PCV7 rates showed similar findings (Tables S2–S5).
Fig. 8. All serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged 18–49 years. 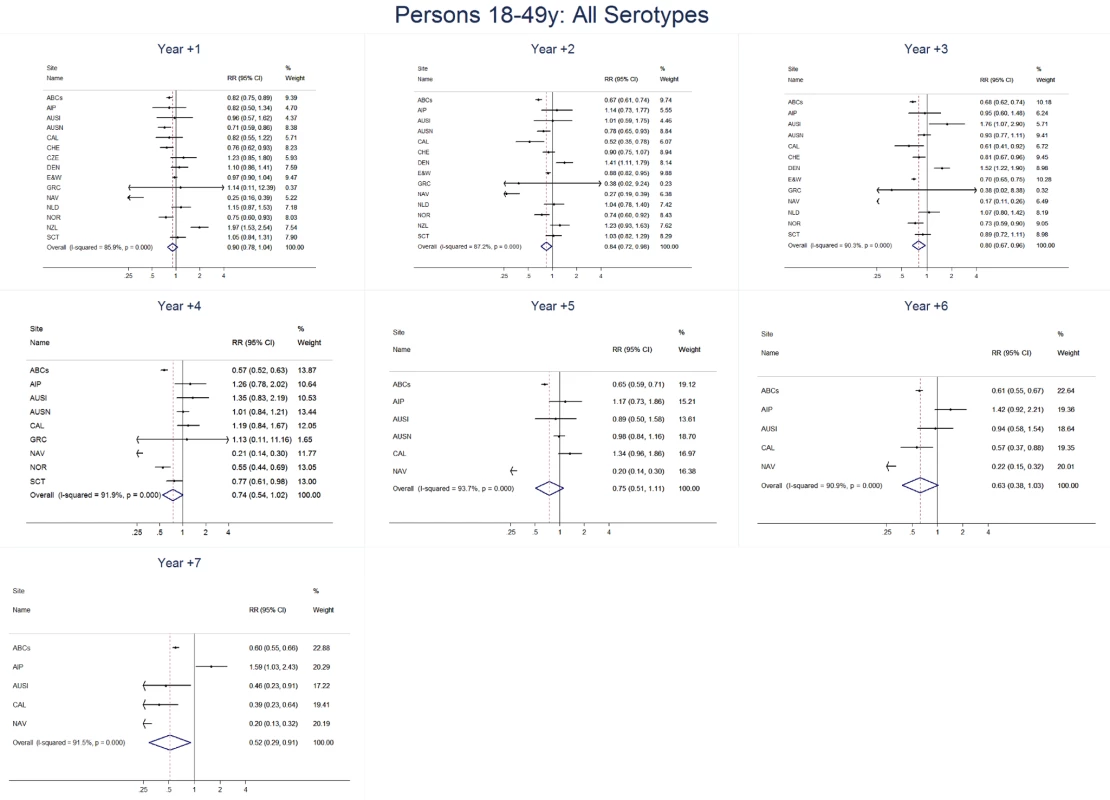
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 9. Vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged 18–49 years. 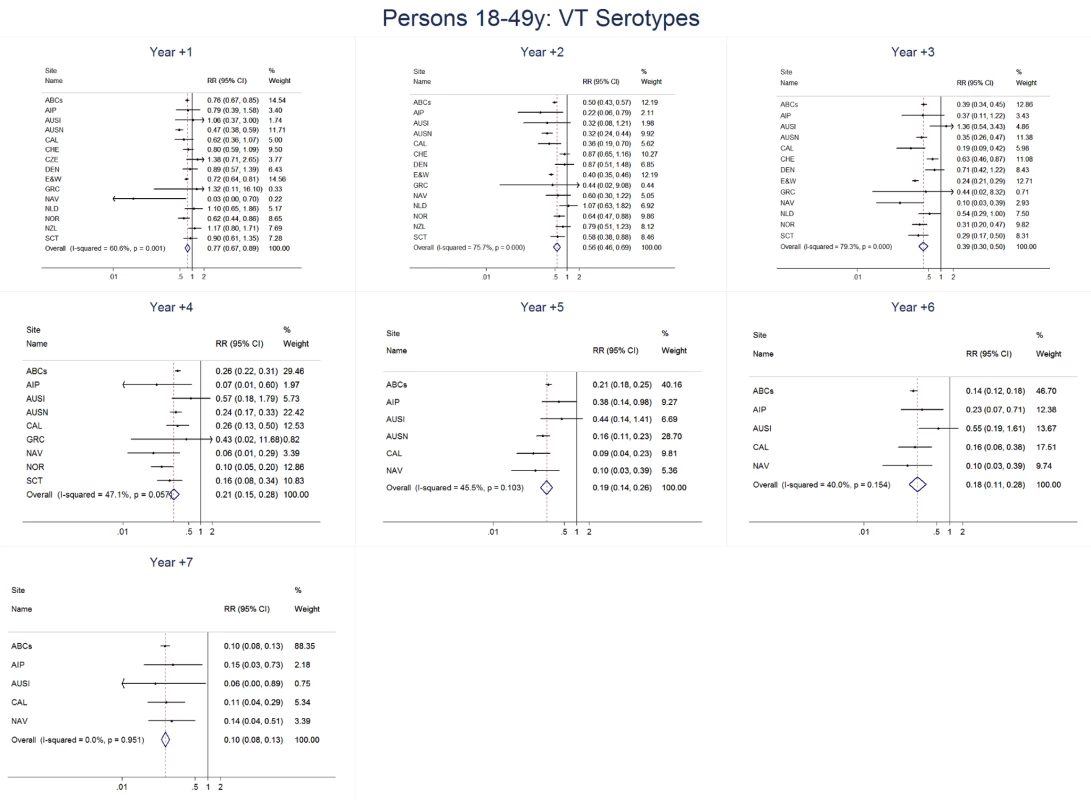
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 10. Non-vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged 18–49 years. 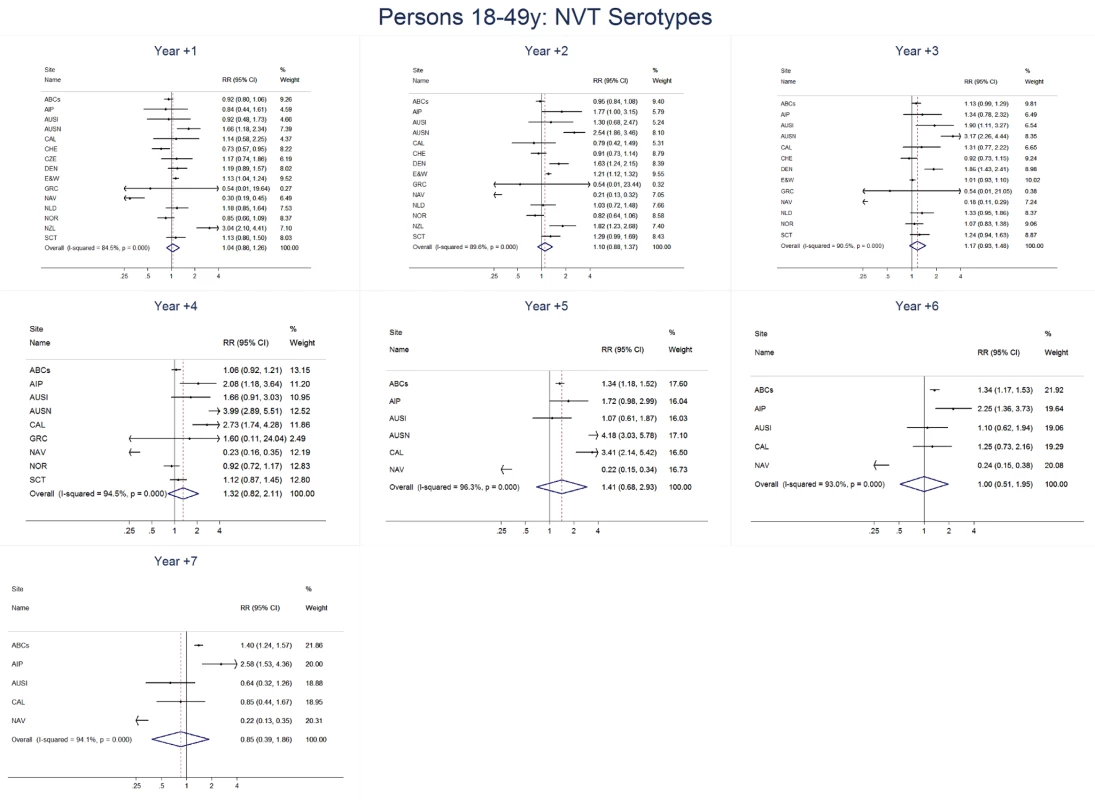
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 11. All serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged 50–64 years. 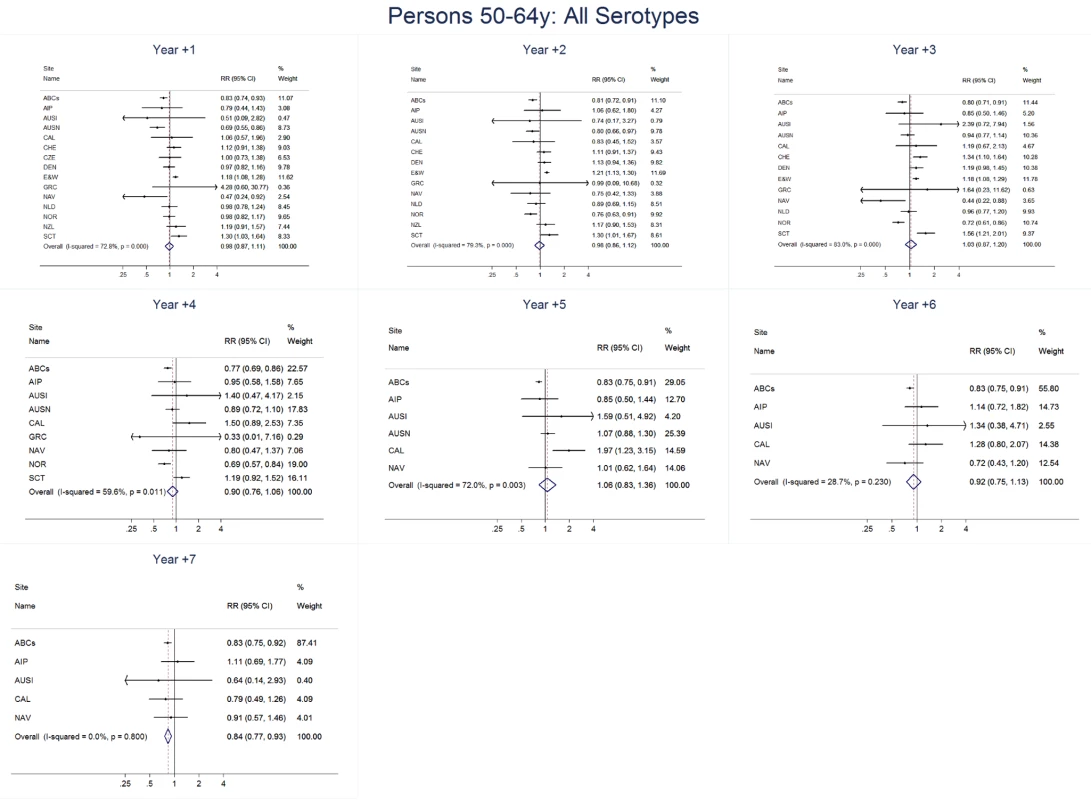
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 12. Vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged 50–64 years. 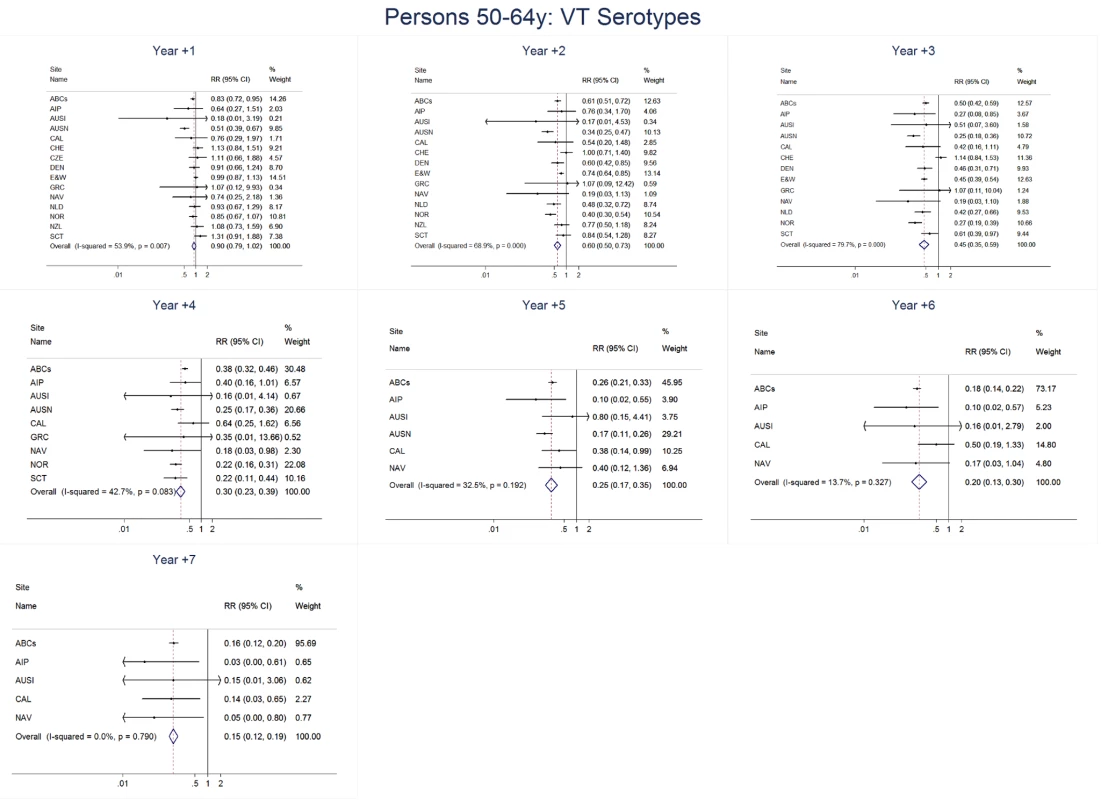
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 13. Non-vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged 50–64 years. 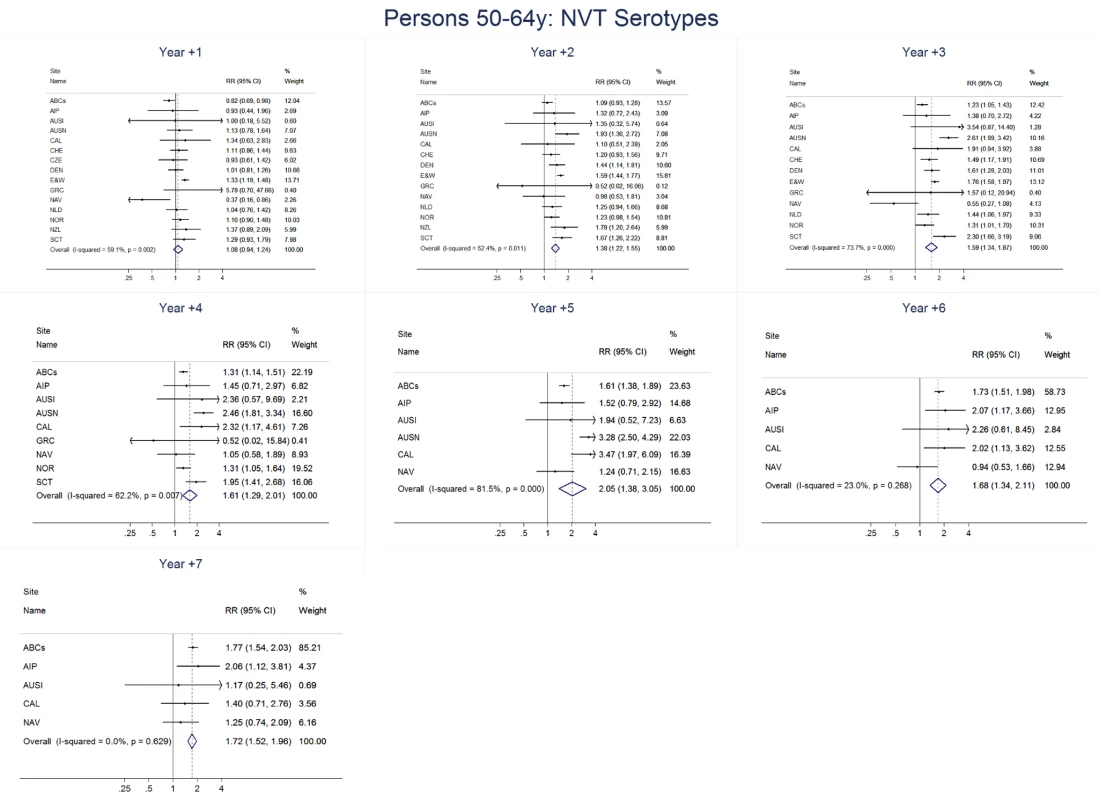
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 14. All serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged ≥65 years. 
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 15. Vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged ≥65 years. 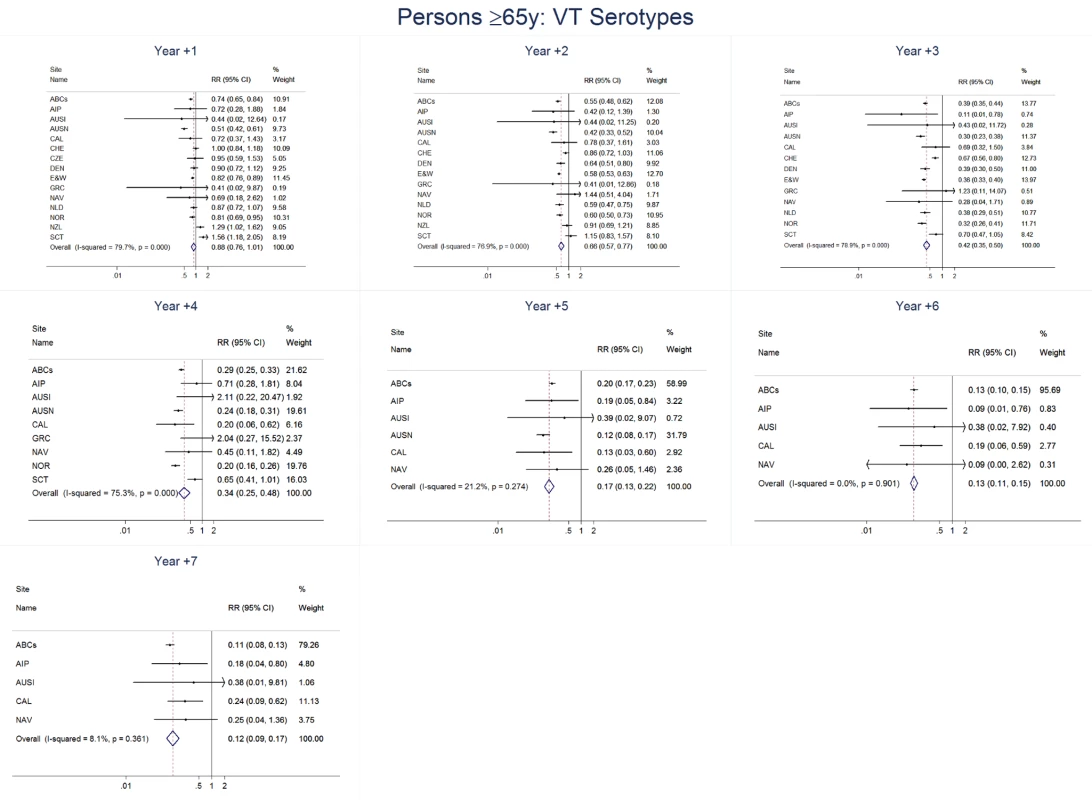
