-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Serious and Life-Threatening Pregnancy-Related Infections: Opportunities to Reduce the Global Burden
article has not abstract
Published in the journal: . PLoS Med 9(10): e32767. doi:10.1371/journal.pmed.1001324
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001324Summary
article has not abstract
Summary Points
-
Pregnancy-related infections are one of the leading causes of maternal mortality worldwide, with the burden falling disproportionately on low - and middle-income countries.
-
Such infections can be categorized into four different syndromes occurring at distinct times during pregnancy: puerperal sepsis, septic abortion, pyelonephritis/urosepsis, and rapidly progressive soft tissue infections.
-
Bundled packages of interventions targeted at these different syndromes should be integrated into antenatal care at two time points: initiation of antenatal care and onset of labor.
-
Affordable, point-of-service diagnosis and treatment, coupled with improved descriptive and microbiologic data, are urgently needed to further reduce deaths from pregnancy-related infections.
Introduction
Infection is an important, potentially preventable, and yet often overlooked cause of maternal mortality and morbidity as well fetal and neonatal well-being. Puerperal sepsis, narrowly defined by the World Health Organization (WHO) as infection of the genital tract occurring any time between the rupture of membranes or labor and the 42nd day postpartum, is the third leading cause of maternal mortality, responsible for 10%–12% of maternal deaths [1]. Deaths due to puerperal sepsis disproportionately occur in low - and middle-income countries (LMICs). The risk of death from puerperal sepsis is 2.7-fold higher in Africa, 1.9-fold higher in Asia, and 2.1-fold higher in Latin America than in developed countries [2].
However, the impact of infection during pregnancy upon maternal, fetal, and neonatal mortality and morbidity is much greater than that attributed to puerperal sepsis alone—the impact includes deaths and disability from other infections such as urinary tract, soft tissue, and abortion-related infections. Septic abortion, for example, is a significant contributor to maternal infectious mortality, responsible for about 50,000 maternal deaths annually [3]. Intrauterine infection is a risk factor for postpartum endometritis (PPE) as well as a significant contributor to preterm birth, which is now recognized as the second biggest cause of newborn deaths [4]. The burden of pregnancy-related infections goes far beyond puerperal sepsis alone. A useful term that incorporates all of these different infections is “life-threatening pregnancy-related infections,” and we use this term in our article.
This Policy Forum article aims to highlight opportunities for screening and appropriate treatment of life-threatening pregnancy-related interventions. Our policy recommendations are based on a review of the published literature on the disease burden associated with life - threatening pregnancy-related infections and the most common causal pathogens in LMICs. In reviewing maternal infections, when they occur in pregnancy, and their associated pathogens, we sought to identify appropriate strategies for detecting infections and providing effective interventions to help reduce the burden of these mostly preventable maternal, fetal, and neonatal deaths and disabilities. We pay particular attention to Africa and Asia, two regions that together account for 80% of global maternal mortality and where puerperal sepsis accounts for 10%–12% of all maternal deaths [5].
Infections not related specifically to pregnancy (e.g., HIV, curable sexually transmitted infections, tuberculosis, and malaria) are also important contributors to maternal mortality and morbidity. These important non-pregnancy-related infections are beyond the scope of “life-threatening pregnancy-related infections” included in our analysis here, but they indirectly contribute to puerperal sepsis. For example, the risk of puerperal sepsis is increased among women with HIV [6], while chlamydial and gonoccocal infections are important causes of septic abortion [7]. Maternal infections, both pregnancy-related and non-pregnancy-related, remain an important and potentially preventable cause of maternal mortality, especially in LMICs. The review methods are summarized in Figure 1.
Fig. 1. Literature review strategy. 
