-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Setting Research Priorities to Reduce Global Mortality from Childhood Pneumonia by 2015
article has not abstract
Published in the journal: . PLoS Med 8(9): e32767. doi:10.1371/journal.pmed.1001099
Category: Guidelines and Guidance
doi: https://doi.org/10.1371/journal.pmed.1001099Summary
article has not abstract
Summary Points
-
This paper aims to identify health research priorities that could assist the rate of progress in childhood pneumonia mortality reduction globally, as set out in the United Nation's Millennium Development Goal 4.
-
The authors applied the Child Health and Nutrition Research Initiative methodology for setting priorities in health research investments. The process was coordinated by the World Health Organization.
-
Forty-five leading childhood pneumonia researchers suggested more than 500 research ideas, which were merged into 158 research questions that spanned the broad spectrum of epidemiological research, health policy and systems research, improvement of existing interventions, and development of new interventions.
-
Within the short time frame in which gains were expected globally, the research priorities were dominated by health systems and policy research topics (e.g., studying barriers to health care seeking and access, as well as barriers to increased coverage with available vaccines; and evaluating the potential to safely scale up antibiotic treatment through community health workers).
-
These were followed by epidemiological questions to identify the main gaps in knowledge (e.g., predictors of severe pneumonia that requires hospitalisation); priorities for improvement of the existing interventions (e.g., training of community health workers to recognise danger signs, refer, and treat sick children); and identifying cost reduction mechanisms for the available conjugate vaccines.
-
Among the new interventions, the greatest support was shown for the development of low-cost conjugate vaccines and cross-protective common protein vaccines against the pneumococcus.
Introduction
Pneumonia is the leading single cause of mortality in children aged less than 5 years with approximately 1.6 million children dying each year [1]. This accounts for almost one in five under-5 deaths worldwide. Furthermore, approximately 155 million new episodes of clinical pneumonia occur in children under 5 years of age annually [2]. It is estimated that 7%–13% of episodes are severe enough to be life-threatening and require hospitalisation [3]. Studies have identified Streptococcus pneumoniae, Haemophilus influenzae, and respiratory syncytial virus (RSV) as the main pathogens associated with severe childhood pneumonia [4]–[6]. Future studies, with new molecular techniques to detect infections due to a wider range of pathogens, will improve our understanding of the cause of pneumonia [7]. The leading risk factors contributing to pneumonia incidence are lack of exclusive breastfeeding, undernutrition, exposure to indoor air pollution, low birth weight, crowding, and absence of immunisation [3].
Initiatives to Control Childhood Pneumonia
The United Nation's (UN) Millennium Development Goal 4 (MDG4) states that childhood mortality should be reduced by two-thirds between 1990 and 2015, but recent estimates show that the progress in mortality reduction has been disappointing in some countries [8],[9]. Key reasons are lack of knowledge on how to implement existing cost-effective interventions and to achieve greater coverage of these interventions in low-resource settings [10], and the need to develop new effective interventions to amplify case management and immunisation strategies. In an attempt to accelerate progress in tackling childhood pneumonia, two major initiatives have been taken. A Global Action Plan for the Prevention and Control of Pneumonia (GAPP) was launched late in 2009 by the World Health Organization (WHO) and UNICEF in collaboration with other global partners, with a multitude of aims and several ongoing activities (see Box 1) [11]. The second major initiative was the successful passage of a resolution on the prevention and control of childhood pneumonia at the 2010 World Health Assembly. The resolution calls on the WHO to strengthen human resources in tackling this problem and to create an international forum to coordinate action. It calls on WHO Member States to create evidence-based and multi-sectoral action plans and to monitor progress [12].
Box 1. Global Action Plan for the Prevention and Control of Pneumonia (GAPP)
GAPP aims to increase awareness of pneumonia as a major cause of child death, calls for the scaling up of the use of the interventions of proven benefit, and provides guidance on how this can be done. The GAPP calls to action a broad coalition of global and government policy-makers, donor agencies, and civil society. GAPP recommends that every child is protected against pneumonia through a healthy environment, and has access to preventive and treatment measures. The key GAPP strategies for treating, preventing, and protecting from pneumonia are case management at all levels, vaccination, prevention and management of HIV infection, improvement of nutrition and breastfeeding, reduction of low birth weight, and control of indoor air pollution. Furthermore, pneumonia is recognised as a common and serious consequence of pandemic influenza, and preparedness for pandemic influenza should include prevention and control of pneumonia [11].
Mismatch of Pneumonia Mortality Burden and Research Investment
The positive initiatives need research and investment, but neither has been commensurate with the importance of pneumonia as the leading child killer [13]. It has been shown that the amount of available research funds per disability-adjusted life year (DALY) of pneumonia is orders of magnitude lower compared to many other diseases today [14],[15]. To assist policy-makers and donors alike in understanding the potential of different research avenues to contribute to reducing the burden of disease and disability, the Child Health and Nutrition Research Initiative (CHNRI) recently developed a methodology that allows systematic listing and transparent scoring of many competing research options, thus exposing their strengths and weaknesses [16]–[18]. The Department of Child and Adolescent Health and Development (CAH) of WHO has used this methodology to identify health research priorities to tackle all the major causes of child deaths, and some of the exercises have already been published [19]–[21]. In this paper, we present the results of the CHNRI research priority-setting process for childhood pneumonia.