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Fig. 16. Non-vaccine serotype invasive pneumococcal disease summary rate ratio forest plots by post-introduction year from random effects meta-analysis for adults aged ≥65 years. 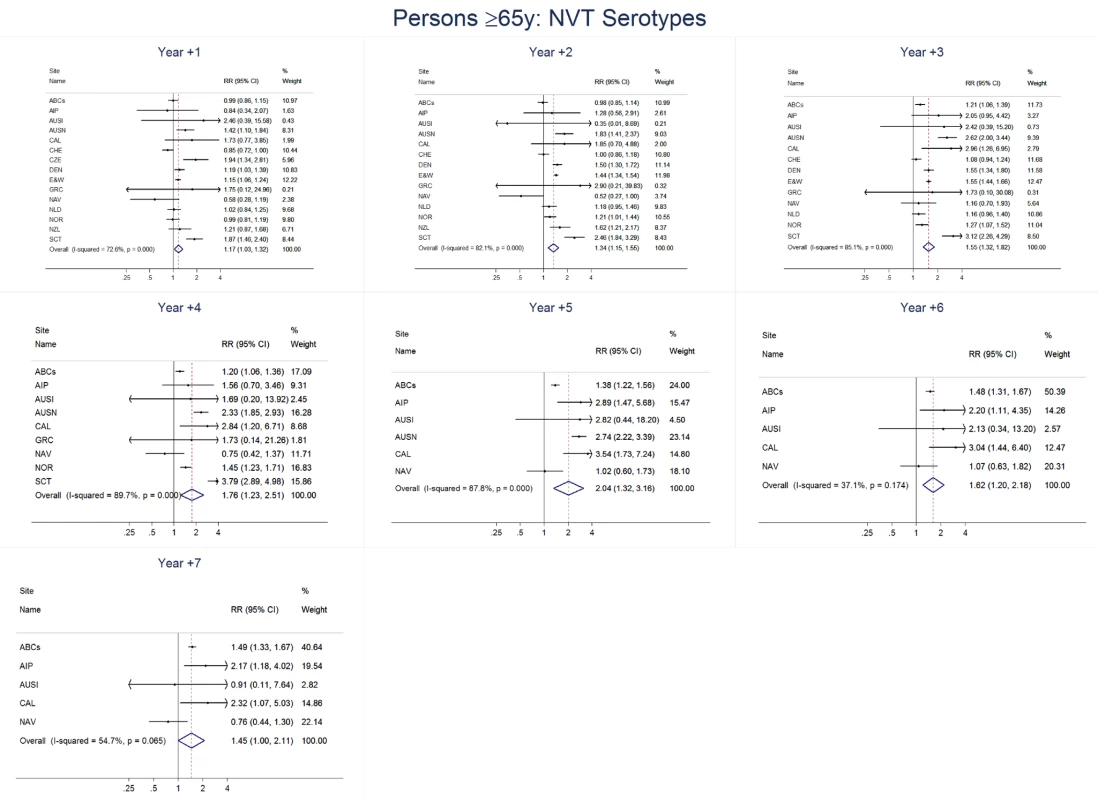
Site abbreviations: ABCs, USA Active Bacterial Core Surveillance; AIP, USA Alaska; AUSI, Australian Indigenous Northern Territory; AUSN, Australian Non-Indigenous; CAL, Canada Calgary; CHE, Switzerland; CZE, Czech Republic; DEN, Denmark; E&W, England and Wales; GRC, Greece; ISR, Israel; NAV, USA Navajo; NCK, USA Kaiser Permanente Northern California; NLD, The Netherlands; NOR, Norway; NZL, New Zealand; SCT, Scotland; URY, Uruguay; UTA, USA Utah. Among all adults in the pre-PCV7 period, the percentage of IPD due to meningitis ranged from 0%–8% by site (Table 2). The findings for meningitis were similar to overall IPD for 18–49 year olds, with statistically significant reductions at 7 years post-PCV7 introduction (RR 0·61, 95% CI 0·40–0·95) (Figure 7; Tables 3 and 4). For persons 50–64 years old, in most years the increase in NVT meningitis tended to be higher than for NVT IPD, resulting in some early years (i.e., years 2 and 3) when there was an increase in overall pneumococcal (i.e., any serotype) meningitis, although this significant increase was not sustained in subsequent years (Figure 7; Tables 3 