Relevant studies from the years 1980 to 2012 were identified by searching PubMed, Medline, Embase, and WHO publications. The search strategy used was “([infectious syndrome]) AND (Africa OR Asia OR India OR Thailand OR Bangladesh)”; the infectious syndromes included in our search were those that cause the most morbidity and mortality: puerperal sepsis (PPE, chorioamnionitis), septic abortion, pyelonephritis or urosepsis, and soft tissue infections (necrotizing fasciitis, group A streptococcal infection, and methicillin-resistant staphylococcal infection). All titles and abstracts were reviewed, and those including etiology, prevalence, or pregnancy were selected for further analysis. Distinct Maternal Infectious Syndromes
Our review identified four clinical syndromes, and microbes associated with them, that appear to be responsible for most cases of life-threatening pregnancy-related infections in LMICs: (1) puerperal sepsis (chorioamnionitis and PPE), (2) pyelonephritis/urosepsis, (3) septic abortion, and (4) skin and soft tissue infections (SSTIs) (Table 1). Each of these occurs at a distinct time during pregnancy, providing opportunities for screening and prevention, which we discuss further below (Figure 2; Table 2). Although we found only 55 studies that included microbial culture results, many of which had design weaknesses (e.g., inadequate culture techniques, inappropriate culture sites), we identified several commonalities between the four syndromes discussed above that could be exploited to develop an effective intervention strategy. A consistent pattern of genital-urinary pathogens associated with the four syndromes discussed above was identified that was similar to that of pathogens reported in high-income countries (Table 1). Most serious maternal infections were polymicrobial and involved both facultative and anaerobic bacteria indigenous to the lower genital tract or rectum, with the exception of pyelonephritis/urosepsis, which was associated with a single pathogen in most cases, usually Escherichia coli. Sexually transmitted pathogens, including Neisseria gonorrhoeae and Chlamydia trachomatis, were important pathogens in septic abortion.
Fig. 2. Timing of four clinical syndromes and their pathogens, and potential opportunities for “bundled” diagnostic–therapeutic interventions. 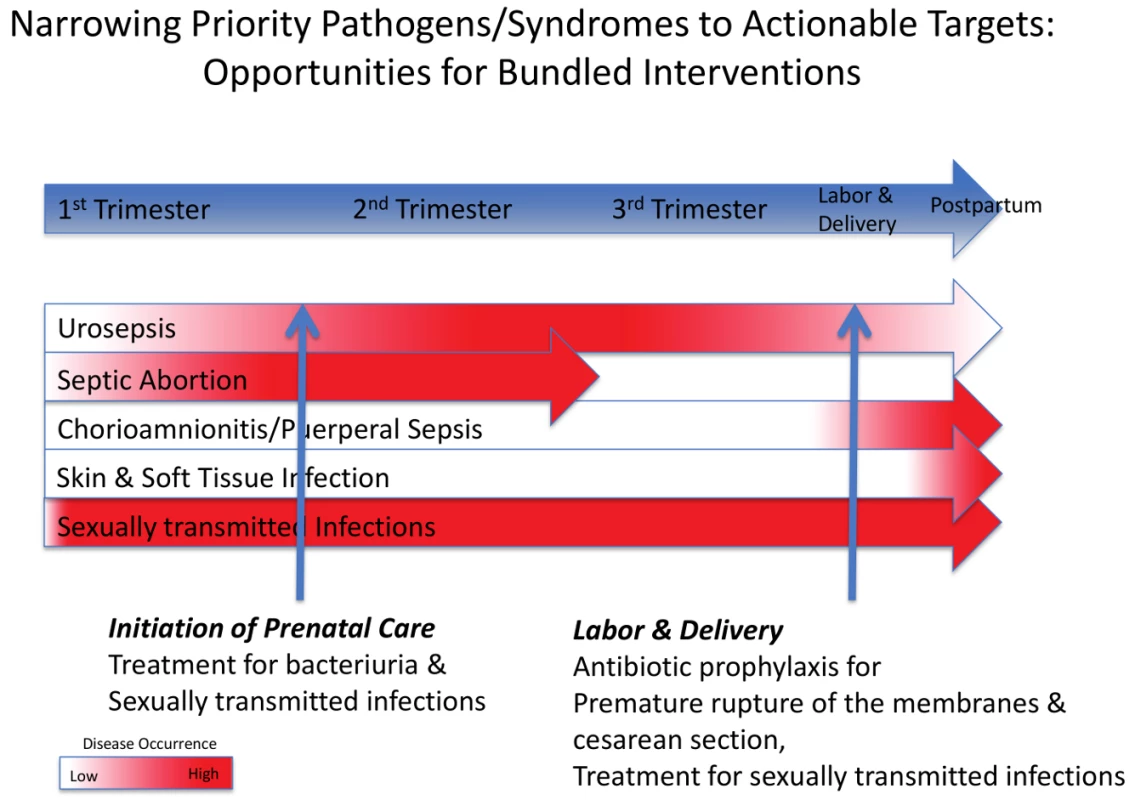
See Table 2 for further details of the interventions. Tab. 1. Common syndromes associated with life-threatening maternal infections and the pathogens most frequently associated with these. 
