-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Monitoring the Introduction of Pneumococcal Conjugate Vaccines into West Africa: Design and Implementation of a Population-Based Surveillance System
article has not abstract
Published in the journal: . PLoS Med 9(1): e32767. doi:10.1371/journal.pmed.1001161
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1001161Summary
article has not abstract
Summary Points
-
Routine use of pneumococcal conjugate vaccines (PCVs) in developing countries is expected to lead to a significant reduction in childhood deaths. However, PCVs have been associated with replacement disease with non-vaccine serotypes.
-
We established a population-based surveillance system to document the direct and indirect impact of PCVs on the incidence of invasive pneumococcal disease (IPD) and radiological pneumonia in those aged 2 months and older in The Gambia, and to monitor changes in serotype-specific IPD.
-
Here we describe how this surveillance system was set up and is being operated as a partnership between the Medical Research Council Unit and the Gambian Government.
-
This surveillance system is expected to provide crucial information for immunisation policy and serves as a potential model for those introducing routine PCV vaccination in diverse settings.
Introduction and Rationale
The introduction of routine immunisation with pneumococcal conjugate vaccines (PCVs) in developing countries is expected to significantly reduce childhood deaths [1]. The 7-valent pneumococcal conjugate vaccine (PCV-7), containing serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, is highly efficacious against invasive pneumococcal disease (IPD) of vaccine serotype, plus serotype 6A [2],[3]. Routine immunisation with PCV-7 has led to dramatic decreases in IPD due to pneumococci of vaccine serotype, but variable increases in IPD due to pneumococci of non-vaccine serotype [4]–[6]. Therefore, it is essential to monitor the introduction of PCVs in different settings. Demonstrating the impact of PCVs will be crucial for ongoing immunisation policy.
In 2009, the Government of The Gambia introduced routine PCV-7 vaccination into the national Expanded Programme of Immunisation (EPI) with support from the GAVI Alliance. Three doses of PCV-7 are given at 2, 3, and 4 months of age. A limited catch-up campaign gave at least two doses of vaccine to approximately 50% of children aged 2–11 months and one dose of vaccine to approximately 10% of children aged 12–23 months. PCV-13 replaced PCV-7 in April 2011 without a catch-up campaign.
The importance of pneumococcal disease in The Gambia was indicated by a 16% reduction in all-cause mortality in children 2–29 months of age in a trial of PCV-9 conducted in Upper and Central River Regions [7]. There was a 71% reduction in IPD of vaccine serotype and a 35% reduction in radiological pneumonia. The incidence of IPD and radiological pneumonia in the placebo arm was 390 per 100,000 person years and 37 per 1,000 person years, respectively. The incidence of IPD in this part of The Gambia was estimated previously to be 554 and 240 per 100,000 person years in under 1 and under 5 year-old children, respectively [7]. Neither the burden of IPD, nor the extent of the indirect (herd) effect of PCVs, are known in older children and adults in The Gambia, although PCV-7 vaccination of Gambian infants has recently been shown to reduce carriage of vaccine serotypes in older subjects [8].
Serotype coverage of IPD in The Gambia with PCV-7 and PCV-13 is approximately 30% and 70%, respectively [9]. Consequently, there is a large reservoir of pneumococci of non-vaccine serotypes available for replacement [10]. We developed a surveillance system to monitor the incidence of vaccine and non-vaccine type IPD and radiological pneumonia in those aged 2 months and older, before and after the introduction of PCVs. The data, when available, will provide key information to support PCV immunisation policy in The Gambia and elsewhere in Africa. Here we describe how this surveillance system was set up and is being operated.
Purpose
The surveillance system monitors two key outcome measures before and after the introduction of PCVs: (1) the incidence of IPD due to vaccine and non-vaccine serotypes and (2) the incidence of radiological pneumonia. Secondary aims are to monitor: (1) pneumococcal antimicrobial resistance and (2) child mortality.
Population under Surveillance
Surveillance Site
The Gambia is a small West African country of 1.5 million people. The surveillance system was established in the Upper River Region and is integrated with the local health system. The only major health centre in the Upper River Region is in the town of Basse, serving a largely rural population of approximately 191,000 people on both banks of the river Gambia. The Basse Health and Demographic Surveillance System (BHDSS) is restricted to the population on the south bank of the river (Figure 1). Five peripheral clinics in the BHDSS area provide inpatient and outpatient care and refer patients to Basse health centre.