and 4). In contrast to 50–64 year olds, among persons ≥65 years there was less of an increase in NVT meningitis than NVT IPD in most years, resulting in relatively greater reductions in overall meningitis due to all serotypes, although never reaching a statistically significant decrease (Figure 7; Tables 3 and 4).
NVT Serotypes Included in Higher Valency Conjugate Vaccines
The magnitude of increases in IPD rates due to the subset of NVT included in higher valency conjugate vaccines but not PCV7 (i.e., serotypes 1, 3, 5, 7F, 19A) was similar to the increases among all the other NVT not in the higher valency vaccines (Table 5). However, the rates due to IPD caused by the five NVT included in higher valency vaccines were higher than rates of the NVT not in the higher valency vaccines in most post-PCV7 years for children (Table 6). In contrast, among adults aged 50–64 years and ≥65 years old, IPD rates of NVT not in the higher valency vaccines were higher than rates caused by the NVT in the higher valency vaccines for most years (Table 6).
Tab. 6. Summary rate ratios from random effects meta-analysis, comparing observed over expected rates for non-vaccine serotypes, divided into those in higher valency vaccines and those not, by age, serotype group, and post-PCV7 introduction year for all sites. 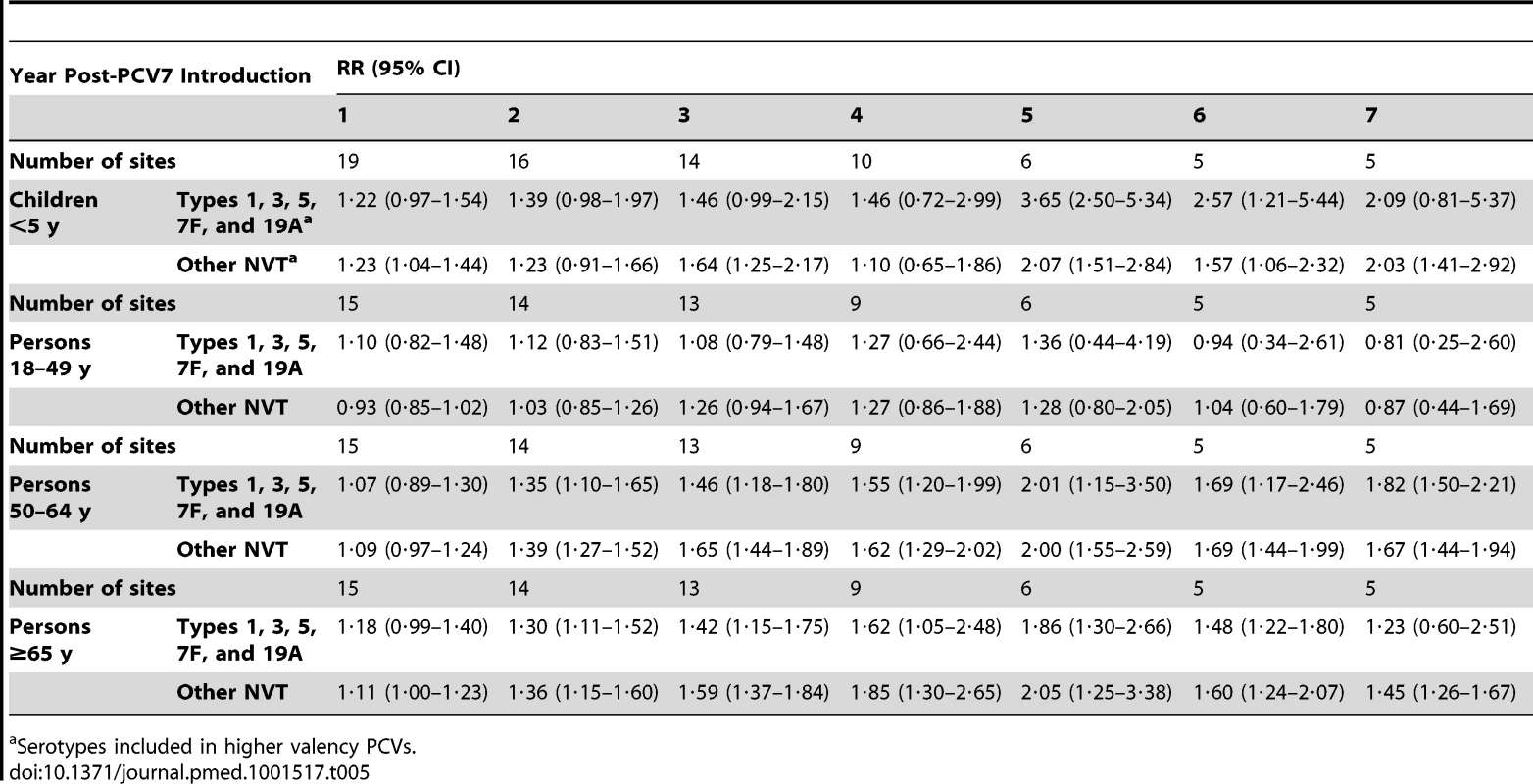
