-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Use of Non-Steroidal Anti-Inflammatory Drugs That Elevate Cardiovascular Risk: An Examination of Sales and Essential Medicines Lists in Low-, Middle-, and High-Income Countries
Background:
Certain non-steroidal anti-inflammatory drugs (NSAIDs) (e.g., rofecoxib [Vioxx]) increase the risk of heart attack and stroke and should be avoided in patients at high risk of cardiovascular events. Rates of cardiovascular disease are high and rising in many low - and middle-income countries. We studied the extent to which evidence on cardiovascular risk with NSAIDs has translated into guidance and sales in 15 countries.Methods and Findings:
Data on the relative risk (RR) of cardiovascular events with individual NSAIDs were derived from meta-analyses of randomised trials and controlled observational studies. Listing of individual NSAIDs on Essential Medicines Lists (EMLs) was obtained from the World Health Organization. NSAID sales or prescription data for 15 low-, middle-, and high-income countries were obtained from Intercontinental Medical Statistics Health (IMS Health) or national prescription pricing audit (in the case of England and Canada). Three drugs (rofecoxib, diclofenac, etoricoxib) ranked consistently highest in terms of cardiovascular risk compared with nonuse. Naproxen was associated with a low risk. Diclofenac was listed on 74 national EMLs, naproxen on just 27. Rofecoxib use was not documented in any country. Diclofenac and etoricoxib accounted for one-third of total NSAID usage across the 15 countries (median 33.2%, range 14.7–58.7%). This proportion did not vary between low - and high-income countries. Diclofenac was by far the most commonly used NSAID, with a market share close to that of the next three most popular drugs combined. Naproxen had an average market share of less than 10%.Conclusions:
Listing of NSAIDs on national EMLs should take account of cardiovascular risk, with preference given to low risk drugs. Diclofenac has a risk very similar to rofecoxib, which was withdrawn from worldwide markets owing to cardiovascular toxicity. Diclofenac should be removed from EMLs.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(2): e32767. doi:10.1371/journal.pmed.1001388
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001388Summary
Background:
Certain non-steroidal anti-inflammatory drugs (NSAIDs) (e.g., rofecoxib [Vioxx]) increase the risk of heart attack and stroke and should be avoided in patients at high risk of cardiovascular events. Rates of cardiovascular disease are high and rising in many low - and middle-income countries. We studied the extent to which evidence on cardiovascular risk with NSAIDs has translated into guidance and sales in 15 countries.Methods and Findings:
Data on the relative risk (RR) of cardiovascular events with individual NSAIDs were derived from meta-analyses of randomised trials and controlled observational studies. Listing of individual NSAIDs on Essential Medicines Lists (EMLs) was obtained from the World Health Organization. NSAID sales or prescription data for 15 low-, middle-, and high-income countries were obtained from Intercontinental Medical Statistics Health (IMS Health) or national prescription pricing audit (in the case of England and Canada). Three drugs (rofecoxib, diclofenac, etoricoxib) ranked consistently highest in terms of cardiovascular risk compared with nonuse. Naproxen was associated with a low risk. Diclofenac was listed on 74 national EMLs, naproxen on just 27. Rofecoxib use was not documented in any country. Diclofenac and etoricoxib accounted for one-third of total NSAID usage across the 15 countries (median 33.2%, range 14.7–58.7%). This proportion did not vary between low - and high-income countries. Diclofenac was by far the most commonly used NSAID, with a market share close to that of the next three most popular drugs combined. Naproxen had an average market share of less than 10%.Conclusions:
Listing of NSAIDs on national EMLs should take account of cardiovascular risk, with preference given to low risk drugs. Diclofenac has a risk very similar to rofecoxib, which was withdrawn from worldwide markets owing to cardiovascular toxicity. Diclofenac should be removed from EMLs.
Please see later in the article for the Editors' SummaryIntroduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely used of therapeutic agents. Taken singly or in combination with other classes of drug, they relieve symptoms across multiple clinical indications, including short and long term pain states and a range of musculoskeletal disorders.