Methods
The CHNRI methodology for setting priorities in health research investments was proposed to inform those who develop research policy and/or invest in health research [16]–[18]. This aims to assist policy makers to understand the full spectrum of research investment options and the potential risks and benefits that can result from investments in different research. As shown in the published CONSORT diagram [13], the CHNRI methodology has four stages: (i) input from investors/policy-makers (who define the context and the criteria for priority setting); (ii) input from a larger group of technical experts (who propose, list systematically, and then independently score many research ideas); (iii) input from other stakeholders (who agree on differential weights for the chosen priority-setting criteria according to a wider societal system of values) [16]–[18],[22]; and (iv) computation and discussion of the scores and analysis of the agreement between experts. The conceptual framework for the CHNRI methodology is shown in Figure 1. More detailed explanation has been published elsewhere [16]–[18],[22] and is also available in Table S1.
Fig. 1. CHNRI's conceptual framework showing key steps required to get from investments in health research options to decrease in burden of death, disease, or disability. 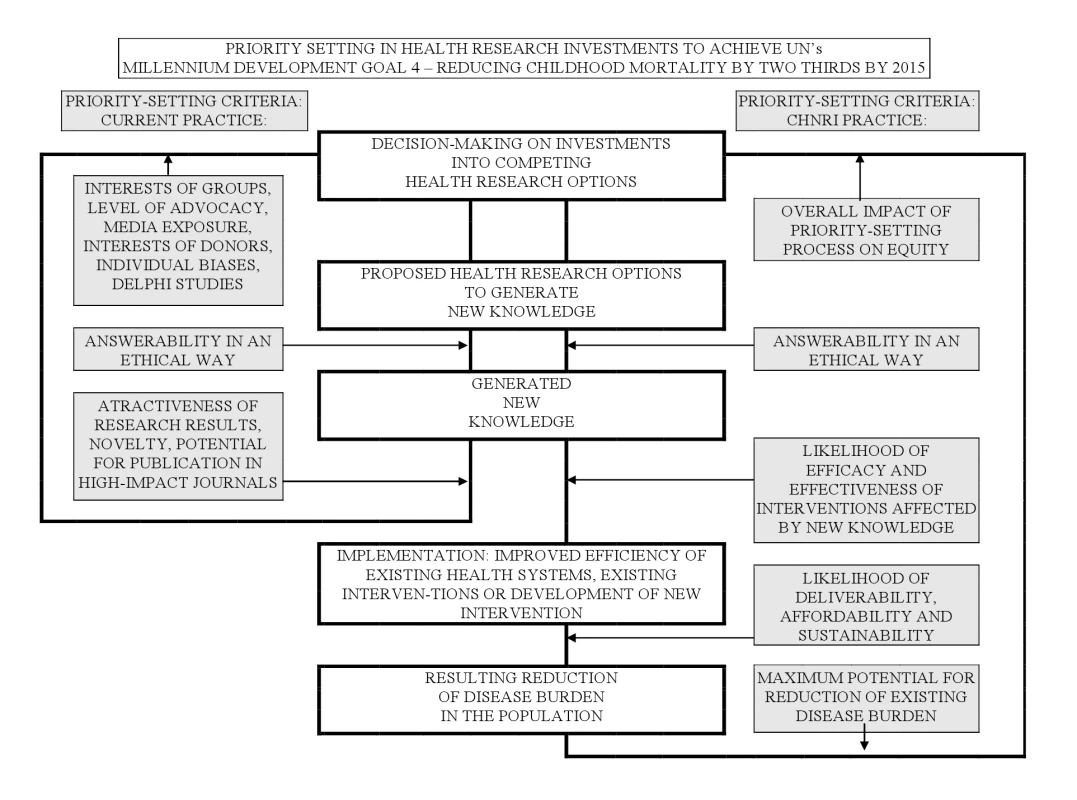
The framework identifies criteria to discriminate between likelihoods of success of competing research options: (i) answerability; (ii) effectiveness; (iii) deliverability; (iv) maximum potential for disease burden reduction; and (v) predicted impact on equity in the population (right side). These criteria are not necessarily what drives investment decisions in health research today (left side) [13],[16]–[18]. (i) Input from Investors/Policy-Makers
The WHO CAH programme coordinated a large international exercise, involving more than 200 experts from about 80 different countries, to identify health research priorities that could directly tackle the main causes of global child mortality. The aim was to inform key global donors, public investors in health research, and international agencies on research investment policies that could support efforts to accelerate the progress towards MDG4. Thus, the context for this exercise was a short-term one, set within MDG4 and requiring an urgent and rapid progress in mortality reduction from childhood pneumonia. While defining this context, the WHO also recognised the importance of context-specific issues at local or regional levels, the large problem of pneumonia morbidity, and the beneficial effects of investments in the improvement of malnutrition and other cross-cutting and cross-sectoral issues [17],[18]. Further details are provided in Table S1.
(ii) Input from Technical Experts
Individuals with a wide range of technical expertise and regional representation were recruited to participate. A large list of research questions was drafted by the technical expert group based on recent systematic reviews and a survey of experts. Initially, more than 500 questions were proposed. They were organised using the CHNRI framework for listing research questions, shown in Table S2. They were then compressed into a smaller number (158 questions) that still represented the broad spectrum of health research areas, topics, and instruments. The expert group then reviewed the questions, refining and reformulating them to allow the scoring. The final questions were sent to each technical group member for scoring. The criteria that were adopted were: (i) answerability (which captures the likelihood that each proposed research question can indeed be answered through a well designed study and in an ethical way, using the existing level of research capacity); (ii) likelihood of effectiveness; (iii) likelihood of deliverability, affordability, and sustainability; (iv) maximum potential impact on mortality reduction; and (v) predicted impact on equity. The CHNRI framework for scoring research questions is shown in Table S3 [17],[18]. Further details are provided in Table S1.