Serotypes included in higher valency PCVs. Tab. 7. Summary rate ratios comparing the rate of serotypes 1, 3, 5, 7F, and 19A over the rate of all other non-vaccine types in each year post-PCV7 introduction, from random effects meta-analysis. 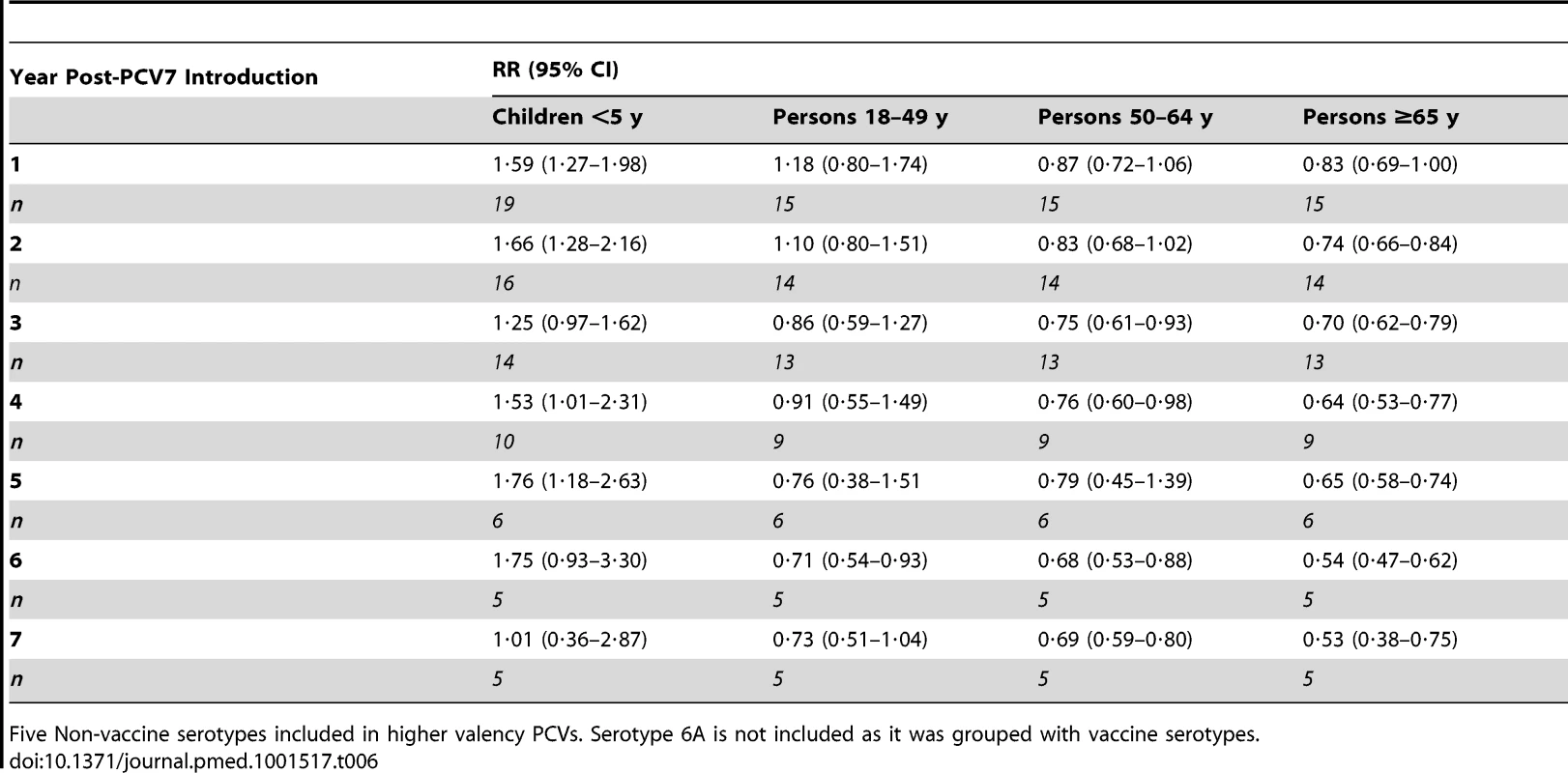
Five Non-vaccine serotypes included in higher valency PCVs. Serotype 6A is not included as it was grouped with vaccine serotypes. Discussion
This study was unique in being able to collect, restrict, adjust, and analyze multiple IPD surveillance datasets in a standardized and systematic way, allowing summary estimates and cross-site comparisons of PCV7 impact on IPD rates that are not possible from individual site-specific publications [4],[14],[38]. The most important public health implication of our analysis was that decreases in overall IPD rates in children–the group targeted for PCV7 vaccination–occurred quickly and were sustained after vaccine introduction despite increases in NVT rates. The summary reduction in the rate of overall IPD in children was 50%–60% compared with pre-introduction rates through 7 years after PCV7 introduction. We found similar overall rate reductions for pneumococcal meningitis as for overall IPD; meningitis might be less susceptible to changes over time in clinical practice and reporting compared to bacteremia. Over a half million children still die annually from pneumococcal disease, mostly in low-income countries [1], and WHO’s SAGE urges all countries to implement routine immunization with PCVs [39], a recommendation supported by this study’s finding that PCV introduction has resulted in sustained, widespread reduction in overall IPD rates in children despite the occurrence of some serotype replacement.
The relative stability in overall IPD reductions from years one to seven after PCV7 introduction belies changes in both VT and NVT IPD incidence that occurred over the years. Point estimates of VT disease continued to decrease out to seven years when VT IPD became uncommon in most sites. Point estimates of NVT, on the other hand, increased out to at least 5 years after vaccine introduction, albeit with variable magnitude across sites. This increase in NVT IPD across sites is consistent with serotype replacement, but the magnitude of those increases was smaller than the reductions in VT disease, thereby resulting in a reduction of overall IPD rates. The temporal association of the rise in NVT IPD following PCV7 introduction suggests a causal relationship. In our analysis, increases in NVT among children under 5 years were seen within 2–3 years of PCV7 introduction in all sites. The lag between the decrease in VT IPD and rise in NVT IPD, as shown here, has been pointed out previously [14].