Tab. 2. Interventions for reducing serious morbidities and mortalities from pregnancy-related infections. 
pPROM, preterm premature rupture of membranes; PROM, premature rupture of membranes. Diagnosis and management of these infections in LMICs is based upon recognition of the clinical syndrome because culture is often not available and treatment cannot be delayed while waiting for culture results. This syndromic approach to managing pregnancy-related infections relies on early recognition of clinical signs and symptoms and the ability to aggressively intervene when infection is suspected. The WHO Integrated Management of Pregnancy and Childbirth guidelines [8] (provided in Text S1) outline diagnostic and treatment options relevant to LMICs.
Puerperal Sepsis
The reported incidence of puerperal sepsis in developing countries ranges from 0.1% to 10%, but the true incidence is difficult to determine [1]. The wide incidence range likely reflects discrepancies in diagnostic criteria, lack of access to health care, and incomplete case ascertainment. Case fatality rates for puerperal sepsis as high as 30% to 50% have been reported in LMICs [9].
Most puerperal sepsis in reviewed reports occurred as two distinct clinical syndromes: (1) intrapartum uterine infection preceding or during labor (clinical chorioamnionitis), and (2) early postpartum infection following birth (PPE), as summarized in Table 3. These infections usually represent ascending infections from the lower genital tract. They share a common microbial etiology, many risk factors, and clinical features (fever and uterine tenderness), and both are associated with neonatal infectious sequelae. Chorioamnionitis is a common complication of pregnancy that may lead to serious adverse maternal, fetal, and neonatal outcomes. Chorioamnionitis is usually the result of ascending polymicrobial infection from the lower genital tract into the chorioamnion. The resulting infection triggers a maternal and fetal inflammatory response and may lead to infection of the fetus, premature rupture of the membranes, preterm labor, and neonatal complications. PPE is an infection of the uterus that occurs in 5% of all vaginal births and 10% of cesarean deliveries in high-income countries [10], but is inconsistently reported in LMICs. PPE is usually caused by ascending infections from bacteria indigenous to the lower genital tract flora, although exogenous, sexually transmitted microorganisms including N. gonorrhoeae and C. trachomatis may also cause PPE [11]. Cesarean section is an important risk factor for PPE.
Tab. 3. Puerperal sepsis: chorioamnionitis. 
HICs, high-income countries; GBS, group B streptococcus; IAI, intra-amniotic infection; PROM, premature rupture of membranes. Pyelonephritis/Urosepsis
Acute pyelonephritis is one of the most common severe complications of pregnancy, occurring in 1%–2% of all pregnancies [12] and may result in serious maternal and fetal morbidity if not diagnosed and treated appropriately. Pregnant women are at increased risk of acute pyelonephritis due to normal anatomic and physiologic changes that occur during pregnancy and make the urinary tract system more vulnerable to infection. Pyelonephritis occurs when bacteria normally present in the vagina and distal urethra ascend the urethra and colonize the upper urologic system [13],[14]. Asymptomatic bacteriuria (ASB) commonly precedes pyelonephritis, and screening and treatment of ASB is a recommended and effective practice to prevent the development of pyelonephritis in pregnancy [15]. Table 4 summarizes the risk factors, complications, and etiology of pyelonephritis.
Tab. 4. Pyelonephritis/urosepsis. 