(iii) Solicited Input from Other Societal Stakeholders
The five criteria for scoring may be perceived to be of varying importance and the value given to each criterion may vary with the perspective of stakeholders. For example, parents who have experienced a pneumonia-associated death may rate mortality reduction much higher than a research funder who may value answerability, or a health system planner who may be most concerned with deliverability. Hence, CHNRI undertook an exercise to poll a wide range of stakeholders and to weight the criteria based on values assigned by these stakeholders, as described elsewhere [22]. The weights applied in this exercise are explained in detail in Table S1.
(iv) Computation of the “Research Priority Scores” and Average Expert Agreement
Completed worksheets were returned to the group coordinator. The overall research priority score (RPS) was computed as the mean of the scores for the five criteria [18], weighted according to the input from the stakeholders [22], according to the following formula:
Average expert agreement (AEA) scores were also computed for each research question as the average proportion of scorers that gave the most common answer while scoring that particular research question. This is computed for each scored research investment option as:(where q is a question that experts are being asked to evaluate competing research investment options, ranging from 1 to 15). For further details regarding the choice of methods, agreement statistics, and interpretation, see Table S1.
Results
Table 1 shows the top 10% of the 158 research questions, and Table S4 shows the complete list of ranks and scores. Both tables present the perceived likelihood that each research question will comply with each of the five chosen priority-setting criteria. Research questions from all four broad research domains (epidemiological research; health systems and policy research; research to improve the existing interventions; and research to develop new interventions) feature in the top 10% research questions. When the 30 questions with highest overall scores are considered (see Table S4), there is a predominance of research questions from the domain of “epidemiological research” (12/30) and health systems and policy research (8/30), while a smaller number came from the domain of “research to improve the existing interventions” (6/30) and “research to develop new interventions” (4/30). These results reflect the context of the exercise, i.e. expectation of short to medium term impact, within 5–10 years. This short time frame benefited epidemiological questions to assess and confirm the value of existing and available cost-effective interventions; health systems and policy research to identify key obstacles to delivery of those interventions on a larger scale; and optimising the use of those interventions (alone or in combination) in different contexts. The highest ranked questions address issues related to improving current case management and immunisation interventions, including systems-based approaches. The highest ranked issue is the study of barriers to care-seeking, an issue that is rarely given high funding priority by international agencies.
Tab. 1. The top 10% of research questions according to their achieved research priority score (RPS), with average expert agreement (AEA) related to each question. 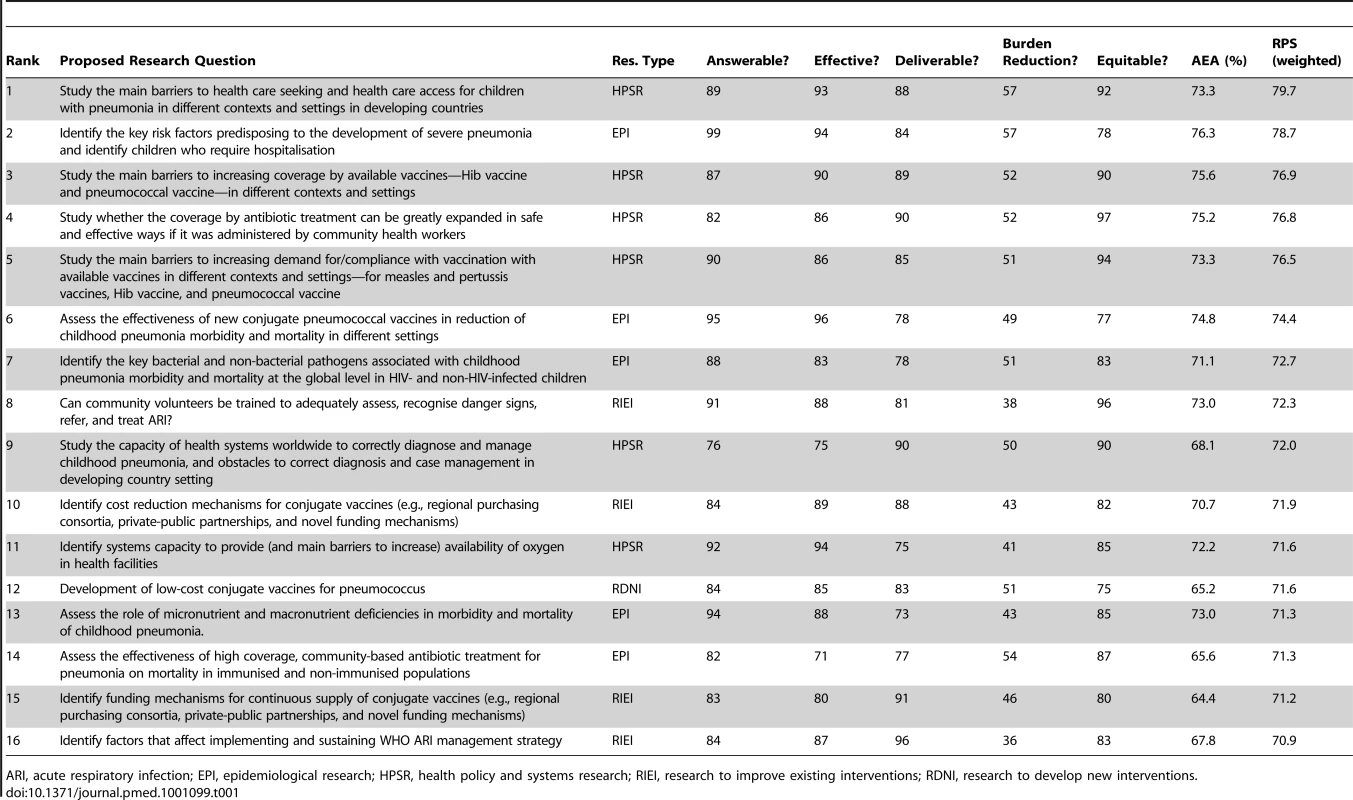
ARI, acute respiratory infection; EPI, epidemiological research; HPSR, health policy and systems research; RIEI, research to improve existing interventions; RDNI, research to develop new interventions. Research questions seeking to develop new interventions had only four representatives among the 30 highest ranked questions. This is not surprising given the short specified time frame (i.e., 5–10 years) by when it is really difficult to envisage new interventions that could have substantial impact. The four ideas that were strongly encouraged by the experts were development of: (i) low-cost conjugate vaccines for pneumococcus; (ii) low-cost cross-protective common protein vaccines for pneumococcus; (iii) combination vaccine against common bacterial pathogens of acute lower respiratory infections (ALRIs); and (iv) a new approach to culture-appropriate health education on health-seeking behaviour change. Among the bottom ranked 30 research options, the majority proposed development of entirely new interventions (20/30). In addition, six from the domain of “epidemiological research”, and four from the domain of “improvement of existing interventions” were given low priority. In