Our data suggest that much of the NVT IPD occurring after PCV7 introduction will likely be prevented by the current use of higher valency conjugate vaccine formulations [40]–[42]. The NVT pneumococci most frequently observed to increase in carriage in areas using PCV7 are generally less likely to result in invasive disease in children than those serotypes included in PCV7 [43]–[46]. Nonetheless, our data show that serotypes other than those in PCV13 also can cause serotype replacement. Whether the higher valency vaccines will ultimately lead to further sustained reductions in overall IPD than those observed after PCV7 introduction is not yet clear and should be carefully monitored in the years ahead.
Our findings among adults showed a similar trend as in children, with some notable differences. There was a lag of at least 2 years before significant decreases in VT IPD rates were observed, an expected finding as the level of herd protection will depend on the accumulated size of the vaccinated group [47]. Moreover, the relative reduction in VT IPD, although substantial, was not of the same magnitude as in children. The variability of the changes in NVT IPD rate was greater in adults, with some sites having increases and others having decreases. Moreover, some differences in adult age groups were noticeable, with 50–64 year olds having the most modest decrease in overall IPD and meningitis, which has been shown before; this perhaps reflects the greater contribution of underlying illness to IPD in this age group [48],[49]. With increased susceptibility, this population might be more likely to show increases in IPD from less invasive replacing NVTs. These differences in VT and NVT IPD rate changes post-PCV7 among adults resulted in the finding that although overall IPD decreased in adults, there was more variability in the magnitude of the decrease by site and age group. Though the majority of sites showed a decrease in overall IPD among adults, there were a few sites in which adults had an increase in overall IPD in some post-PCV7 years, emphasizing the need for ongoing, methodologically sound and consistent surveillance among not just children but adults to document the full population impact of PCVs.
Despite the evidence from both IPD and carriage studies that PCV7 leads to some serotype replacement, other factors can also contribute to the observed increases in NVT disease rates. First, secular trends in serotype prevalence occur over time, absent vaccine, as has been shown in Spain, Denmark, Chile, and the US [38],[50]–[53]. One cause of short-term fluctuations in IPD is outbreaks, particularly due to serotypes 1, 5, 8, and 12F [54]. Removal of serotypes 1 and 5 from our analyses did not alter the overall findings, suggesting outbreaks of these two serotypes did not account for the increases in NVT incidence. Second, rapid temporal changes in antibiotic use could lead to competitive advantage of serotypes commonly resistant to antibiotics. This mechanism, particularly increased macrolide use in some countries, has been postulated as contributing to the rapid rise of serotype 19A [38],[55],[56]. Third, certain characteristics of surveillance systems can significantly influence whether changes in NVT IPD rates are identified. For example, if serotyping is performed only on the most severe cases, or if the selection of isolates for serotyping changes over time, then the observed distribution of serotypes in any given year may not reflect the true distribution in the population. Additionally, if sensitivity of case ascertainment changes over time, then findings are likely biased. For example, if clinical investigation of suspected cases, or reporting of known pneumococcal cases increases because of publicity surrounding a national vaccination program or if identification of cases decreases because of changing clinical practices (e.g., blood culturing frequency), then identification of NVT IPD cases over time will increase or decrease, respectively. Lastly, if the susceptibility of the population to pneumococcal diseases changes, for example by increased use of antiretroviral therapy in persons with HIV infection, then the rates of IPD in the population can change over time. Similarly, if the prevalence of underlying or immunocompromising illness increases over time, the population might become more susceptible to IPD from less invasive NVT serotypes, leading to an apparent increase in serotype replacement. Although these non-vaccine factors might have played a part in the observed IPD rates post-vaccination, we attempted to eliminate or adjust for them in multiple ways, leading us to believe that their overall contribution to the observed serotype-specific IPD changes, including serotype replacement, were secondary.
This analysis had certain limitations. First, as mentioned, this review includes only data from programs using PCV7. PCV7 is no longer produced and so it will be important to be cautious when extrapolating to programs using the newer PCV10 and PCV13 formulations. Nonetheless, if PCV10 and PCV13 affect nasopharyngeal colonization in a manner similar to that of PCV7, IPD serotype replacement will likely occur to some degree following immunization with the higher valency formulations; the epidemiology and the policy implications of serotype replacement learned from PCV7 will continue to be relevant. Second, we may not have fully identified or controlled for temporal trends in IPD surveillance or possible outbreaks of serotypes besides 1 and 5 in some datasets. Third, these data represent the experience in high-income countries. Findings from the two indigenous populations (i.e., Navajo and Australian Indigenous), known to be at high risk of IPD and to share pneumococcal epidemiologic characteristics with lower-income settings, did not diverge substantially from the findings of the overall analysis. Nonetheless, the results of this analysis might differ in developing countries, where there are differences in the pressure of pneumococcal carriage, serotype distributions, prevalence of risk factors, and co-morbidities. To assess the impact of pneumococcal conjugate vaccines in such populations, longitudinal surveillance of serotype-specific disease will be important. Fourth, only five sites had data out to 6 and 7 years post-introduction, which might have limited the representativeness of the findings for those years, although these five sites showed similar results to all sites in years 1–5 post-introduction (Table S8). Fifth, we could not control for inherent differences in clinical practice across sites, such as the criteria for hospitalization and performing lumbar punctures and blood cultures, which might, in part, explain heterogeneity of findings across sites. The focus of our analysis was to describe post-PCV IPD epidemiology across many sites, rather than identify site-specific variables that might predict serotype replacement. Finally, these conclusions apply only to IPD and may not be fully representative of serotype replacement in non-bacteremic pneumococcal pneumonia, the most important cause of pneumococcal morbidity and mortality worldwide [8],[57].
Based on our experience in reviewing many datasets for this evaluation, we have several recommendations for the collection and interpretation of IPD surveillance data (Table 7). In settings where these recommendations cannot be implemented, introduction of PCV should still occur as quickly as possible. However, attempts to identify and characterize serotype replacement using surveillance systems that do not meet these criteria could lead to erroneous conclusions. With so many countries having introduced or about to introduce PCV, and with the need for multiple years of stable and complete pre - and post-IPD rate data, it may be too late to establish many new surveillance sites to monitor serotype replacement. Many countries have existing systems, however, which can be assessed and enhanced to meet the rigorous, high-quality IPD surveillance requirements to monitor the impact of PCVs. Optimizing surveillance data that allows for valid interpretations of the vaccine effect on disease is essential for sound policy decisions [58].