Fig. 1. Map of catchment area for the surveillance system in The Gambia, including settlements, primary health care (PHC), and other health facilities. 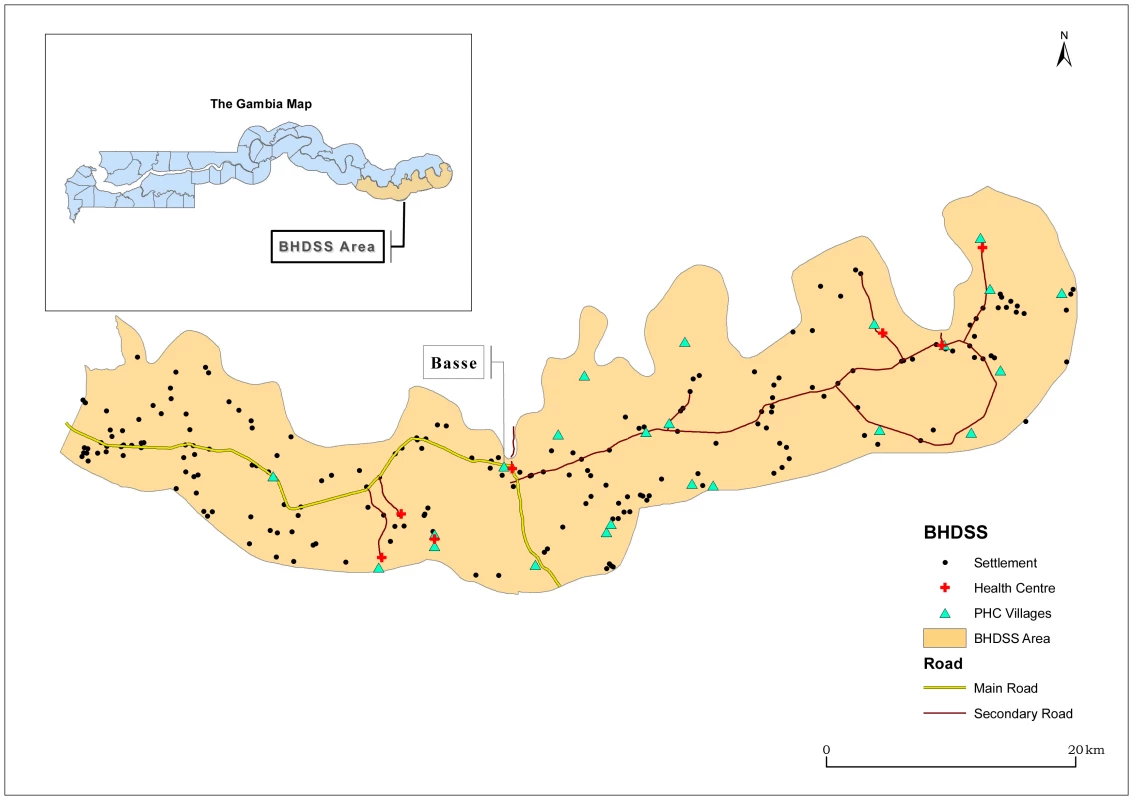
Demographic Surveillance
To provide precise estimates of the population denominator, demographic surveillance commenced in July 2007 with visits to each household every 4 months. The total population initially enumerated was 136,387. All deaths, births, in - and out-migrations, pregnancies and marriages, and the vaccination status of the children, are recorded during visits. Verbal autopsies are conducted using questionnaires designed by the INDEPTH Network (http://www.indepth-network.org). The Global Positioning System coordinates of all households have been recorded.
Sample Size Considerations
We estimated that the surveillance system would identify approximately 75 cases of IPD in children under 5 years of age per year [7], 45 of whom would be under 2 years of age, prior to the introduction of PCV-7 [3]. Using incidence data from the placebo arm of the PCV-9 trial in The Gambia, we calculated that we would have approximately 85% power to detect a 50% decrease in vaccine type IPD in under 2-year-old children over 2 years of before and after surveillance, at the 5% level of significance. We calculated that we would have over 90% power to detect a 20% reduction in radiological pneumonia in children under 2 years old and 60%–70% power to detect a 40% increase in non-vaccine type IPD. Increased power of the study has resulted from extension beyond 2 years of follow-up and the growth in the population under surveillance to over 160,000 in 2011. In addition, the introduction of the higher valency PCV-13 (containing the additional serotypes 1, 3, 5, 6A, 7F, and 19A) in mid-2011, should further reduce vaccine type IPD and radiological pneumonia. Sample size considerations assumed vaccination coverage of over 85%.
Case Definitions and Screening Criteria
Case definitions
Case definitions were developed to ensure international comparability while being measurable locally (Table 1). IPD includes any case of clinically suspected pneumonia, meningitis, or septicaemia from whom S. pneumoniae is isolated from a normally sterile site. The case definition for radiological pneumonia is aligned to the World Health Organization (WHO) consensus definition, as used previously [3],[11].