HICs, high-income countries. Septic Abortion
WHO estimates that 21.6 million unsafe abortions take place every year, with 98% of these occurring in developing countries, where abortion is either illegal or accessibility to legal abortions is limited [3]. Unsafe abortion is a major contributor to maternal mortality, accounting for 68,000 maternal deaths worldwide, 5 million hospital admissions, and 1.7 million cases of secondary infertility in developing countries each year [3]. Septic abortion is a common complication of unsafe abortion. Septic abortion has been reported to be responsible for up to 88% of complications from unsafe abortions [16] and is responsible for most deaths due to abortion complications in LMICs [17]. The risk of septic abortion increases as gestation progresses. Many cases go unreported or are diagnosed too late because women are reluctant to seek care following clandestine abortions. In LMICs, sexually transmitted pathogens, especially N. gonorrhoeae and C. trachomatis, are an important cause of septic abortion (see Table 5 for other risk factors and complications). When women do seek a provider before or after an abortion, providers should take advantage of the opportunity to intercept women for screening and treatment of infections.
Tab. 5. Septic abortion. 
HICs, high-income countries. Skin and Soft Tissue Infections
SSTIs are a rare complication of pregnancy, but are associated with high mortality and morbidity. SSTIs are classified as either uncomplicated or complicated. Uncomplicated SSTIs are superficial infections such as simple abscesses, mastitis, and cellulites, and are associated with a low risk of life - or limb-threatening complications. Complicated SSTIs, including streptococcal cellulites, clostridial myonecrosis, and necrotizing fasciitis, are characterized by involvement of deep soft tissue and are associated with a high risk of life - or limb-threatening complications that typically require surgical intervention [18] (Table 6). Many SSTIs originate at infected wound sites following surgical procedures or traumatic vaginal births.
Tab. 6. Complicated SSTIs (necrotizing fasciitis, cellulites, and myositis) during pregnancy. 
HICs, high-income countries. Discussion
Our landscape review found significant gaps in knowledge about the burden, etiology, and diagnosis of maternal life-threatening infections and about how best to prevent and treat these infections in women in low-resource settings. There are few reliable epidemiologic data from LMICs about the incidence of life-threatening pregnancy-related infections and their microbial etiology. Many births occur at home or in community clinics, which may lead to ascertainment bias that may overestimate maternal infections (e.g., individuals are disproportionately referred to facilities and infections are then reported) or underestimate them (e.g., individuals die at home or infections go unreported). Studies from LMICs usually report only those patients that either deliver in a facility or are referred to a facility because of infection.
There were also significant gaps in the descriptive microbial etiology of pregnancy-related infections in LMICs. Microbial data are lacking, or inadequately assessed, in many studies. Obtaining appropriate samples for bacterial culture is time-consuming, technically challenging, and expensive. The upper genital tract and endometrium are relatively inaccessible; cultures obtained through the cervix, a frequently reported method, are contaminated with indigenous lower genital tract microflora and may not represent upper genital tract pathogens. Anaerobic bacteria and genital mycoplasmas, both important causes of chorioamnionitis and endometritis, have not been consistently sought or identified in most studies from LMICs because of the difficulty in culturing these organisms. Treatment of life-threatening pregnancy-related infections is therefore largely empiric, based upon clinical diagnosis and syndromic management of maternal infections [8].
The lack of reliable population-based incidence estimates and accurate microbiological data hinder development of efficacious treatment and prevention programs that may be readily implemented at the community level (e.g., screening for asymptomatic bacteria). Despite the challenges in detecting and preventing pregnancy-related infections, a few meaningful commonalities and trends point toward a targeted strategy for reducing serious pregnancy-related infections.
Most notably, in our review, we identified a limited number of discrete clinical syndromes that account for most life-threatening pregnancy-related infections in LMICs, each of which tends to cluster at a certain point in gestation. This provides the opportunity for focused programs in screening and intervention across the continuum of care that lend themselves to “bundled” diagnostic–therapeutic interventions, either singly or as combinations of multiple testing and treatment regimens (Figure 2). We have recognized two such points for bundled interventions that target maternal infections and coincide with when a pregnant woman might seek health care: the initiation of prenatal care and the onset of labor. These two time points represent the periods of highest disease occurrence for life-threatening maternal infections, and therefore the times when screening, diagnosis, and treatment are most critical.
