-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Risk of Violent Crime in Individuals with Epilepsy and Traumatic Brain Injury: A 35-Year Swedish Population Study
Background:
Epilepsy and traumatic brain injury are common neurological conditions, with general population prevalence estimates around 0.5% and 0.3%, respectively. Although both illnesses are associated with various adverse outcomes, and expert opinion has suggested increased criminality, links with violent behaviour remain uncertain.Methods and Findings:
We combined Swedish population registers from 1973 to 2009, and examined associations of epilepsy (n = 22,947) and traumatic brain injury (n = 22,914) with subsequent violent crime (defined as convictions for homicide, assault, robbery, arson, any sexual offense, or illegal threats or intimidation). Each case was age and gender matched with ten general population controls, and analysed using conditional logistic regression with adjustment for socio-demographic factors. In addition, we compared cases with unaffected siblings.
Among the traumatic brain injury cases, 2,011 individuals (8.8%) committed violent crime after diagnosis, which, compared with population controls (n = 229,118), corresponded to a substantially increased risk (adjusted odds ratio [aOR] = 3.3, 95% CI: 3.1–3.5); this risk was attenuated when cases were compared with unaffected siblings (aOR = 2.0, 1.8–2.3). Among individuals with epilepsy, 973 (4.2%) committed a violent offense after diagnosis, corresponding to a significantly increased odds of violent crime compared with 224,006 population controls (aOR = 1.5, 1.4–1.7). However, this association disappeared when individuals with epilepsy were compared with their unaffected siblings (aOR = 1.1, 0.9–1.2). We found heterogeneity in violence risk by age of disease onset, severity, comorbidity with substance abuse, and clinical subgroups. Case ascertainment was restricted to patient registers.Conclusions:
In this longitudinal population-based study, we found that, after adjustment for familial confounding, epilepsy was not associated with increased risk of violent crime, questioning expert opinion that has suggested a causal relationship. In contrast, although there was some attenuation in risk estimates after adjustment for familial factors and substance abuse in individuals with traumatic brain injury, we found a significantly increased risk of violent crime. The implications of these findings will vary for clinical services, the criminal justice system, and patient charities.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(12): e32767. doi:10.1371/journal.pmed.1001150
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001150Summary
Background:
Epilepsy and traumatic brain injury are common neurological conditions, with general population prevalence estimates around 0.5% and 0.3%, respectively. Although both illnesses are associated with various adverse outcomes, and expert opinion has suggested increased criminality, links with violent behaviour remain uncertain.Methods and Findings:
We combined Swedish population registers from 1973 to 2009, and examined associations of epilepsy (n = 22,947) and traumatic brain injury (n = 22,914) with subsequent violent crime (defined as convictions for homicide, assault, robbery, arson, any sexual offense, or illegal threats or intimidation). Each case was age and gender matched with ten general population controls, and analysed using conditional logistic regression with adjustment for socio-demographic factors. In addition, we compared cases with unaffected siblings.
Among the traumatic brain injury cases, 2,011 individuals (8.8%) committed violent crime after diagnosis, which, compared with population controls (n = 229,118), corresponded to a substantially increased risk (adjusted odds ratio [aOR] = 3.3, 95% CI: 3.1–3.5); this risk was attenuated when cases were compared with unaffected siblings (aOR = 2.0, 1.8–2.3). Among individuals with epilepsy, 973 (4.2%) committed a violent offense after diagnosis, corresponding to a significantly increased odds of violent crime compared with 224,006 population controls (aOR = 1.5, 1.4–1.7). However, this association disappeared when individuals with epilepsy were compared with their unaffected siblings (aOR = 1.1, 0.9–1.2). We found heterogeneity in violence risk by age of disease onset, severity, comorbidity with substance abuse, and clinical subgroups. Case ascertainment was restricted to patient registers.Conclusions:
In this longitudinal population-based study, we found that, after adjustment for familial confounding, epilepsy was not associated with increased risk of violent crime, questioning expert opinion that has suggested a causal relationship. In contrast, although there was some attenuation in risk estimates after adjustment for familial factors and substance abuse in individuals with traumatic brain injury, we found a significantly increased risk of violent crime. The implications of these findings will vary for clinical services, the criminal justice system, and patient charities.
: Please see later in the article for the Editors' SummaryIntroduction
Despite evidence demonstrating an association between certain severe mental illnesses and violence [1],[2], much less is known about the relationship of neurological disorders with violent and other antisocial behaviour [3],[4]. Despite this, expert opinion has suggested that some neurological conditions increase the risk of violence. Reviews and modern textbooks assert that epilepsy is associated with violence risk [3],[5]–[8], a view widely held in the 19th century [9]. However, a recent systematic review suggested caution in drawing any conclusions about the relationship between epilepsy and violence [10], as the small evidence base is based on prisoner samples [11],[12] or individuals with childhood epilepsy [13]. For traumatic brain injury, the theoretical basis for an association with violence is much stronger. Influential case reports, such as that for Phineas Gage [14], case–control studies of war veterans who experienced frontal lobe damage [15], and case series of murderers [16] and brain injured prisoners [17] have provided some support. On the other hand, there is little evidence of causal mechanisms, and there are, for example, socio-economic differences between head injured persons and others [18]. Yet, as with epilepsy, a recent review found no published population-based or longitudinal surveys [10], and the potential role of injuries incurred in childhood is unknown [19].