Tab. 1. Case definitions for pneumonia, meningitis, and septicaemia. 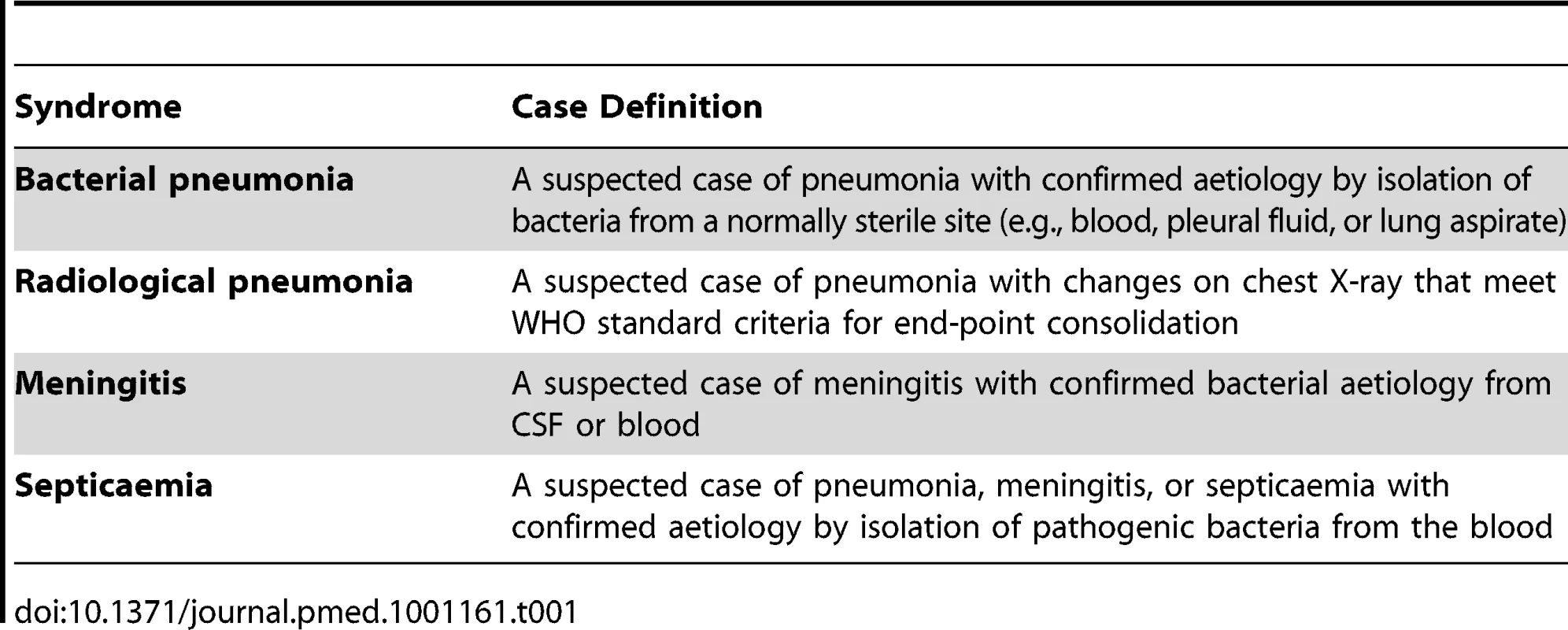
Screening criteria for nursing assessment
Figure 2 illustrates the flow from presentation of a case to formal reporting of the clinical and laboratory data. All individuals in the BHDSS population who present to health facilities at any time of day are evaluated by a dedicated surveillance system nurse to determine if referral to a surveillance system clinician at Basse is indicated. The nurses also conduct daily rounds of all inpatients to identify any cases missed at outpatient screening or who have developed criteria for referral. Criteria for referral are presented in Table 2, and these took into consideration the capabilities of local nurses. Separate criteria were developed for patients aged 2–59 months and for those 5 years and older. Patients who do not meet referral criteria proceed to evaluation and treatment by other health facility staff.
Fig. 2. Flow chart for the Gambian pneumococcal surveillance system. 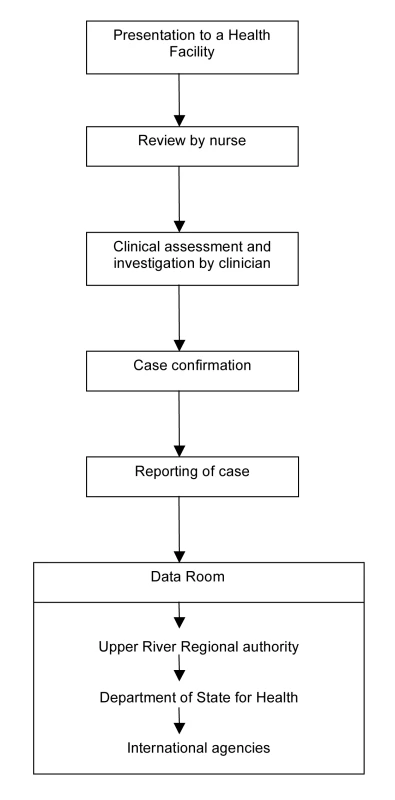
Tab. 2. Criteria developed for nurses to identify patients who should be referred to a clinician for assessment of suspected pneumonia, meningitis, or septicaemia. 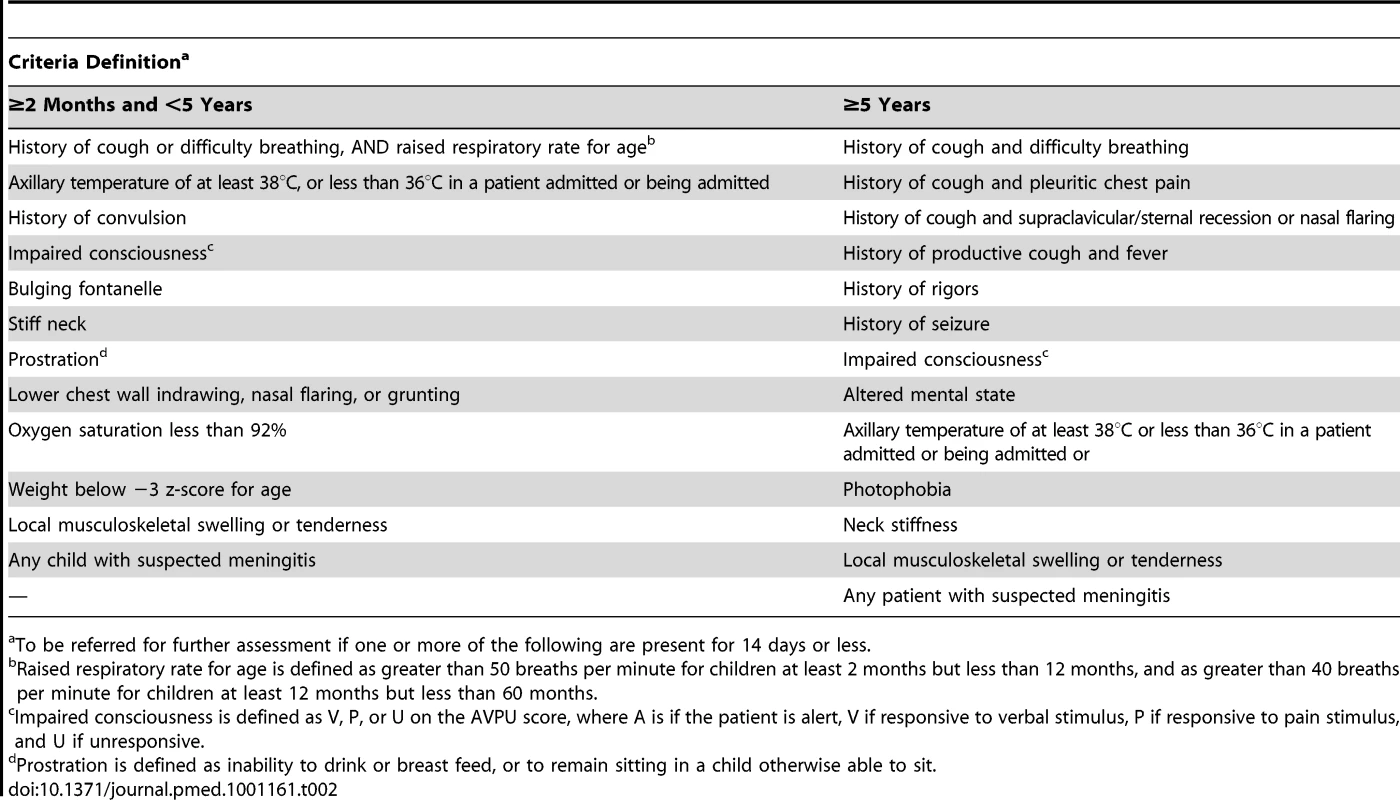
To be referred for further assessment if one or more of the following are present for 14 days or less. Screening criteria for clinical assessment