Women should be screened for ASB and curable sexually transmitted infections at the time of their first antenatal visit to prevent pyelonephritis and reduce septic abortion. Screening and treatment of ASB by urinalysis or culture at the first prenatal contact substantially reduces the risk of subsequent pyelonephritis and is recommended for all pregnant women. Screening for N. gonorrhoeae and C. trachomatis at the first prenatal visit or prior to a scheduled abortion has been shown to reduce post-abortion infections in developed countries [11]. Simple bundled interventions during labor and delivery also afford the opportunity to reduce maternal infections. A single dose of prophylactic antibiotics given at the time of cesarean delivery reduces the risk of PPE. Similarly, prophylactic antibiotics given following preterm premature rupture of the membranes reduces maternal and neonatal infectious morbidity in developed countries and should also be efficacious in LMICs. Finally, although rapidly progressive soft tissue infections occur sporadically, and do not lend themselves to a systematic preventive strategy, education of providers and patients as part of a bundled package at delivery may lead to earlier recognition and prompt treatment, with improved survival.
To reduce the burden of life-threatening pregnancy-related infections, significant limitations in our knowledge and delivery of care need to be directly addressed. There is a great need for comprehensive studies in LMICs exploring the epidemiology, risk factors, and microbiology of life-threatening maternal infections. These studies should include infections occurring at the level of the community and household, where most births occur, as well as facility-based programs. The lack of readily available microbiologic data points to the need for rapid, reliable point-of-care adjunctive diagnostic tests. The attributes of an ideal diagnostic test have been outlined by WHO as an acronym, ASSURED, standing for affordable, sensitive, specific, user-friendly, robust and rapid, equipment-free, and deliverable to those in need [19]. Except for detection of bacteriuria and detection of certain sexually transmitted infections, tests meeting these criteria do not currently exist for life-threatening maternal infections.
Regional differences in pathogens and antimicrobial susceptibility and resistance patterns should be specifically addressed in high-burden LMICs. Ongoing microbiologic surveillance surveys are also necessary to identify shifts in microbial flora or emergence of microbial resistance. Without such data, women will continue to be treated inappropriately and experience potentially preventable mortality and morbidity.
Supporting Information
Zdroje
1. Hussein J, Walker L (2010) Puerperal sepsis in low - and middle - income settings: past, present and future. In: Kehoe S, Neilson JP, Norman JE, editors. Maternal and infant deaths: chasing Millennium Development Goals 4 and 5. London: RCOG Press.
2. KhanKS, WojdylaD, SayL, GulmezogluAM, Van LookPF (2006) WHO analysis of causes of maternal death: a systematic review. Lancet 367 : 1066–1074.
3. World Health Organization Department of Reproductive Health and Research (2011) Unsafe abortion: global and regional estimates of incidence of unsafe abortion and associated mortality in 2008, 6th edition. Geneva: World Health Organization.
4. World Health Organization (2012) Born too soon: the global action report on preterm birth. Geneva: World Health Organization.
5. HoganMC, ForemanKJ, NaghaviM, AhnSY, WangM, et al. (2010) Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 375 : 1609–1623.
6. ZvandasaraP, HargroveJW, NtoziniR, ChidawanyikaH, MutasaK, et al. (2006) Mortality and morbidity among postpartum HIV-positive and HIV-negative women in Zimbabwe: risk factors, causes, and impact of single-dose postpartum vitamin A supplementation. J Acquir Immune Defic Syndr 43 : 107–116.
7. RahangdaleL (2009) Infectious complications of pregnancy termination. Clin Obstet Gynecol 52 : 198–204.
8. World Health Organization, United Nations Population Fund, United Nations Children's Fund, World Bank (2003) Managing complications in pregnancy and childbirth: a guide for midwives and doctors. Geneva: World Health Organization.
9. Dolea C, Stein C (2003) Global burden of maternal sepsis in the year 2000. Geneva: World Health Organization.
10. DuffP (1986) Pathophysiology and management of postcesarean endomyometritis. Obstet Gynecol 67 : 269–276.
11. BlackwellAL, ThomasPD, WarehamK, EmerySJ (1993) Health gains from screening for infection of the lower genital tract in women attending for termination of pregnancy. Lancet 342 : 206–210.