Tab. 8. Recommendations for maximizing the interpretability of surveillance data on invasive pneumococcal disease rates in the context of serotype replacement. 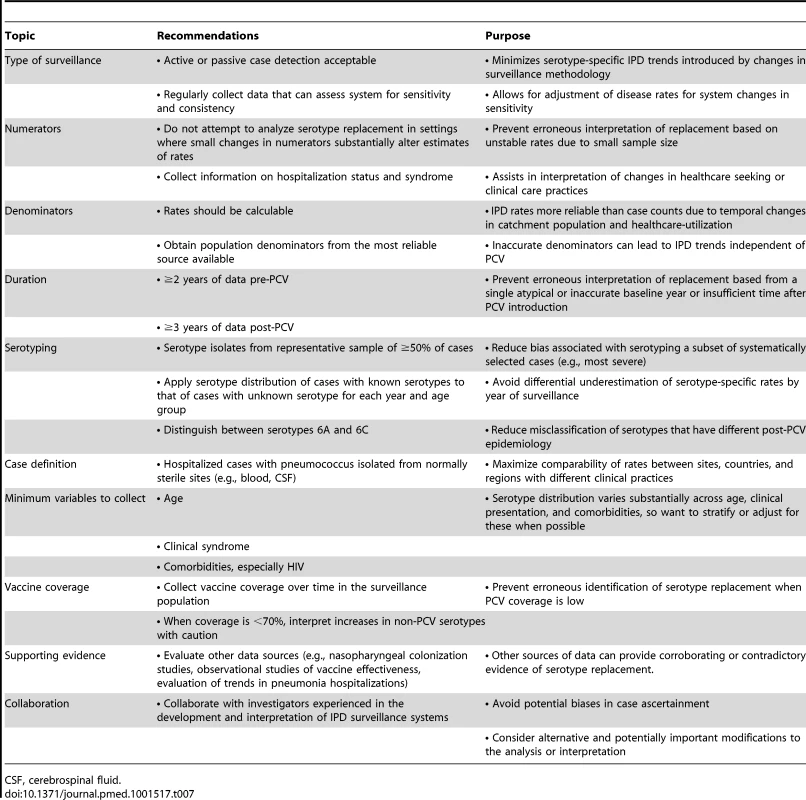
CSF, cerebrospinal fluid. Supporting Information
Zdroje
1. World Health Organization (2012) Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. Available: http://www.who.int/immunization_monitoring/burden/Pneumo_hib_estimates/en/index.html. Accessed 18 February 2013.
2. PilishviliT, LexauC, FarleyMM, HadlerJ, HarrisonLH, et al. (2010) Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201 : 32–41.
3. KellnerJD, VanderkooiOG, MacDonaldJ, ChurchDL, TyrrellGJ, et al. (2009) Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis 49 : 205–212.
4. SingletonRJ, HennessyTW, BulkowLR, HammittLL, ZulzT, et al. (2007) Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297 : 1784–1792.
5. O’BrienKL, MillarEV, ZellER, BronsdonM, WeatherholtzR, et al. (2007) Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis 196 : 1211–1220.
6. HuangSS, PlattR, Rifas-ShimanSL, PeltonSI, GoldmannD, et al. (2005) Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116: e408–e413.
7. FlascheS, Van HoekAJ, SheasbyE, WaightP, AndrewsN, et al. (2011) Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med 8: e1001017 doi:10.1371/journal.pmed.1001017
8. O'BrienKL, WolfsonLJ, WattJP, HenkleE, Deloria-KnollM, et al. (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374 : 893–902.
9. AbdullahiO, KaraniA, TigoiCC, MugoD, KunguS, et al. (2012) The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One 7: e30787 doi:10.1371/journal.pone.0030787
10. World Health Organization (2010) Changing epidemiology of pneumococcal serotypes after introduction of conjugate vaccine: July 2010 report. Wkly Epidemiol Rec 85 : 434–436.
11. Conklin L, Knoll MD, Loo JD, Fleming-Dutra K, Park D, et al.. (2011) Landscape analysis of pneumococcal conjugate vaccine dosing schedules: A systematic review (Report to the Strategic Advisory Group of Experts on Immunization (SAGE), World Health Organization). Available: http://www.who.int/immunization/sage/3_Conklin_L_PCV_Dosing_Landscape_Report_Oct_17_2011_FINAL_nov11.pdf. Accessed 18 February 2013.
12. ParkIH, MooreMR, TreanorJJ, PeltonSI, PilishviliT, et al. (2008) Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis 198 : 1818–1822.
13. HausdorffWP, SiberG, ParadisoPR (2001) Geographical differences in invasive pneumococcal disease rates and serotype frequency in young children. Lancet 357 : 950–952.
14. WeinbergerDM, MalleyR, LipsitchM (2011) Serotype replacement in disease after pneumococcal vaccination. Lancet 378 : 1962–1973.
15. HanquetG, LernoutT, VergisonA, VerhaegenJ, KisslingE, et al. (2011) Impact of conjugate 7-valent vaccination in Belgium: addressing methodological challenges. Vaccine 29 : 2856–2864.
16. MillerE, AndrewsNJ, WaightPA, SlackMP, GeorgeRC (2011) Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 11 : 760–768.
17. CoxC (1990) Fieller’s Theorem, the Likelihood and the Delta Method. Biometrics 46 : 709–718.
18. Beaton GH, United Nations. Subcommittee on Nutrition (1992) Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. Nutrition policy discussion paper number 13. Geneva: ACC/SCN. 120p.
19. SweetingMJ, SuttonAJ, LambertPC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23 : 1351–1375.
20. Borenstein M, Hedges L., Rothstein H. (2007) Introduction to meta-analysis. pp.2–303. Available: http://www.meta-analysis.com/downloads/Meta%20Analysis%20Fixed%20vs%20Random%20effects.pdf. Accessed 18 February 2013.
21. KrauseVL, ReidSJ, MerianosA (2000) Invasive pneumococcal disease in the Northern Territory of Australia, 1994–1998. Med J Aust 173 Suppl: S27–31.
22. BarryC, KrauseVL, CookHM, MenziesRI (2012) Invasive pneumococcal disease in Australia 2007 and 2008. Commun Dis Intell Q Rep 36: E151–165.
23. MotlovaJ, BenesC, KrizP (2009) Incidence of invasive pneumococcal disease in the Czech Republic and serotype coverage by vaccines, 1997–2006. Epidemiol Infect 137 : 562–569.
24. HarboeZB, Valentiner-BranthP, IngelsH, RasmussenJN, AndersenPH, et al. (2013) Pediatric invasive pneumococcal disease caused by vaccine serotypes following the introduction of conjugate vaccination in Denmark. PLoS ONE 8: e51460 doi:10.1371/journal.pone.0051460
25. GrallN, HurmicO, Al NakibM, LongoM, PoyartC, et al. (2011) Epidemiology of Streptococcus pneumoniae in France before introduction of the PCV-13 vaccine. Eur J Clin Microbiol Infect Dis 30 : 1511–1519.
26. ZissisNP, SyriopoulouV, KafetzisD, DaikosGL, TsilimingakiA, et al. (2004) Serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae causing invasive infections and acute otitis media in children. Eur J Pediatr 163 : 364–368.
27. VickersI, FitzgeraldM, MurchanS, CotterS, O'FlanaganO’FlanaganD, et al. (2011) Serotype distribution of Streptococcus pneumoniae causing invasive disease in the Republic of Ireland. Epidemiol Infect 139 : 783–790.
28. Ben-ShimolS, GreenbergD, Givon-LaviN, EliasN, GlikmanD, et al. (2012) Rapid reduction in invasive pneumococcal disease after introduction of PCV7 into the National Immunization Plan in Israel. Vaccine 30 : 6600–6607.
29. van DeursenAM, van MensSP, SandersEA, VlaminckxBJ, de MelkerHE, et al. (2012) Invasive pneumococcal disease and 7-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis 18 : 1729–1737.