Serious adverse effects of NSAIDs are well understood, being related largely to their underlying mechanisms of action [1]. Extensive pharmaco-epidemiological studies and meta-analyses have documented hazards, in particular serious gastrointestinal [2],[3] and cardiovascular complications [4]–[9]. These studies have enabled some discrimination in risk between individual members of this large class of drugs, providing guidance on selection according to patient risk profiles [10]. Gastrointestinal damage can be reduced by co-prescription of proton pump inhibitors [11]. In contrast, there is no convincing evidence that low dose aspirin mitigates the cardiovascular risk of NSAIDs [12],[13]. Faced with patients at high risk of cardiovascular events, prescribers have a choice of advising against use of a NSAID or recommending a drug with a lower risk. This dilemma is not limited to doctors working in high-income countries. With the widespread use of NSAIDs and a steep rise in cardiovascular disease, it is a particular concern in low - and middle-income countries [14].
Precise summary information on cardiovascular risk with NSAIDs has been available since 2006 and current evidence suggests that there are significant differences between commonly used members of the class [4]–[9],[13]. There are strong reasons for choosing low risk NSAIDs in those at high risk of cardiovascular events. Our interest here is the extent to which this is reflected in Essential Medicines Lists (EMLs) and in data on sales across several countries.
Methods
Estimating Cardiovascular Risk with Individual Drugs
We ranked NSAIDs by cardiovascular risk (with non-use as reference) using relative risk (RR) values derived from published meta-analyses of randomised trials and controlled observational studies that reported RR for three or more individual drugs [4]–[9]. Data on pairwise comparisons of individual agents, representing the least confounded comparisons of RR, were obtained from the most recent meta-analysis [9].
Essential Medicines Lists
Essential medicines are those satisfying the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness. We determined the NSAIDs recommended by the World Health Organization (WHO) in its Model List of Essential Medicines [15], and by individual countries with published national EMLs [16]. National EMLs are informed by the WHO Model List of Essential Medicines and modified to reflect national health care priorities [17]. For the countries with published EMLs, we compared the information on cardiovascular risk with individual NSAIDs and their inclusion in the country EML.
Measuring Sales of NSAIDs in Different Countries
Intercontinental Medical Statistics Health (IMS Health) tracks over 80% of global pharmaceutical use by sampling individual country sales through multiple supply routes to retail pharmacies and hospitals (http://www.imshealth.com/portal/site/ims/). These sales include both indirect sales from wholesalers and direct sales from manufacturers. In some countries, hospital audits are based on data sourced from hospital pharmacies. In each country, the sampling data are projected to estimate sales for the whole country. We purchased data from IMS Health on the mass of individual NSAIDs sold in 2011 in 13 countries in the South Asian, Southeast Asian, and Asian Pacific regions (Multinational Integrated Data Analysis, MIDAS). The countries included in the analyses were: Australia, Bangladesh, China, China (Hong Kong), Indonesia, Malaysia, New Zealand, Pakistan, Philippines, Singapore, Taiwan, Thailand, and Vietnam. The data reflected retail pharmacy and hospital sales in all countries except Bangladesh and Pakistan (retail pharmacy sales) and China (hospital sales). Defined daily doses (DDD), established by the WHO Collaborating Centre for Drug Statistics Methodology (WHOCC), permit comparisons of use between different drugs and across different countries [18]. We calculated the numbers of DDD of individual NSAIDs for each country using the values published by the WHOCC [18].
Data on NSAID prescriptions dispensed in the community in England during 2011 were obtained from public prescription cost analysis reports [19]. For Canada, we purchased data on NSAID prescriptions dispensed in the community during 2011 from IMS Brogan (IMS Brogan Inc., Ottawa, Canada). We did not have sufficient information to convert prescription data to DDD, but assumed that the proportionality of market shares for individual NSAIDs calculated from prescriptions would be equivalent to that derived from sales data.
Results
Cardiovascular Risk with Individual NSAIDs
The meta-analyses were fairly constant in their findings (Table 1). The NSAIDs that had consistently higher cardiovascular risks (RRs) were rofecoxib, etoricoxib, and diclofenac. All were found to have a higher RR than naproxen in pairwise analyses in the most recent published meta-analysis [9]. Indometacin and meloxicam had moderately elevated RR values that were significantly greater than naproxen [9]. Etodolac was found to have an elevated risk but in pairwise analysis, it did not have a statistically significantly higher RR than naproxen [9]. Celecoxib and ibuprofen were associated with elevated RR values when used in clinical trials in high doses but not in the lower doses typically used in the community. We judged naproxen to have the lowest risk. Five of the six meta-analyses found it to be risk-neutral (Table 1). We classified rofecoxib, etoricoxib, and diclofenac as “high risk” drugs for the purpose of analysis. This is conservative in that other NSAIDs could also be considered high risk.