the large majority of cases, the main reason for this was minimal, or entirely non-existent, optimism towards their possible impact on reduction of pneumonia within the context defined above (i.e., by 2015). This was coupled with concerns over effectiveness and deliverability of many of the proposed new interventions, such as anti-inflammatory agents, antioxidants, or other ideas, leading from fundamental molecular research of uncertain answerability and effectiveness that seeks to identify novel disease mechanisms and approaches to treatment. Another common concern was that they would be the least likely to improve equity, at least by the year 2015. For example, new interventions are very likely to be initially available only to those who can afford them.
Good discrimination between the levels of agreement among the scorers on the priority of the 158 questions was achieved by calculating AEA (Table 1; Table S4). The scores ranged from 46.7% to 76.3%, indicating the proportion of scorers that gave the most common answer to an average question they were asked in relation to a specific research investment option. AEA values are also presented for the top 10% of research questions in Table 1. Generally, the questions over which the greatest level of overall agreement was observed among the experts were those that also achieved very high overall research priority scores. The greatest points of controversy were the research questions related to development of entirely new interventions or some controversial topics (e.g. antiviral drugs, exposure to cold air, the role of air pollutants or combustion of biomass fuels, transdermal delivery of antibiotics, or genetically modified crops for improved nutrition).
The scores given to all 158 research questions from individual experts and their level of agreement for each research question are presented in Table S5. The full list of technical experts who were invited to participate, their expertise, and reasons for non-participation from those who declined are presented in Table S6. It is difficult to suimultaneously discuss strengths and weaknesses of many proposed research questions that were ranked in the middle of the list. Generally, these comprised a very broad mix of ideas of possible novel interventions and diagnostic tests of uncertain answerability and effectiveness; health policy and systems research ideas with a very limited potential impact on overall mortality reduction; support for new ideas that may not be affordable, sustainable, or improve equity; and improvements to existing interventions of uncertain deliverability and improved effect on mortality. Table S4 offers many examples of such research proposals.
The results of this exercise, which involved a substantial number of researchers active in studying the problem of childhood pneumonia, exposed how entirely different research questions can be considered research priorities depending on the criterion used. Box 2 shows the three highest scoring research questions within each of the five priority-setting criteria used. The research that would be most answerable is related to determining risk factors for severe pneumonia and referring sick children to a hospital. This question was also among those most likely to be effective, and carrying the greatest potential for disease burden reduction. Other highly answerable questions were improving the definition of an episode in a community and quantifying the problem of antibiotic resistance. The ideas that were considered most likely to be effective were studies to assess effectiveness of new conjugate pneumococcal vaccines in different contexts and studying health systems capacity to provide oxygen. The questions that would contribute to improved outreach and delivery were those studying factors that affect implementing and sustaining WHO's acute respiratory infections management strategy, studying the main barriers to increase coverage by available vaccines, and assessing the effectiveness of existing WHO treatment algorithms and guidelines. The greatest potential for disease burden reduction was assigned to research studying the main barriers to health care seeking and access, and the development of combo-vaccines against common bacterial pathogens. Research that would contribute mostly to improving equity was a study of expanded diagnosis, referral, and antibiotic treatment in a safe and effective way through community health workers' training, and evaluating culture-appropriate health education and public health messages on health-seeking behaviour change and hospitalisation.