Patients referred by the nurses are evaluated by clinicians using clinical criteria for suspected pneumonia, meningitis, and septicaemia (Table 3). These were derived from WHO definitions [12] and previous studies [3],[7]. In addition, cough and/or difficulty breathing with an oxygen saturation <92% on breathing air was included as a criterion for suspected pneumonia [13]. We adapted criteria for lumbar puncture from a study of adults in Malawi [14]. For children under 5 years of age, severe malnutrition was included as an indication for blood culture [15].
Tab. 3. Clinical criteria for suspected pneumonia, meningitis, and septicaemia. 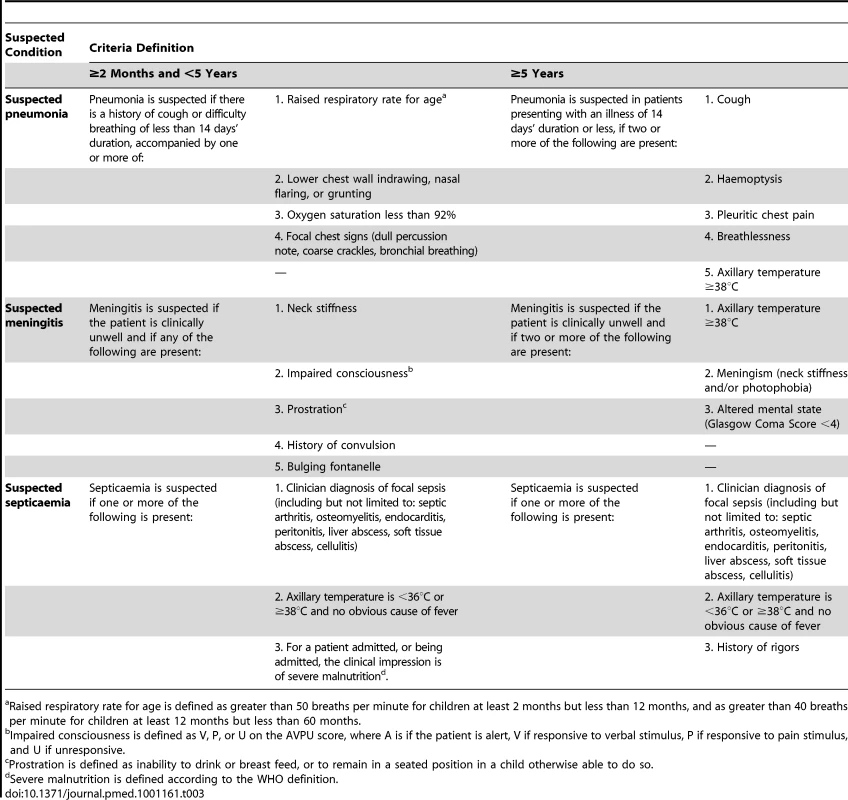
Raised respiratory rate for age is defined as greater than 50 breaths per minute for children at least 2 months but less than 12 months, and as greater than 40 breaths per minute for children at least 12 months but less than 60 months. We initially adopted a temperature criterion of <36°C or ≥38°C for referral as a suspected case of bacteraemia, following a previous study in Gambian children [16]. However, because this criterion overwhelmed the surveillance capacity during piloting, with little added yield of cases of IPD, it was applied to inpatients only. Similarly, “excessive crying” was omitted because of a low yield and a large number of inappropriate referrals.