Tab. 1. Summary of relative risk estimates for cardiovascular events with individual NSAIDs (versus non-use). 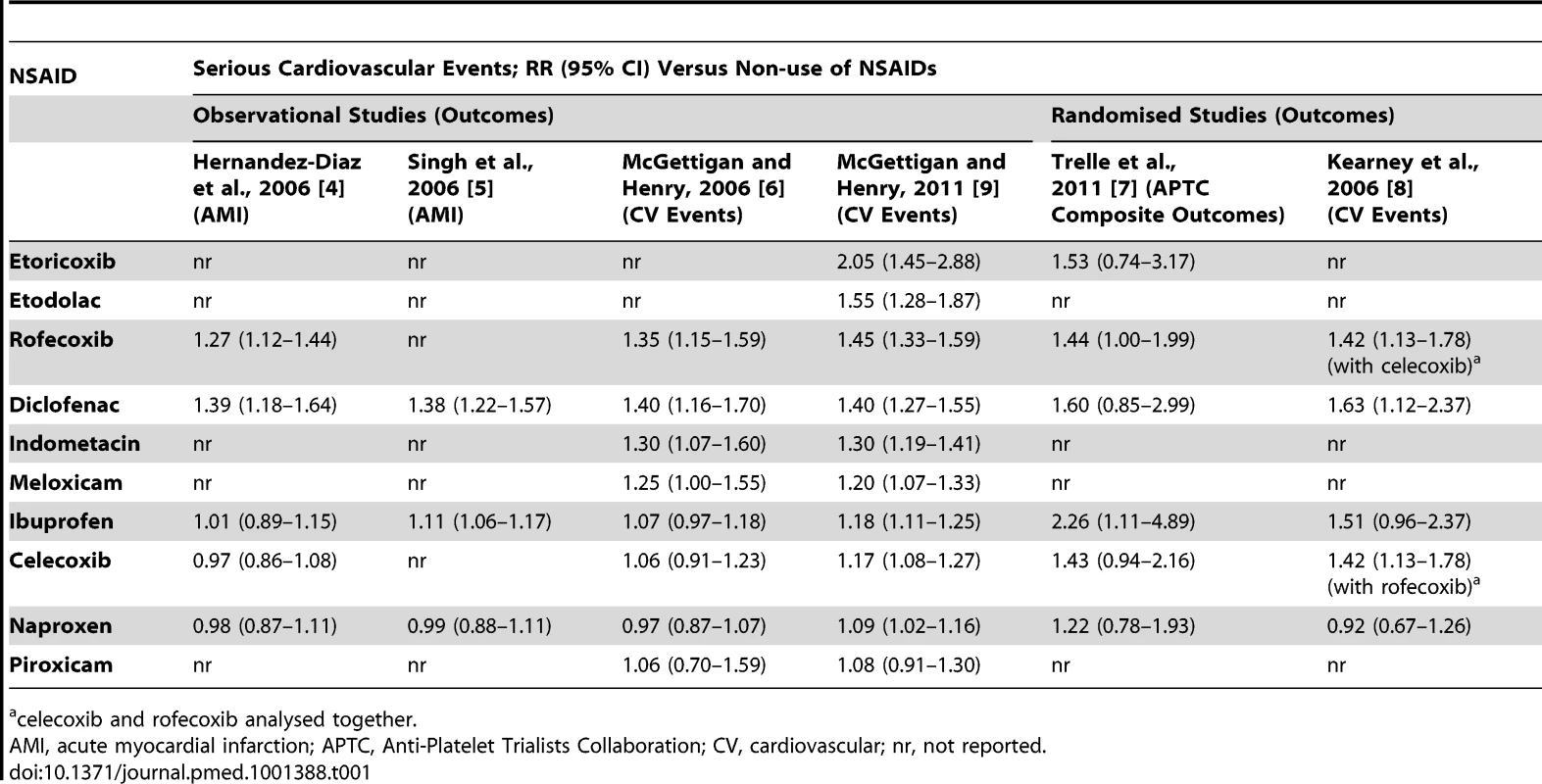
celecoxib and rofecoxib analysed together. EML Inclusions
The WHO Model List of Essential Medicines includes three drugs, paracetamol, acetyl salicylic acid (aspirin), and ibuprofen, in the category “non-opioids and non-steroidal anti-inflammatory medicines.” Of 100 countries with EMLs published on the WHO website, most included fewer than six agents in this class. The NSAIDs most commonly recommended were: aspirin (88 countries), ibuprofen (90 countries), diclofenac (74 countries), indometacin (56 countries), and naproxen (27 countries) (Table S2). Significantly, 51 of the countries that listed diclofenac did not list naproxen. Selective cyclooxygenase-2 (cox-2) inhibitors were included on the EMLs of 12 countries. Of 86 EMLs published or updated since 2007, diclofenac was listed on 74, naproxen on 27.