Box 2. The Three Highest Scoring Research Questions within Each of the Five Priority-Setting Criteria (CHNRI Scores Can Range from 0 to 100)
ANSWERABILITY
-
Identify the key risk factors predisposing to the development of severe pneumonia and identify children who require hospitalisation (99/100)
-
Measure and compare the burden of pneumonia using existing WHO definition and newer alternate definitions of ALRI/clinical pneumonia that use X-ray or laboratory diagnostics and/or correct for diseases that mimic the presence of pneumonia (96/100)
-
Measure the frequency of antibiotic resistance among cases of pneumonia caused by common respiratory bacterial pathogens (96/100)
EFFECTIVENESS
-
Assess the effectiveness of new conjugate pneumococcal vaccines in reduction of childhood pneumonia morbidity and mortality in different settings (96/100)
-
Identify the key risk factors predisposing to the development of severe pneumonia and identify children who require hospitalisation (94/100)
-
Identify systems capacity to provide (and main barriers to increase) availability of oxygen in health facilities (94/100)
DELIVERABILITY
-
Identify factors that affect implementing and sustaining WHO's acute respiratory infections management strategy (96/100)
-
Study the main barriers to increase coverage by available vaccines—measles and pertussis vaccines—in different contexts and settings (94/100)
-
Assess the effectiveness of existing WHO treatment algorithms and guidelines on preventing pneumonia-related deaths, unnecessary referrals, and unnecessary antibiotic use (93/100)
MAXIMUM POTENTIAL FOR MORTALITY REDUCTION
-
Identify the key risk factors predisposing to the development of severe pneumonia and identify children who require hospitalisation (57/100)
-
Study the main barriers to health care seeking and health care assess for children with pneumonia in different contexts and settings in developing countries (57/100)
-
Development of combo-vaccine against common bacterial pathogens of ALRI (57/100)
EQUITY
-
Study whether the coverage by antibiotic treatment can be greatly expanded in a safe and effective way if it was administered by community health workers (97/100)
-
Can community volunteers be trained to adequately assess, recognise danger signs, refer, and treat acute respiratory infections? (96/100)
-
Investigate efficacy of the impact of culture-appropriate health education and public health messages on health-seeking behaviour change, hospitalisation, and mortality from childhood pneumonia (95/100)
Another sub-analysis that was allowed by the CHNRI process was evaluating the research ideas related to increased oxygen provision, which has often been a point of disagreement between donors, researchers, and implementors. Table 2 suggests that health systems research to improve availability of oxygen in health facilities and on the (cost) effectiveness of pulse oximeter technology should be given high investment priority within the short-term context; research on improving oxygen concentrator and other related technology be given medium priority; and research to define thresholds and improve user acceptability be given low priority. The exercise also illustrates the potential of this simple structured scoring system to give clear prioritisation among research options within a narrow research field and to give guidance on strengths and weaknesses of individual research questions to research policy-makers; in doing so, it limits individual biases by drawing together a larger number of experts from different backgrounds.
Tab. 2. An example of oxygen-related questions. 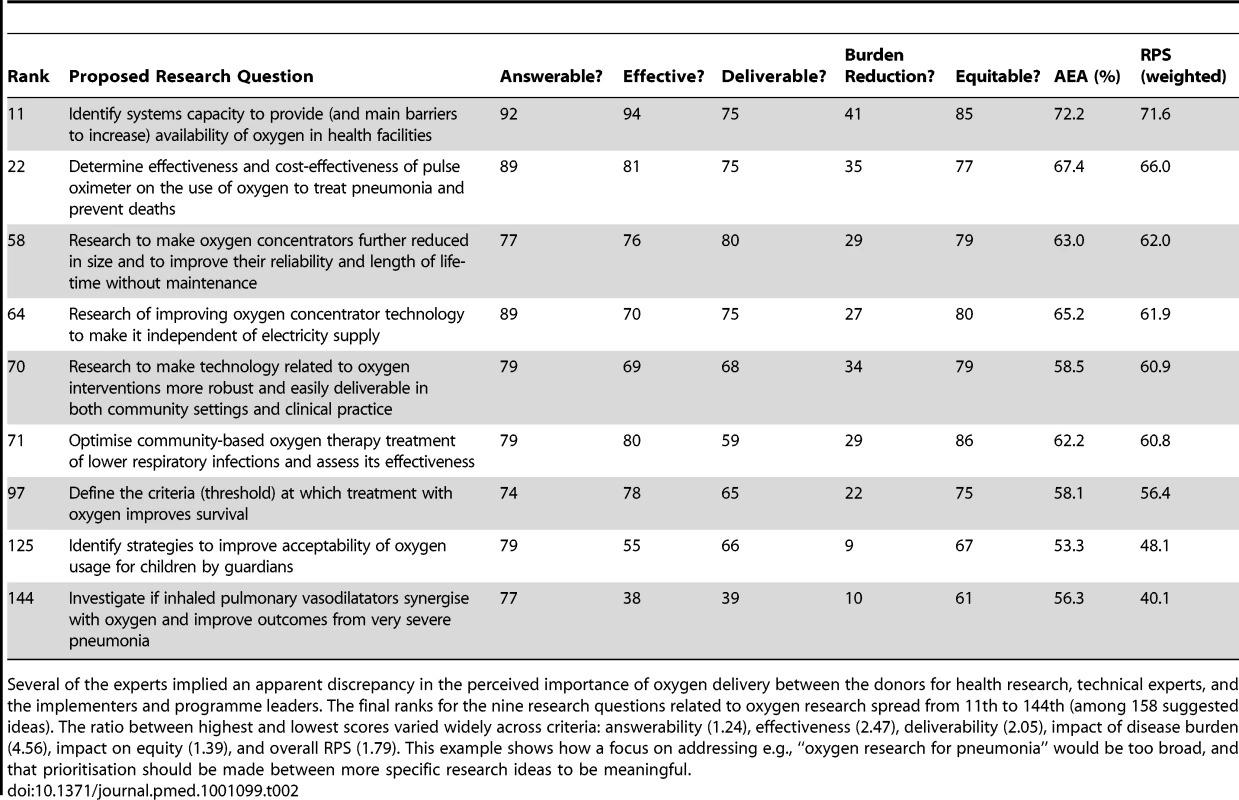
Several of the experts implied an apparent discrepancy in the perceived importance of oxygen delivery between the donors for health research, technical experts, and the implementers and programme leaders. The final ranks for the nine research questions related to oxygen research spread from 11th to 144th (among 158 suggested ideas). The ratio between highest and lowest scores varied widely across criteria: answerability (1.24), effectiveness (2.47), deliverability (2.05), impact of disease burden (4.56), impact on equity (1.39), and overall RPS (1.79). This example shows how a focus on addressing e.g., “oxygen research for pneumonia” would be too broad, and that prioritisation should be made between more specific research ideas to be meaningful. Discussion
The highest ranked questions in our priority-setting exercise address issues related to improving current case management and immunisation interventions, including systems-base approaches. This is not surprising, given that the context of the exercise was defined with a very short time frame (5–10 years), to which political commitment has been made through the support for the idea of MDGs. It is of interest that the highest ranked issue is the study of barriers to care-seeking, an issue that is rarely given high funding priority by international agencies. The process clearly showed how different research ideas can be seen as priorities based on different criteria, but also how some research questions satisfy most criteria and should represent apparent research priorities.