30. HeffernanHM, MartinDR, WoodhouseRE, MorganJ, BlackmoreTK (2008) Invasive pneumococcal disease in New Zealand 1998–2005: capsular serotypes and antimicrobial resistance. Epidemiol Infect 136 : 352–359.
31. VestrheimDF, LovollO, AabergeIS, CaugantDA, HoibyEA, et al. (2008) Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine 26 : 3277–3281.
32. Lamb KE, Flasche S, Diggle M, Inverarity D, Greenhalgh D, et al.. (2013) Trends in serotypes and sequence types among cases of invasive pneumococcal disease in Scotland, 1999–2010. Vaccine. In press.
33. PilishviliT, ZellER, FarleyMM, SchaffnerW, LynfieldR, et al. (2010) Risk factors for invasive pneumococcal disease in children in the era of conjugate vaccine use. Pediatrics 126: e9–e17.
34. WeatherholtzR, MillarEV, MoultonLH, ReidR, RudolphK, et al. (2010) Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis 50 : 1238–1246.
35. BlackS, FranceEK, IsaacmanD, BrackenL, LewisE, et al. (2007) Surveillance for invasive pneumococcal disease during 2000–2005 in a population of children who received 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 26 : 771–777.
36. ByingtonCL, SamoreMH, StoddardGJ, BarlowS, DalyJ, et al. (2005) Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis 41 : 21–29.
37. GAVI Alliance (2011) GAVI Alliance Country Eligibility Policy. Available: www.gavialliance.org/about/governance/programme-policies/country-eligibility/. Accessed 31 May 2013.
38. BlackS (2010) The volatile nature of pneumococcal serotype epidemiology: potential for misinterpretation. Pediatr Infect Dis J 29 : 301–303.
39. World Health Organization (2012) Pneumococcal vaccine WHO position paper - 2012. Wkly Epidemiol Rec 14 : 129–144.
40. MillerE, AndrewsNJ, WaightPA, SlackMP, GeorgeRC (2011) Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 29 : 9127–9131.
41. PalmuAA, JokinenJ, BorysD, NieminenH, RuokokoskiE, et al. (2012) Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 381 : 214–222.
42. SingletonR, WengerJ, KlejkaJA, BulkowLR, ThompsonA, et al. (2013) The 13-valent pneumococcal conjugate vaccine for invasive pneumococcal disease in Alaska Native children: results of a clinical trial. Pediatr Infect Dis J 32 : 257–263.
43. BrueggemannAB, PetoTE, CrookDW, ButlerJC, KristinssonKG, et al. (2004) Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis 190 : 1203–1211.
44. BogaertD, ThompsonCM, TrzcinskiK, MalleyR, LipsitchM (2010) The role of complement in innate and adaptive immunity to pneumococcal colonization and sepsis in a murine model. Vaccine 28 : 681–685.
45. SleemanKL, GriffithsD, ShackleyF, DiggleL, GuptaS, et al. (2006) Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis 194 : 682–688.
46. WeinbergerDM, HarboeZB, FlascheS, ScottJA, LipsitchM (2011) Prediction of serotypes causing invasive pneumococcal disease in unvaccinated and vaccinated populations. Epidemiology 22 : 199–207.
47. WhitneyCG, FarleyMM, HadlerJ, HarrisonLH, BennettNM, et al. (2003) Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 348 : 1737–1746.
48. HsuHE, ShuttKA, MooreMR, BeallBW, BennettNM, et al. (2009) Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 360 : 244–256.
49. CohenAL, HarrisonLH, FarleyMM, ReingoldAL, HadlerJ, et al. (2010) Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS 24 : 2253–2262.
50. FeikinDR, KlugmanKP (2002) Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis 35 : 547–555.
51. FenollA, Martin BourgonC, MunozR, ViciosoD, CasalJ (1991) Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979–1989. Rev Infect Dis 13 : 56–60.
52. HarboeZB, BenfieldTL, Valentiner-BranthP, HjulerT, LambertsenL, et al. (2010) Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis 50 : 329–337.
53. LagosR, MunozA, San MartinO, MaldonadoA, HormazabalJC, et al. (2008) Age - and serotype-specific pediatric invasive pneumococcal disease: insights from systematic surveillance in Santiago, Chile, 1994–2007. J Infect Dis 198 : 1809–1817.
54. HausdorffWP, FeikinDR, KlugmanKP (2005) Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 5 : 83–93.
55. DaganR, Givon-LaviN, LeibovitzE, GreenbergD, PoratN (2009) Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis 199 : 776–785.
56. Hausdorff WP, Van Dyke MK, Van Effelterre T (2012) Serotype replacement after pneumococcal vaccination. Lancet 379: : 1387–1388; author reply 1388–1389.
57. BlackRE, CousensS, JohnsonHL, LawnJE, RudanI, et al. (2010) Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375 : 1969–1987.
58. World Health Organization (2012) Review of serotype replacement in the setting of 7-valent pneumococcal conjugate vaccine (PCV-7) use and implications for the PCV10/PCV13 era. Wkly Epidemiol Rec 87 : 12–13.
59. RobinsonKA, BaughmanW, RothrockG, BarrettNL, PassM, et al. (2001) Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: Opportunities for prevention in the conjugate vaccine era. JAMA 285 : 1729–1735.
60. van DeursenAM, van MensSP, SandersEA, VlaminckxBJ, de MelkerHE, et al. (2012) Invasive pneumococcal disease and 7-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis 18 : 1729–1737.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa
- Translating Cochrane Reviews to Ensure that Healthcare Decision-Making is Informed by High-Quality Research Evidence
- Physician Emigration from Sub-Saharan Africa to the United States: Analysis of the 2011 AMA Physician Masterfile
- Acupuncture and Counselling for Depression in Primary Care: A Randomised Controlled Trial
- Serotype-Specific Changes in Invasive Pneumococcal Disease after Pneumococcal Conjugate Vaccine Introduction: A Pooled Analysis of Multiple Surveillance Sites
- Risk Factors and Outcomes for Late Presentation for HIV-Positive Persons in Europe: Results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE)
- Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis
- Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China
- Feasibility of Mass Vaccination Campaign with Oral Cholera Vaccines in Response to an Outbreak in Guinea
- Preconception Care in Low- and Middle-Income Countries: New Opportunities and a New Metric
- How to Stir Up Trouble…while Riding a Rollercoaster
- Health Workforce Brain Drain: From Denouncing the Challenge to Solving the Problem
- Association of the ANRS-12126 Male Circumcision Project with HIV Levels among Men in a South African Township: Evaluation of Effectiveness using Cross-sectional Surveys
- Setting Research Priorities for Preconception Care in Low- and Middle-Income Countries: Aiming to Reduce Maternal and Child Mortality and Morbidity
- Transnational Tobacco Company Interests in Smokeless Tobacco in Europe: Analysis of Internal Industry Documents and Contemporary Industry Materials
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China
- Translating Cochrane Reviews to Ensure that Healthcare Decision-Making is Informed by High-Quality Research Evidence
- Physician Emigration from Sub-Saharan Africa to the United States: Analysis of the 2011 AMA Physician Masterfile
- Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