12. CunninghamFG, LucasMJ (1994) Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol 8 : 353–373.
13. MillarLK, CoxSM (1997) Urinary tract infections complicating pregnancy. Infect Dis Clin North Am 11 : 13–26.
14. PattersonTF, AndrioleVT (1997) Detection, significance, and therapy of bacteriuria in pregnancy. Update in the managed health care era. Infect Dis Clin North Am 11 : 593–608.
15. LeJ, BriggsGG, McKeownA, BustilloG (2004) Urinary tract infections during pregnancy. Ann Pharmacother 38 : 1692–1701.
16. RanaA, PradhanN, GurungG, SinghM (2004) Induced septic abortion: a major factor in maternal mortality and morbidity. J Obstet Gynaecol Res 30 : 3–8.
17. StubblefieldPG, GrimesDA (1994) Septic abortion. N Engl J Med 331 : 310–314.
18. VinhDC, EmbilJM (2005) Rapidly progressive soft tissue infections. Lancet Infect Dis 5 : 501–513.
19. PeelingRW, HolmesKK, MabeyD, RonaldA (2006) Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect 82 Suppl 5v1–v6.
20. GibbsRS, DuffP (1991) Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol 164 : 1317–1326.
21. TitaAT, AndrewsWW (2010) Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 37 : 339–354.
22. NewtonER (2005) Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol 32 : 571–600.
23. BejarR, WozniakP, AllardM, BenirschkeK, VaucherY, et al. (1988) Antenatal origin of neurologic damage in newborn infants. I. Preterm infants. Am J Obstet Gynecol 159 : 357–363.
24. GretherJK, NelsonKB (1997) Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 278 : 207–211.
25. KenyonS, BoulvainM, NeilsonJP (2010) Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev 2010: CD001058.
26. RouseDJ, HauthJC, AndrewsWW, MillsBB, MaherJE (1997) Chlorhexidine vaginal irrigation for the prevention of peripartal infection: a placebo-controlled randomized clinical trial. Am J Obstet Gynecol 176 : 617–622.
27. FaroS (2005) Postpartum endometritis. Clin Perinatol 32 : 803–814.
28. MaharajD (2007) Puerperal pyrexia: a review. Part I. Obstet Gynecol Surv 62 : 393–399.
29. SealeAC, MwanikiM, NewtonCR, BerkleyJA (2009) Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis 9 : 428–438.
30. SmaillFM, GyteGM (2010) Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev 2010: CD007482.
31. LumbiganonP, ThinkhamropJ, ThinkhamropB, TolosaJE (2004) Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV). Cochrane Database Syst Rev 2004: CD004070.
32. SaleemS, RouseDJ, McClureEM, ZaidiA, RezaT, et al. (2010) Chlorhexidine vaginal and infant wipes to reduce perinatal mortality and morbidity: a randomized controlled trial. Obstet Gynecol 115 : 1225–1232.
33. LumbiganonP, LaopaiboonM, ThinkhamropJ (2010) Screening and treating asymptomatic bacteriuria in pregnancy. Curr Opin Obstet Gynecol 22 : 95–99.
34. McGreadyR, WuthiekanunV, AshleyEA, TanSO, PimanpanarakM, et al. (2010) Diagnostic and treatment difficulties of pyelonephritis in pregnancy in resource-limited settings. Am J Trop Med Hyg 83 : 1322–1329.
35. PitukkijronnakornS, ChittacharoenA, HerabutyaY (2005) Maternal and perinatal outcomes in pregnancy with acute pyelonephritis. Int J Gynaecol Obstet 89 : 286–287.
36. SharmaP, ThapaL (2007) Acute pyelonephritis in pregnancy: a retrospective study. Aust N Z J Obstet Gynaecol 47 : 313–315.
37. GilstrapLC3rd, RaminSM (2001) Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am 28 : 581–591.
38. SchnarrJ, SmaillF (2008) Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest 38 Suppl 250–57.
39. PenneyGC (1997) Preventing infective sequelae of abortion. Hum Reprod 12 : 107–112.
40. GuleriaK, BansalS, AgarwalN, GroverV (2006) Women with septic abortion: who, how and why? A prospective study from tertiary care hospital in India. Indian J Public Health 50 : 95–96.