In this paper, we were primarily interested in research priorities that have a potential to reduce mortality from childhood pneumonia globally, thus contributing to achievement of MDG4. According to the most recent estimates, more than 99% of all pneumonia deaths occur in low - and middle-income countries. Because of this, addressing pneumonia deaths in wealthy countries by health research would not carry any potential to contribute to the main aim of our paper, and this is why the research on pneumonia in the high-income context hasn't been discussed. Furthermore, it takes a considerable amount of time to translate the outcomes of health research into interventions that, when rolled out, would indeed achieve measurable impact on the burden of any disease at the global level within a short time frame. This is why the proposed research agenda presented in Table 1 should merely be regarded as investments to accelerate progress toward the MDGs and beyond.
Towards Transparent and Systematic Priority Setting In Global Health Research
The CHNRI methodology is a serious attempt to characterise many issues in the highly complex process of research investment priority setting; however, its validity is surely imperfect. For example, some good ideas (“research investment options”) may not have been included in the initial list of research options. Some ideas might be included due to excessive media interest. The conclusions represent the opinion of a limited group of involved people. Those and other possible biases and limitations of the method are described and discussed in greater detail in Table S1. Nevertheless, the method has rapidly become the most frequently applied tool to set research priorities at all levels, because it is very cheap and practical, simple to apply via e-mail, transparent and replicable, the output is intuitive and easily understood, and it has been validated and improved through many exercises over the past several years. We believe that it is important to use systematic and transparent methods and processes, and large expert groups, to keep exposing the strengths and weaknesses of different approaches in global health research. This should keep the focus of the donors on the areas where funding is most needed, for as long as the progress in reaching MDGs becomes truly satisfactory, and prevent it from drifting into other areas for which there is a lot of new advocacy, but not much evidence. We feel that the research community has a responsibility to expose strengths and weaknesses of the many competing ideas through transparent processes, and thus to reassure both the donors and the end users of health research investments that they should persist in supporting the activities with true potential to make a difference and save lives. Thus, the main goal of this paper was not to state the obvious, but rather to expose strengths and weaknesses of many competing existing and emerging research ideas. This should reassure the broad global health community on the choices that could be concluded reasonably quickly, and lead to interventions that would be likely to demonstrate measurable impact within a shorter time frame.
A Need for Coordinated, Evidence-Based, and Equitable Research Investment Policies
The amount of funding available today for health research globally is unprecedented and the research investment market has been growing steadily over the past decade [23]. However, large inequities exist between amounts invested in different conditions that contribute to the global burden of disease. For example, while research on diabetes type 2 receives more than US$100 per DALY, research on pneumonia receives less than US$5 per DALY [14],[15],[23]. Perhaps a more pressing issue is the way in which the risk of investing in different health research domains is managed today. Long-term strategic investments in basic research, which are usually seen as highly uncertain, but also potentially highly profitable, may be justified in cases of chronic diseases, because those diseases can already be controlled by changes in diet and lifestyle and do not cause imminent threat to life. However, the situation with childhood diseases such as pneumonia and diarrhoea is quite different. Those two diseases combined continue to cause more child deaths each year worldwide than annual deaths attributable to smoking in all ages, or twice as many annual deaths as HIV/AIDS globally [13]. The persisting high mortality from pneumonia in the presence of existing cost-effective interventions and available resources to implement them represents a continuing scandal [13],[24],[25]. Given the consequences of the disease in terms of persisting child mortality, the level of urgency in dealing with this problem is very different than for other chronic diseases that contribute heavily to DALYs [13]. We believe that this should be reflected in global health research policies and investment strategies.