These criteria are applied in the presence of sound clinical judgement. In particular clinicians are trained that clinical judgement plays an important role in identifying patients who might have meningitis (Table 3). Clinical assessment results in diagnoses of suspected pneumonia, meningitis, septicaemia, or combinations of the three. Standardised investigations are performed on the basis of the surveillance diagnosis. Most specimens and all X-rays are taken at Basse Health Centre. If necessary, blood cultures are collected at peripheral clinics prior to antibiotic administration and transport of the patient. Lung aspiration is undertaken on children with dense and accessible peripheral consolidation on chest X-ray after informed consent.
Implementation of the Surveillance System
Situational analysis
We conducted pre-surveillance site evaluations of clinical and laboratory facilities, including evaluation of patient referral and investigation, transport, electricity supply, staff expertise and training, and existing clinic and laboratory infrastructure. We reviewed patient flows and the systems employed for their transport between facilities.
Project management
We used a project management framework to set up the surveillance system. Figure 3 shows each line of activity as a series of dependency relationships, which were then prioritised according to estimated time to completion. We aimed to complement routine assessment and management, while strengthening existing clinical and laboratory services. We organised meetings at central, regional, and local level, and developed plans in collaboration with staff at clinics, the health centre, and the regional health office. We explained the nature and purpose of the project employing oral and visual presentations at community events and answered participants' questions.
Fig. 3. Project management for the establishment of the Gambian pneumococcal surveillance system: major dependency relationships. 
Facilities
A system was established to transport patients referred to Basse Health Centre for clinical evaluation and a separate space was established at the health centre for the assessment. A digital X-ray system was established for pneumonia surveillance. The MRC Basse laboratory performs all microbiological analyses. The MRC laboratory on the coast performs serotyping and is a WHO reference site for pneumococcal bacteriology and serotyping.
Staff and staff training
On average, four clinicians (all based in Basse) and 16 nurses (11 based in Basse) are employed on the surveillance team, along with auxiliary staff and a medically trained epidemiologist. Surveillance staff are located at each health facility serving the BHDSS population. A radiographer, trained for previous studies, was retained and employed to work on the surveillance system. Training was given to project staff on assessment of patients using surveillance criteria through oral and visual presentations and written material. Training is continuing as frequent turnover of staff, especially clinicians, is a challenge in The Gambia.
Data management and analysis
Nurses complete screening forms for each patient referred to a clinician. Clinicians complete case report forms and investigation request forms. Nurse coordinators take all these forms and specimens to the data management team and laboratory respectively (Figure 2). Specific forms have been created for microbiological analysis and serotyping. Data are double entered and backed up, and paper forms are stored securely. All laboratory and radiological information are fed back by nurse coordinators to those responsible for the patients' clinical management. Formal classification of X-rays is conducted at a later date by trained clinicians blinded to the date of investigation. The data management team produces 3-monthly reports to a steering committee that grants access to the data to investigators and others as requested. The reports include analyses of the main and secondary outcomes and the numbers of suspected and confirmed cases. Formal publication of pre - and post-vaccine introduction data will be in peer-reviewed open access journals.
Piloting
After a 1-year preparation period, we established surveillance progressively over a 7-month piloting period. During this time, we screened over 1,000 patients among whom 37 cases of IPD were identified. As a result, minor adjustments were made to the system, including to the screening criteria, forms, and standard operating procedures. Key areas for further training of staff were identified and appropriate training provided. A lack of awareness of the surveillance system among non-surveillance clinic staff was identified and addressed. Formal surveillance began on 12 May 2008.
Cost
The capital cost of establishing the system was approximately US$500,000 with annual expenditure of approximately US$1.3 million. While the surveillance system provides clinical and laboratory investigations for those presenting to health centres with suspected IPD, their treatment is undertaken by the government health system.
Ethics
While the majority of the activities of the surveillance system are simply enhanced routine care, specific patient consent is obtained for any invasive procedures (such as lung aspiration), for storage of samples for future analysis, and for secure storage of identifiable records.