41. SoodM, JunejaY, GoyalU (1995) Maternal mortality and morbidity associated with clandestine abortions. J Indian Med Assoc 93 : 77–79.
42. VasquezDN, EstenssoroE, CanalesHS, ReinaR, SaenzMG, et al. (2007) Clinical characteristics and outcomes of obstetric patients requiring ICU admission. Chest 131 : 718–724.
43. KonjeJC, ObisesanKA (1991) Septic abortion at University College Hospital, Ibadan, Nigeria. Int J Gynaecol Obstet 36 : 121–125.
44. GallupDG, FreedmanMA, MeguiarRV, FreedmanSN, NolanTE (2002) Necrotizing fasciitis in gynecologic and obstetric patients: a surgical emergency. Am J Obstet Gynecol 187 : 305–310.
45. TharpeN (2008) Postpregnancy genital tract and wound infections. J Midwifery Womens Health 53 : 236–246.
46. LegboJN, LegboJF (2007) Bacterial isolates from necrotizing fasciitis: a clinico-pathological perspective. Niger J Med 16 : 143–147.
47. LegboJN, ShehuBB (2005) Necrotizing fasciitis: a comparative analysis of 56 cases. J Natl Med Assoc 97 : 1692–1697.
48. McHenryCR, AzarT, RamahiAJ, CollinsPL (1996) Monomicrobial necrotizing fasciitis complicating pregnancy and puerperium. Obstet Gynecol 87 : 823–826.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- The Double Burden of Obesity and Malnutrition in a Protracted Emergency Setting: A Cross-Sectional Study of Western Sahara Refugees
- PRISMA-Equity 2012 Extension: Reporting Guidelines for Systematic Reviews with a Focus on Health Equity
- Human Rights Research and Ethics Review: Protecting Individuals or Protecting the State?
- Serious and Life-Threatening Pregnancy-Related Infections: Opportunities to Reduce the Global Burden
- Donor Funding for Newborn Survival: An Analysis of Donor-Reported Data, 2002–2010
- The Potential Impact of Pre-Exposure Prophylaxis for HIV Prevention among Men Who Have Sex with Men and Transwomen in Lima, Peru: A Mathematical Modelling Study
- Removing the Age Restrictions for Rotavirus Vaccination: A Benefit-Risk Modeling Analysis
- Where There Is No Paramedic: The Sachigo Lake Wilderness Emergency Response Education Initiative
- Associations between Mode of HIV Testing and Consent, Confidentiality, and Referral: A Comparative Analysis in Four African Countries
- Strengthening Medical Product Regulation in Low- and Middle-Income Countries
- Bringing Clarity to the Reporting of Health Equity
- Developing a National Mental Health Policy: A Case Study from Uganda
- Psychosocial Factors That Shape Patient and Carer Experiences of Dementia Diagnosis and Treatment: A Systematic Review of Qualitative Studies
- Research Conducted Using Data Obtained through Online Communities: Ethical Implications of Methodological Limitations
- Genetic Predictors of Response to Serotonergic and Noradrenergic Antidepressants in Major Depressive Disorder: A Genome-Wide Analysis of Individual-Level Data and a Meta-Analysis
- Risk of Cardiovascular Disease and Total Mortality in Adults with Type 1 Diabetes: Scottish Registry Linkage Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Rights Research and Ethics Review: Protecting Individuals or Protecting the State?
- The Potential Impact of Pre-Exposure Prophylaxis for HIV Prevention among Men Who Have Sex with Men and Transwomen in Lima, Peru: A Mathematical Modelling Study
- Psychosocial Factors That Shape Patient and Carer Experiences of Dementia Diagnosis and Treatment: A Systematic Review of Qualitative Studies
- Genetic Predictors of Response to Serotonergic and Noradrenergic Antidepressants in Major Depressive Disorder: A Genome-Wide Analysis of Individual-Level Data and a Meta-Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