Patterns of NSAID Use
Figure 1 presents the market shares of the nine most widely sold NSAIDs in 15 countries. The analyses are presented in Table 2. Full data are provided in Table S1. Diclofenac was the most popular NSAID, with a market share almost equal to that of the next three most popular NSAIDs combined (ibuprofen, mefenamic acid, naproxen). There was no documented use of rofecoxib. Etoricoxib was commonly sold in Bangladesh, Malaysia, Hong Kong, and Singapore. “High risk” NSAIDs (diclofenac and exoricoxib) comprised about one-third of the market across the 15 countries (median 33.2%, range 14.7–58.7%), and this proportion did not differ between high - and low-income states (Table 2).
Fig. 1. Individual NSAID defined daily doses (DDD) expressed as a percentage of total NSAID DDD sales in each country in 2011. 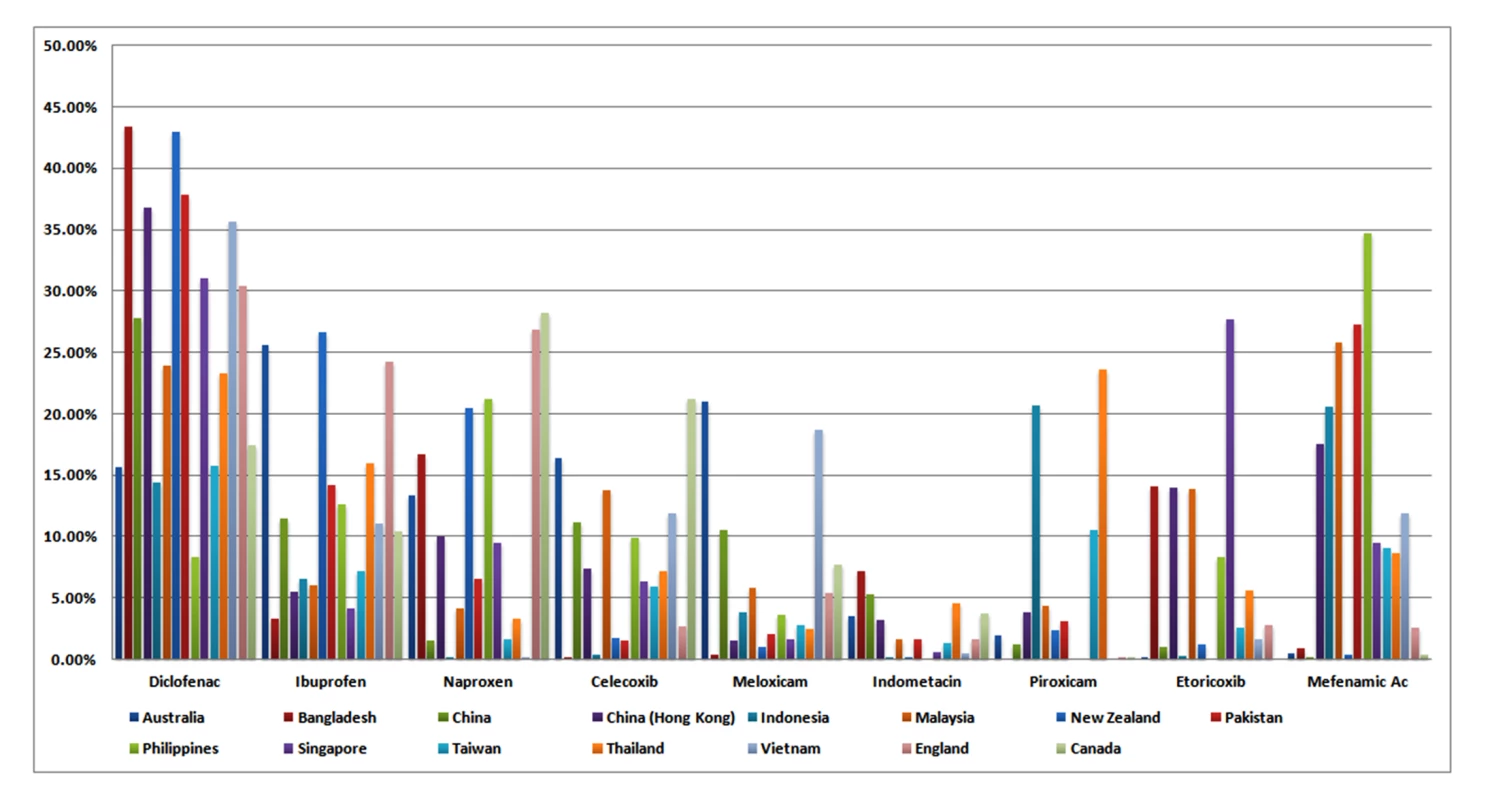
Data reflect retail pharmacy and hospital sales in all countries except Bangladesh and Pakistan [retail pharmacy sales], China [hospital sales], and England and Canada [prescription sales only]. Tab. 2. Analysis of use of selected NSAIDs in 15 countries. 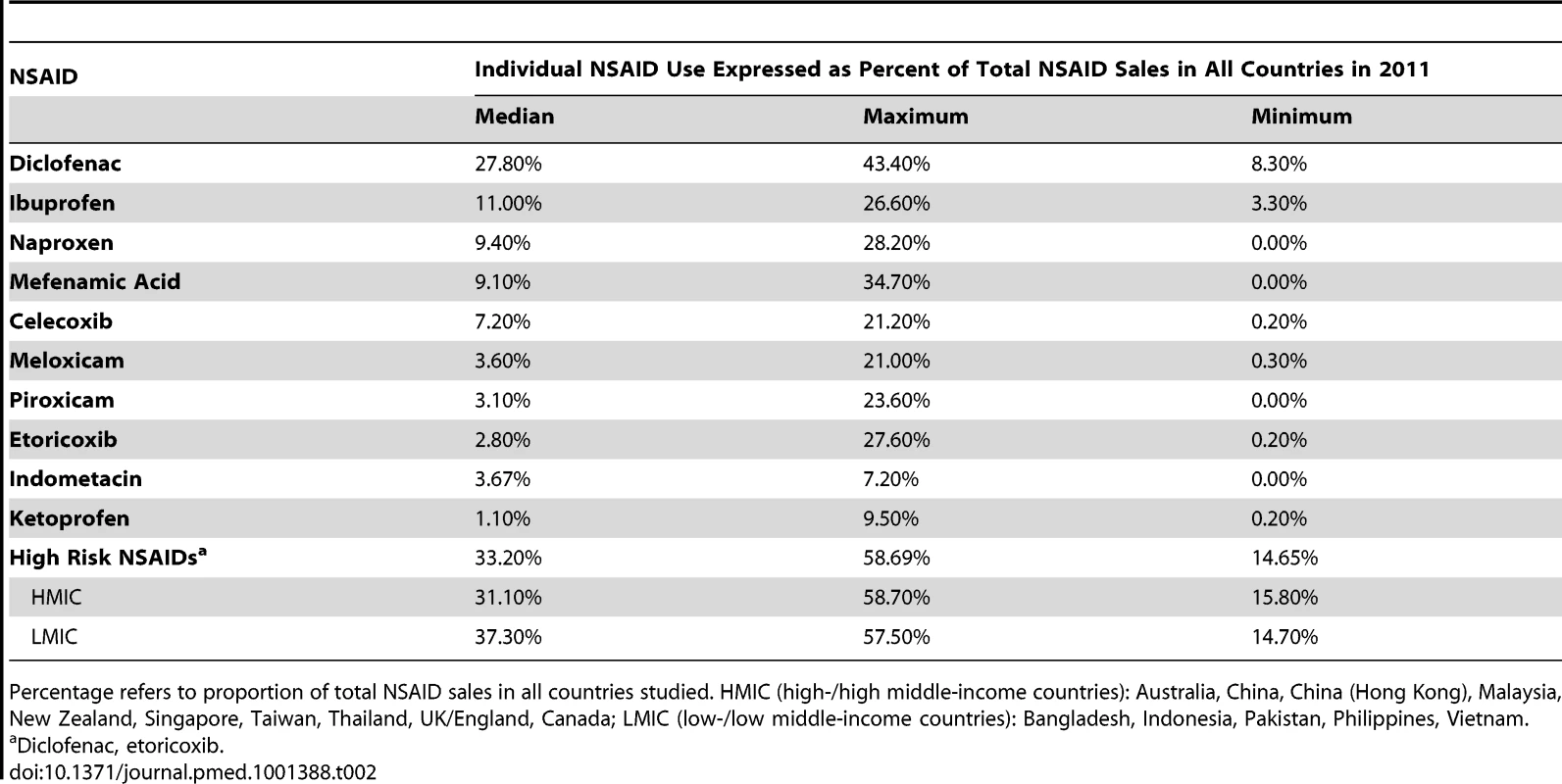
Percentage refers to proportion of total NSAID sales in all countries studied. HMIC (high-/high middle-income countries): Australia, China, China (Hong Kong), Malaysia, New Zealand, Singapore, Taiwan, Thailand, UK/England, Canada; LMIC (low-/low middle-income countries): Bangladesh, Indonesia, Pakistan, Philippines, Vietnam. Discussion