Evaluation of the Surveillance System
System attributes
Table 4 shows the attributes of the system according to US Centers for Disease Control (CDC) evaluation guidelines [17]. These do not include demonstration of the cost-effectiveness of the system and no formal evaluation of this is planned. Standard operating procedures were written for all activities. Interlocking projects include studies of other pathogens and molecular characterisation of pneumococcal isolates.
Tab. 4. Pneumococcal surveillance system attributes. 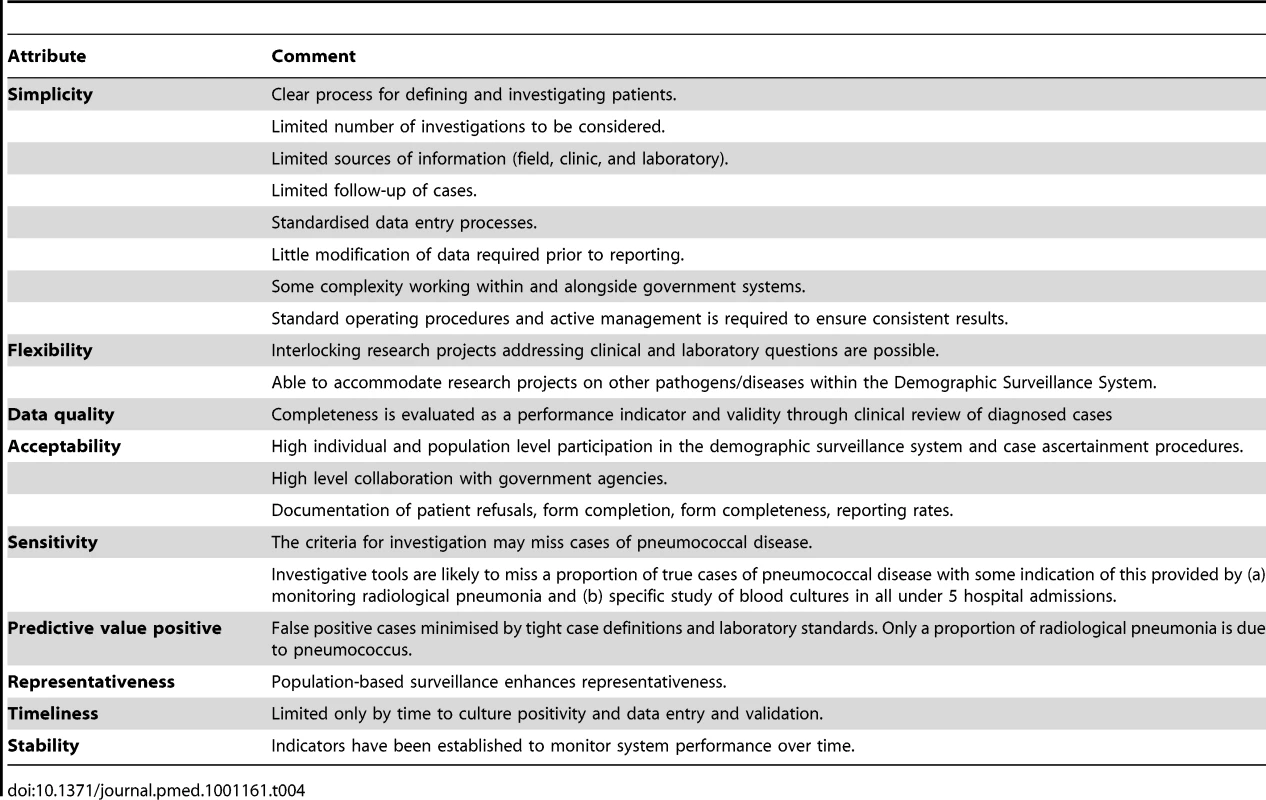
Conventional microbiology provides pneumococcal isolates for serotyping. However, blood culture has sensitivity for IPD of less than 50% [18]. While two-thirds or more of lower respiratory tract infections have a normal chest X-ray [3],[19], radiological pneumonia has been a useful primary outcome in efficacy trials [3]. A clinical diagnosis of pneumonia has been useful for estimating the preventable burden of disease [20].
Laboratory-based systems, and hospital record-based reports, are vulnerable to bias from changing practice [21]. The Gambian surveillance system monitors numbers of patients presenting and has standardised clinical, radiological, and laboratory procedures. The microbiological methods have been consistent over time ensuring data comparability between studies in The Gambia. The surveillance area is typical of large areas of sub-Saharan Africa. However, international comparisons need to take into account local characteristics such as HIV prevalence, which is estimated to be less than 2% in The Gambia.
Performance indicators
Table 5 shows the performance indicators that were developed and the methods used for measuring them [21]. Vaccine supply and delivery are evaluated as part of the assessment of vaccine effectiveness [22]. We produce regular checklists for equipment, staff, sample transport, and other indicators. We developed clinical, X-ray and laboratory log books to enhance consistency between completed forms and data entry. Regular measurement of these indicators facilitates ongoing quality control and the identification of obstacles to the smooth running of the system. Obstacles identified include turnover of staff, laboratory contamination, misunderstandings between surveillance staff and other staff at the clinics, and the logistics of referral and transport of patients. In addition, external factors, such as flooding in the wet season, can affect performance.