Investment in global health research today would benefit from consensus on the context, investment strategies, and coordination to achieve significant reduction of the disease burden in the foreseeable future—both among the investors, policy-makers, and researchers. The present exercise was designed to assist them all in making more informed choices on their investments in health research on pneumonia by exposing the risks and potential benefits associated with a broad spectrum of health research options. The expected “profit” from investments is associated with generating new knowledge that can be translated into development of new (or improvement of existing) interventions that are effective, deliverable, affordable, and can reduce the existing burden of disease and disability in an equitable way. The risk is associated with research that is not likely to satisfy some of those criteria. Investors' preference for high-risk investment in health research is particularly questionable when it is occurring in a context that requires urgent progress, such as childhood pneumonia [13]. The focus on complex challenges of implementation (i.e., improving health systems, training health workers, including poorly educated village health workers, improving drug supply and delivery at the community level, etc.), which the exercise highlighted, was reflected in many research questions being ranked near the top of the list of overall priorities.
The implementation of the CHNRI methodology showed that, within the context of MDG4, a better balance should be achieved between specific domains of health research. Along with continuing strategic long-term investments and new interventions, which represent “high risk - high-profit”, the CHNRI process suggested that more attention should also be given to health policy research, health systems research, operations research, and research that addresses political, economic, social, cultural, behavioural, and infrastructure issues surrounding the problem of child mortality. These domains of health research are rarely recognised as attractive by investors in health research because their results are unlikely to grab the headlines, get considered by journals with high impact factors, lead to patents or commercial products. Yet, they can generate new knowledge that can be exceptionally helpful in achieving real progress in mortality reduction.
This was an exercise aimed mainly at identifying research priorities to improve specific pneumonia prevention and management. If a broader policy context was more inclusive, policy research priorities to address underlying determinants (such as environment, nutrition, women's education, housing, social and political context, etc.) would surely also emerge as very important. A separate CHNRI exercise will investigate broader policies addressing underlying determinants of child health and cross-cutting issues that affect all major child diseases.
Evaluation of the Process and Further Steps
With the emergence of the CHNRI methodology, several group leaders with the WHO spotted the opportunity to conduct an inclusive and systematic exercise to define child health research priorities globally that could help accelerate the progress towards MDG4. They conducted the process from WHO headquarters in Geneva, but included hundreds of external experts globally and collected their opinions. This paper is one of the five papers that resulted from this process, which has been seen as an example of a helpful, systematic, and transparent priority-setting exercise [19]–[24]. The members of the WHO CAH-based group were eventually happy to conclude that the identified priorities were in good agreement with the research that they already support at present. They emphasised the evaluation of existing interventions and the development and testing of new delivery approaches of existing interventions. They also highlighted the value of research on preventive measures, with research on new interventions being downplayed within the short-term context. But in reality, even these “shorter term” priorites (which can have more rapid impact on mortality reduction) would still take 10–20 years to fully explore in a developing country context, and past experiences have shown that each of these top priorities would likely entail a global research programme of a decade or more to see its impact fully realised. Following the completion of the exercise, a large donor conference called “Identifying priorities for Child Health Research to achieve MDG4” was held at the WHO in Geneva on March 26–27, 2009. More than 40 donor organisations were invited to choose and support some of the identified priorities. A publication that will summarise and discuss follow-up activities is in preparation.
Conclusions
The context for this exercise was set within MDG4, requiring an urgent and rapid progress in mortality reduction from childhood pneumonia, rather than identifying long-term strategic solutions of the greatest potential. In a short-term context, the health policy and systems research to improve access and coverage by the existing interventions [25],[26] and epidemiological research to address the key gaps in knowledge [27] were highlighted as research priorities. These questions are mainly targeted at better understanding the barriers towards implementation, effectiveness, and optimisation of use of available interventions and programmes. If progress towards the reduction of global pneumonia mortality is to be improved by 2015, these are the research questions that are most likely to be of greatest importance. However, very few donors agencies recognise the importance of these domains of health research to readily invest in those options [14],[15]. The core group of CHNRI experts made several serious attempts to influence the key donors and point to this gap and serious imbalance in health research investing between long-term, strategic investments in basic research and support for instruments of health research that could contribute to mortality reduction in shorter term. This exercise, which involved much of the pneumonia research community, is the best example to date conducted at the global level.
Supporting Information
Zdroje
1. BlackRECousensSJohnsonHLLawnJERudanI 2010 Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375 1969 1987
2. RudanITomaskovicLBoschi-PintoCCampbellH 2004 Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ 82 895 903
3. RudanIBoschi-PintoCBiloglavZMulhollandKCampbellH 2008 Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 86 408 416
4. O′BrienKLWolfsonLJWattJPHenkleEDeloria-KnollM 2009 Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374 893 902
5. WattJPWolfsonLJO′BrienKLHenkleEDeloria-KnollM 2009 Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 374 903 911
6. NairHNokesDJGessnerBDDheraniMMadhiSA 2010 Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375 1545 1555
7. Johns Hopkins Center for Global Health 2011 Global health research. Project research map. Available: http://research.hopkinsglobalhealth.org/GlobalProjectDetail.cfm?project_id=8074&country_code=BG. Accessed 22 August 2011.