NSAIDs with a high risk of cardiovascular complications are widely used. Diclofenac and etoricoxib together account for approximately one-third of all sales of NSAIDs in the 15 countries included in our analysis. There was no difference between high - and low-income countries. Diclofenac was by far the most popular NSAID, despite having an RR identical to rofecoxib [9], which was withdrawn from world markets 8 years ago owing to cardiovascular toxicity [20]. The information on cardiovascular risk associated with diclofenac has been available to regulators, writers of guidelines and essential medicines lists, and prescribers for at least 5 years [4]–[9]. Calls have been made for its withdrawal [21]. High levels of sales as recently as 2011 suggest that none of this information has resulted in effective action. There has been a slow decline in prescription numbers in England, Australia, and Canada since 2006 (Figure S1), but it remains popular in all three countries, particularly in England where it is the single most-prescribed NSAID (Table S1). While the popularity of diclofenac in high-income countries is well known, to our knowledge this is the first report that highlights the risks associated with its dominant market position in low - and middle-income countries.
Etoricoxib is the other high risk NSAID that features in this study. While there is limited information on its cardiovascular risk, an updated meta-analysis published by us in 2011 found a doubling of cardiovascular risk compared with non-use [9]. It was significantly more harmful than ibuprofen and naproxen in pairwise comparisons. In a large head-to-head randomised clinical trial, it had an identical cardiovascular risk to diclofenac [22]. In the current study, etoricoxib accounted for 28% of NSAID sales in Singapore, and 14% in Bangladesh, Hong Kong, and Malaysia. In England, it is prescribed as often as celecoxib (Table S1), but it is not licensed in North America.