Tab. 5. Performance indicators for the Gambian pneumococcal surveillance system and the methods for measuring them. 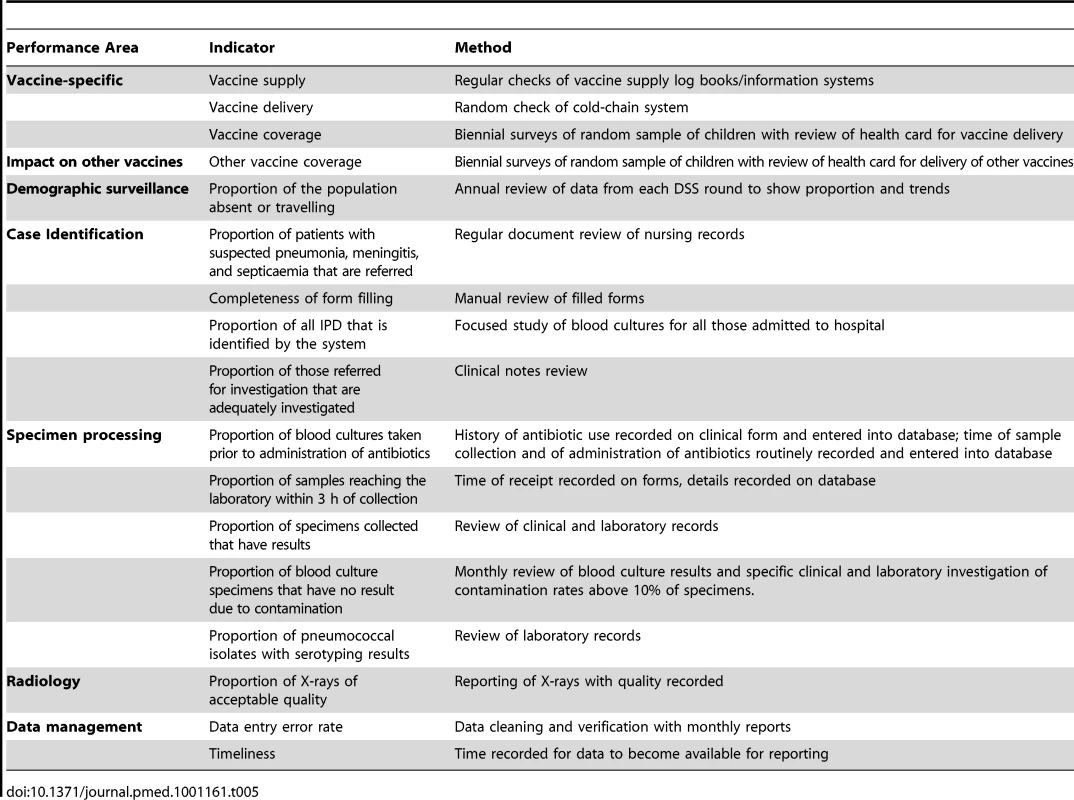
Conclusions
Our surveillance system has been established to document the direct and indirect impact of PCVs on the incidence of IPD and radiological pneumonia in The Gambia. The system is expected to provide crucial information for immunisation policy and to serve as a model for those introducing routine PCV vaccination in diverse settings. It would be helpful if a similar system could be established in a few key locations globally. Evidence of sustained reduction of IPD and radiological pneumonia due to PCVs is important to justify their introduction and ongoing use. Identification of emerging pneumococcal serotypes may also assist the design of future PCVs. Key features of a robust system include standardised case definitions and criteria for screening, investigation, and reporting. It is also important to identify whether available census information can provide reliable estimates of denominator populations for the calculation of incidence rates, in areas where there is no demographic surveillance system. Over the first 2 years of surveillance, 3,938 suspected cases of pneumococcal disease aged 2–59 months, and 707 aged 5 years and over, were screened in the Basse pneumococcal surveillance system. It is expected that the first detailed results from this project will be peer-reviewed and published in 2012 and that the project will continue until at least 2015.