8. BryceJTerreriNVictoraCGMasonEDaelmansB 2006 Countdown to 2015: tracking intervention coverage for child survival. Lancet 368 1067 1076
9. MurrayCJLaaksoTShibuyaKHillKLopezAD 2007 Can we achieve Millennium Development Goal 4? New analysis of country trends and forecasts of under-5 mortality to 2015. Lancet 370 1040 1054
10. BryceJEl ArifeenSPariyoGLanataCGwatkinD 2003 Reducing child mortality: can public health deliver? Lancet 362 159 164
11. WHO 2008 Global action plan for the prevention and control of pneumonia (GAPP): report of an informal consultation. Available: http://whqlibdoc.who.int/publications/2008/9789241596336_eng.pdf. Accessed 22 August 2011.
12. World Health Assembly 2010 Counterfeit medical products. Report by the Secretariat. Document A63/26. Available: http://apps.who.int/gb/e/e_wha63.html. Accessed 22 August 2011.
13. RudanIEl ArifeenSBlackRECampbellH 2007 Childhood pneumonia and pneumonia: setting our priorities right. Lancet Infect Dis 7 56 61
14. MoranMGuzmanJRopars A-L, McDonaldAJamesonN 2009 Neglected disease research and development: how much are we really spending? PLoS Med 6 e30 doi:10.1371/journal.pmed.1000030
15. EnserinkM 2009 Some neglected diseases are more neglected than others. Science 323 700
16. RudanIGibsonJKapiririLLansangMAHyderAA 2007 Setting priorities in global child health research investments: assessment of principles and practice. Croat Med J 48 595 604
17. RudanIChopraMKapiririLGibsonJLansangMA 2008 Setting priorities in global child health research investments: universal challenges and conceptual framework. Croat Med J 49 398 408
18. RudanIGibsonJLAmeratungaSEl ArifeenSBhuttaZA 2008 Setting priorities in global child health research investments: guidelines for implementation of the CHNRI method. Croat Med J 49 720 733
19. BahlRMartinesJAliNBhanMKCarloW 2009 Research priorities to reduce global mortality from newborn infections by 2015. Pediatr Inf Dis J 28(Suppl 1) S43 S48
20. FontaineOKosekMBhatnagarSBoschi-PintoCChanKY 2009 Setting research priorities to reduce global mortality from childhood diarrhoea by 2015. PLoS Med 6 e41 doi:10.1371/journal.pmed.1000041
21. LawnJEBahlRBergstromSBhuttaZADarmstadtGL 2011 Setting research priorities to reduce almost one million deaths from birth asphyxia by 2015. PLoS Med 8 e1000389 doi:10.1371/journal.pmed.1000389
22. KapiririLTomlinsonMGibsonJChopraMEl ArifeenS 2007 Setting priorities in global child health research investments: addressing the values of the stakeholders. Croat Med J 48 618 627
23. De FranciscoAMatlinS 2006 Monitoring financial flows for health research. Geneva Global Forum for Health Research
24. BahlRBhandariNBhuttaZABiloglavZChanKY 2011 Setting research priorities to reduce global mortality from low birth weight by 2015. J Global Health. In press
25. JonesGSteketeeRWBlackREBhuttaZAMorrisSS 2003 How many child deaths can we prevent this year? Lancet 362 65 71
26. BryceJBlackREWalkerNBhuttaZALawnJE 2005 Can the world afford to save the lives of 6 million children each year? Lancet 365 2193 200
27. RudanILawnJCousensSRoweAKBoschi-PintoC 2005 Gaps in policy-relevant information on burden of in children: a systematic review. Lancet 365 2031 2040
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Cost-Effectiveness of Early Versus Standard Antiretroviral Therapy in HIV-Infected Adults in Haiti
- Cardiovascular Risk with Non-Steroidal Anti-Inflammatory Drugs: Systematic Review of Population-Based Controlled Observational Studies
- Assessing and Strengthening African Universities' Capacity for Doctoral Programmes
- Why Drug Safety Should Not Take a Back Seat to Efficacy
- Research Priorities for Mental Health and Psychosocial Support in Humanitarian Settings
- Informing the 2011 UN Session on Noncommunicable Diseases: Applying Lessons from the AIDS Response
- Strengthening the Informed Consent Process in International Health Research through Community Engagement: The KEMRI-Wellcome Trust Research Programme Experience
- Towards Improved Measurement of Financial Protection in Health
- Alcohol Consumption at Midlife and Successful Ageing in Women: A Prospective Cohort Analysis in the Nurses' Health Study
- Dissecting Inflammatory Complications in Critically Injured Patients by Within-Patient Gene Expression Changes: A Longitudinal Clinical Genomics Study
- Net Benefits: A Multicountry Analysis of Observational Data Examining Associations between Insecticide-Treated Mosquito Nets and Health Outcomes
- African Malaria Control Programs Deliver ITNs and Achieve What the Clinical Trials Predicted
- Setting Research Priorities to Reduce Global Mortality from Childhood Pneumonia by 2015
- Living Alone and Alcohol-Related Mortality: A Population-Based Cohort Study from Finland
- , , and Variants Additively Predict Response to Therapy in Chronic Hepatitis C Virus Infection in a European Cohort: A Cross-Sectional Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Living Alone and Alcohol-Related Mortality: A Population-Based Cohort Study from Finland
- Cardiovascular Risk with Non-Steroidal Anti-Inflammatory Drugs: Systematic Review of Population-Based Controlled Observational Studies
- , , and Variants Additively Predict Response to Therapy in Chronic Hepatitis C Virus Infection in a European Cohort: A Cross-Sectional Study
- Towards Improved Measurement of Financial Protection in Health
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