Based on meta-analyses of randomised and non-randomised studies, the greatest amount of evidence supports naproxen as the safest choice to minimize cardiovascular risk. However, it was listed in only 27 out of 86 national EMLs published or updated since 2007. In contrast, diclofenac was included on 74 of these EMLs. On average, diclofenac was used three times as frequently as naproxen. In other words, evidence on the relative cardiovascular safety of this drug has failed to translate into appropriate selection for EMLs or usage. The WHO Model List of Essential Medicines provides limited guidance for selection of NSAIDs on EMLs [15]. It includes aspirin and ibuprofen, but offers no advice on their safety or cost-effectiveness relative to each other or to other NSAIDs.
There are a number of limitations to this work. Most obviously, we do not have information on the risk profiles of patients taking NSAIDs. However, the large and consistent volumes of use of high risk NSAIDs make it very likely that these drugs are being taken by substantial numbers of individuals at high risk of serious cardiovascular events. We relied on sales data for 13 countries and prescription sales for England and Canada. Sales data provide the most comprehensive estimates capturing non-prescription and hospital use in addition to community prescribing, although coverage of all sectors was variable in our study. We could not analyse prevalence of use or dosage, and while it is possible that duration of treatment varies between individual drugs, we don't think this is likely to distort greatly the patterns we have observed in the overall sales data. Importantly, the increase in cardiovascular risk has been reported very early in the course of diclofenac treatment [9],[21].