Zdroje
1. O'BrienKLWolfsonLJWattJPHenkleEDeloria-KnollM 2009 Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374 893 902
2. KlugmanKPMadhiSAHuebnerREKohbergerRMbelleN 2003 A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 349 1341 1348
3. CuttsFTZamanSMEnwereGJaffarSLevineOS 2005 Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365 1139 1146
4. LehmannDWillisJMooreHCGieleCMurphyD 2010 The changing epidemiology of invasive pneumococcal disease in aboriginal and non-aboriginal western Australians from 1997 through 2007 and emergence of nonvaccine serotypes. Clin Infect Dis 50 1477 1486
5. SingletonRJHennessyTWBulkowLRHammittLLZulzT 2007 Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297 1784 1792
6. [No authors listed] 2010 Changing epidemiology of pneumococcal serotypes after introduction of conjugate vaccine: July 2010 report. Wkly Epidemiol Rec 85 434 436
7. O'DempseyTJMcArdleTFLloyd-EvansNBaldehILawrenceBE 1996 Pneumococcal disease among children in a rural area of west Africa. Pediatr Infect Dis J 15 431 437
8. RocaAHillPCTownendJEgereUAntonioM 2011 Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in The Gambia: a cluster-randomized trial. PLoS Med 8 e1001107 doi:10.1371/journal.pmed.1001107
9. AdegbolaRAHillPCSeckaOIkumapayiUNLahaiG 2006 Serotype and antimicrobial susceptibility patterns of isolates of Streptococcus pneumoniae causing invasive disease in The Gambia 1996–2003. Trop Med Int Health 11 1128 1135
10. HillPCAkisanyaASankarehKCheungYBSaakaM 2006 Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin Infect Dis 43 673 679
11. World Health Organization, Department of Vaccines and Biologicals 2001 Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. WHO/V&B/01.35. Available: http://www.who.int/entity/vaccine_research/diseases/ari/www616.pdf. Accessed 10 January 2011
12. BenguiguiYSteinF 2006 Integrated management of childhood illness: an emphasis on the management of infectious diseases. Semin Pediatr Infect Dis 17 80 98
13. JungeSPalmerAGreenwoodBMKim MulhollandEWeberMW 2006 The spectrum of hypoxaemia in children admitted to hospital in The Gambia, West Africa. Trop Med Int Health 11 367 372
14. GordonSBWalshALChapondaMGordonMASokoD 2000 Bacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonal. Clin Infect Dis 31 53 57
15. DukeTMichaelAMgoneJFrankDWalT 2002 Etiology of child mortality in Goroka, Papua New Guinea: a prospective two-year study. Bull World Health Organ 80 16 25
16. BanyaWAO'DempseyTJMcArdleTLloyd-EvansNGreenwoodBM 1996 Predictors for a positive blood culture in African children with pneumonia. Pediatr Infect Dis J 15 292 297
17. GermanRRLeeLMHoranJMMilsteinRLPertowskiCA 2001 Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep 50 1 35; quiz CE31-37
18. SahaSDarmstadtGNaheedAArifeenSIslamM 2011 Improving the sensitivity of blood culture for Streptococcus pneumoniae. J Trop Pediatr 57 192 196
19. MadhiSAKlugmanKP 2007 World Health Organization definition of “radiologically-confirmed pneumonia” may under-estimate the true public health value of conjugate pneumococcal vaccines. Vaccine 25 2413 2419
20. MadhiSAKuwandaLCutlandCKlugmanKP 2005 The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis 40 1511 1518
21. KnollMDMoisiJCMuhibFBWonodiCBLeeEH 2009 Standardizing surveillance of pneumococcal disease. Clin Infect Dis 48 Suppl 2 S37 S48
22. AdegbolaRASeckaOLahaiGLloyd-EvansNNjieA 2005 Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet 366 144 150
Štítky
Interní lékařství
Článek Trends in Compulsory Licensing of Pharmaceuticals Since the Doha Declaration: A Database AnalysisČlánek Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic Review and Meta-AnalysisČlánek A New Year at : Maintaining a Focus on the World's Health Priorities and Identifying the Gaps
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 1- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- What Will It Take to Eliminate Pediatric HIV? Reaching WHO Target Rates of Mother-to-Child HIV Transmission in Zimbabwe: A Model-Based Analysis
- Challenging Medical Ghostwriting in US Courts
- The Inadequate Treatment of Pain: Collateral Damage from the War on Drugs
- A United Nations General Assembly Special Session for Mental, Neurological, and Substance Use Disorders:
- Trends in Compulsory Licensing of Pharmaceuticals Since the Doha Declaration: A Database Analysis
- Monitoring the Introduction of Pneumococcal Conjugate Vaccines into West Africa: Design and Implementation of a Population-Based Surveillance System
- The Role of Health Systems Factors in Facilitating Access to Psychotropic Medicines: A Cross-Sectional Analysis of the WHO-AIMS in 63 Low- and Middle-Income Countries
- Trends in Resource Utilization by Children with Neurological Impairment in the United States Inpatient Health Care System: A Repeat Cross-Sectional Study
- Hitting Hotspots: Spatial Targeting of Malaria for Control and Elimination
- Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic Review and Meta-Analysis
- Effects of Two Commercial Electronic Prescribing Systems on Prescribing Error Rates in Hospital In-Patients: A Before and After Study
- A New Year at : Maintaining a Focus on the World's Health Priorities and Identifying the Gaps
- Long-Term Survival in a Large Cohort of Patients with Venous Thrombosis: Incidence and Predictors
- Ensemble Modeling of the Likely Public Health Impact of a Pre-Erythrocytic Malaria Vaccine
- Adult Mortality Attributable to Preventable Risk Factors for Non-Communicable Diseases and Injuries in Japan: A Comparative Risk Assessment
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Ensemble Modeling of the Likely Public Health Impact of a Pre-Erythrocytic Malaria Vaccine
- Adult Mortality Attributable to Preventable Risk Factors for Non-Communicable Diseases and Injuries in Japan: A Comparative Risk Assessment
- What Will It Take to Eliminate Pediatric HIV? Reaching WHO Target Rates of Mother-to-Child HIV Transmission in Zimbabwe: A Model-Based Analysis
- Challenging Medical Ghostwriting in US Courts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