The findings here have significant implications for public health. For instance, in China the age - and sex-standardised death rate from cardiovascular disease is estimated to be 312/100,000 for males and 260/100,000 for females [23]. Diclofenac is the most commonly used NSAID in hospitals in China. We assume community use follows a similar pattern. If it were taken by only 1% of China's population of approximately 1.3 billion annually, based on the relative risk calculations from meta-analyses it could cause 14,000 additional unintended deaths. These deaths are preventable—lower risk NSAIDs, including naproxen and low-dose ibuprofen, are widely available and are equally efficacious [24],[25]. Both are available as generic products.
There is increasing regulatory concern about diclofenac. The European Medicines Agency has just commenced (as of October 2012) a new review of its cardiovascular safety [26]. In low - and middle-income countries, national EMLs are authoritative influences on drug choice, being used as the basis for procurement of safe, cost-effective medicines for public reimbursement and to guide local medicines production [17]. NSAID recommendations on national EMLs should be based on the optimum balance of benefit and harm and give preference to low risk drugs, in particular to ibuprofen and naproxen. Diclofenac has no advantage in terms of gastrointestinal safety [11] and it has a clear cardiovascular disadvantage [9]. Given the availability of safer alternatives, diclofenac should be de-listed from national EMLs. There are strong arguments to revoke its marketing authorisations globally.
Supporting Information
Zdroje
1. GrosserT, FriesS, FitzGeraldGA (2006) Biological basis for the cardiovascular consequences of Cox 2 inhibition: therapeutic challenges and opportunities. J Clin Invest 116 : 4–15.
2. HenryD, LimL, Garcia RodriguezL, Perez GutthannS, CarsonJL, et al. (1996) Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 312 : 1563–1566.
3. Masso GonzalezE, PatrignaniP, TacconelliS, Garcia RodriguezL (2010) Variability among non-steroidal anti-inflammatory drugs in risk of upper gastrointestinal bleeding. Arth Rheum 62 : 1592–1601.
4. Hernandez-DıazS, Varas-Lorenzo, Garcıa RodrıguezL (2006) Non-steroidal anti-inflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol 98 : 266–274.
5. SinghS, WuO, LanghorneP, MadhokR (2006) Risk of acute myocardial infarction with non-steroidal anti-inflammatory drugs: a meta-analysis. Arthritis Res Ther 8: R153.
6. McGettiganP, HenryD (2006) Cardiovascular risk and inhibition of cyclo-oxygenase: a systematic review of the observational studies of selective anad non-selective inhibitors of cyclo-oxygenase. JAMA 296 : 1633–1644.
7. TrelleS, ReichenbachS, WandelS, HildebrandP, TschannenB, et al. (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: a network meta-analysis. BMJ 342: c7086.
8. KearneyP, BaigentC, GoodwinJ, HallsH, EmbersonJ, et al. (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332 : 1302–1308.
9. McGettiganP, HenryD (2011) Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 8: e1001098 doi:10.1371/journal.pmed.1001098.
10. ScheimanJM, HindleyCE (2010) Strategies to optimise treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin Ther 32 : 667–677.
11. RostomA, MuirK, DubeC, LanasA, JolicoeurE, TugwellP (2009) Prevention of NSAID-related upper gastrointestinal toxicity: a meta-analysis of traditional NSAIDs with gastroprotection and cox-2 inhibitors. Drug Healthcare Patient Safety 1 : 47–71.
12. StrandV (2007) Are COX-2 inhibitors preferable to non-selective non-steroidal anti-inflammatory drugs in patients with risk of cardiovascular events taking low-dose aspirin? Lancet 370 : 2138–51.
13. Garcia RodriguezL, TacconelliS, PatrignaniP (2008) Role of dose potency in the prediction of risk of myocardial infarction associated with non-steroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol 52 : 1628–1636.
14. DansA, NgN, VargheseC, TaiES, FirestoneR, et al. (2011) The rise of chronic non-communicable diseases in Southeast Asia: time for action. Lancet 337 : 680–89.
15. World Health Organization (2011) WHO model list of essential medicines 17th List March 2011. Available: http://whqlibdoc.who.int/hq/2011/a95053_eng.pdf. Accessed 12 September 2012.
16. World Health Organization (2012) Essential medicines selection. Available: http://www.who.int/selection_medicines/country_lists/en/index.html. Accessed 12 September 2012.
17. World Health Organization (2010) Fact sheet number 325. Medicines: essential medicines. Available: http://www.who.int/mediacentre/factsheets/fs325/en/index.html. Accessed 4 November 2012.
18. World Health Organization (2012) ATC/DDD Index 2012. Available: http://www.whocc.no/atc_ddd_index/. Accessed 12 September 2012.
19. NHS (2011) Prescription cost analysis England 2011. Available: http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions/prescription-cost-analysis-england-2011. Accessed 12 November 2012.
20. Merck (2004) Merck announces voluntary worldwide withdrawal of Vioxx. Available: http://www.merck.com/newsroom/vioxx/pdf/vioxx_press_release_final.pdf. Accessed 12 September 2012.
21. FosbolEL, FolkeF, JacobsenS, RasmussenJN, SørensenR, et al. (2010) Cause-specific cardiovascular risk associated with nonsteroidal anti-inflammatory drugs among healthy individuals. Circ Cardiovasc Qual Outcomes 3 : 395–405.
22. CannonCP, CurtisSP, FitzGeraldGA, KrumH, KaurA, BologneseJA, et al. (2006) Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 368 : 1771–1781.
23. World Health Organization (2012) NCD mortality 2008. Available: http://gamapserver.who.int/gho/interactive_charts/ncd/mortality/cvd/atlas.html. Accessed 12 September 2012.
24. DeeksJJ, SmithLA, BradleyMD (2002) Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for the treatment of osteoarthritis and rheumatoid arthritis: a systematic review of randomised controlled trials. BMJ 325 : 619–623.
25. CollinsSL, MooreRA, McQuayHJ, WiffenPJ (1998) Oral ibuprofen and diclofenac in post-operative pain: a quantitative systematic review. Eur J Pain 2 : 285–291.
26. European Medicines Agency (2012) Questions and answers on the review of non-selective non-steroidal anti-inflammatory drugs (NSAIDs) and cardiovascular risk: outcome of a procedure under Article 5(3) of Regulation (EC) No 726/20041. Available: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2012/10/news_detail_001637.jsp&mid=WC0b01ac058004d5c1. Accessed 11 November 2012.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 2- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Prevalence of Age-Related Macular Degeneration in Nakuru, Kenya: A Cross-Sectional Population-Based Study
- Socioeconomic Inequalities in Lung Cancer Treatment: Systematic Review and Meta-Analysis
- Cardiovascular Risk of NSAIDs: Time to Translate Knowledge into Practice
- Prognosis Research Strategy (PROGRESS) 3: Prognostic Model Research
- Who Should Pay for Global Health, and How Much?
- Global Estimates of Syphilis in Pregnancy and Associated Adverse Outcomes: Analysis of Multinational Antenatal Surveillance Data
- Prehospital Lactated Ringer's Solution Treatment and Survival in Out-of-Hospital Cardiac Arrest: A Prospective Cohort Analysis
- Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts
- A Reality Checkpoint for Mobile Health: Three Challenges to Overcome
- Use of Non-Steroidal Anti-Inflammatory Drugs That Elevate Cardiovascular Risk: An Examination of Sales and Essential Medicines Lists in Low-, Middle-, and High-Income Countries
- Scaling Up mHealth: Where Is the Evidence?
- Prognosis Research Strategy (PROGRESS) 2: Prognostic Factor Research
- Whole Genome Sequencing versus Traditional Genotyping for Investigation of a Outbreak: A Longitudinal Molecular Epidemiological Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Prognosis Research Strategy (PROGRESS) 3: Prognostic Model Research
- Prognosis Research Strategy (PROGRESS) 2: Prognostic Factor Research
- Whole Genome Sequencing versus Traditional Genotyping for Investigation of a Outbreak: A Longitudinal Molecular Epidemiological Study
- Prevalence of Age-Related Macular Degeneration in Nakuru, Kenya: A Cross-Sectional Population-Based Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