There are a number of reasons why examining the association of neurological disorders with violence is potentially considerable. First, considerable stigma is associated with epilepsy [20] and traumatic brain injury [21], and accurate information on risk for adverse outcomes could be relevant in addressing this. Second, it may provide information on mechanisms underlying violence, assisting the understanding of the neurobiological basis of violent behaviour. Third, information on the prevalence and relative risks of violence and criminality could inform neurology, emergency medicine, rehabilitation medicine, and general and forensic psychiatric services in determining when violence risk assessment and management might be most valuable, based on the specific neurological disorder a patient has. Finally, the public health impact of associations between these two common neurological conditions and violence is potentially important. The prevalence of hospitalised traumatic brain injury is around 8 million in Europe [22] (0.2%–0.3% of the general population), and 0.5% of the population is estimated to suffer from epilepsy [23]. Furthermore, brain injury is one of the most common chronic conditions among prisoners [24], and the most frequent reason for presentation of individuals to emergency departments [25]. Thus, understanding associations and mechanisms could assist in improved risk assessment and management of the large numbers of individuals presenting to health services with these two disorders. In the present study, we used longitudinal total population designs to examine the relationship of epilepsy and traumatic brain injury with violent crime in Sweden from 1973 until 2009.
Methods
The Regional Ethics Committee at the Karolinska Institutet approved the study (2009/939-31/5). Data were merged and anonymized by an independent government agency (Statistics Sweden), and the code linking the personal identification numbers to the new case numbers was destroyed immediately after merging. Therefore, informed consent was not required.
Study Setting
We linked several longitudinal, nationwide population-based registries in Sweden: the National Patient Register (held at the National Board of Health and Welfare), the Crime Register (National Council for Crime Prevention), the National Censuses from 1970 and 1990 (Statistics Sweden), and the Multi-Generation Register (Statistics Sweden). The Multi-Generation Register connects each person born in Sweden in 1933 or later and ever registered as living in Sweden after 1960 to their parents [26]. For immigrants, similar information exists for those who became citizens of Sweden before age 18 y, together with one or both parents. In Sweden, all residents including immigrants have a unique ten-digit personal identification number that is used in all national registers, thus making the linking of data in these registers possible. We restricted the population to individuals born between 1958 and 1994, so that all individuals who were at least 15 y (the age of criminal responsibility in Sweden) were included from 1973 onwards, to the end of follow-up in 2009 (n = 5,665,112).
Individuals with Neurological Disorder
We identified cases with epilepsy or traumatic brain injury from the National Patient Register, which includes individuals admitted to any hospital (since 1973) or having outpatient appointments (since 2001) in Sweden [27]. Cases with epilepsy had to have at least two separate patient episodes according to International Classification of Diseases (ICD) ICD-8 (1973–1986; diagnostic codes 345.00–345.99), ICD-9 (1987–1996; codes 345J, K, L, M, N, P, Q, W, X), or ICD-10 (from 1997 onwards; codes G40.1–G40.9, G41). We decided that epilepsy had to be diagnosed at two separate occasions to increase diagnostic precision by minimising false positive diagnoses; hence, those with only one diagnosis were excluded (n = 22,084). For traumatic brain injury (not including concussion), we selected cases based on one or more patient episodes according to ICD-8 (1973–1986; diagnostic codes 851–852), ICD-9 (1987–1996; codes 851–854), or ICD-10 (from 1997 onwards; codes S06.01–S06.09). We also investigated comorbidity with drug and alcohol use disorders. Data were also extracted for every individual on all inpatient (1973–2009) and outpatient (2001–2009) diagnoses with principal or comorbid diagnoses of alcohol abuse or dependence (ICD-8 : 303; ICD-9 : 303, 305.1; ICD-10: F10, except x.5) or drug abuse or dependence (ICD-8 : 304; ICD-9 : 304, 305.9; ICD-10: F11–F19, except x.5). This information was used as a marker for comorbid alcohol and/or drug abuse disorders.
Diagnostic Validity
Swedish patient register data on diagnoses have good to excellent validity for a range of conditions, such as acute myocardial infarction [28], injuries [29], acute stroke [30], Guillain-Barré syndrome [31], and schizophrenia [32],[33]. Overall, the positive predictive value of the inpatient register, in a recent review, was found to be 85%–95% for most diagnoses [34]. Little information exists on diagnostic validity of comorbid conditions in neurological disorders. However, fair to moderate agreement for comorbid substance abuse has been found in schizophrenia (κ of 0.37, standard error = 0.23, p<0.001, corresponding to 68% full agreement) [35]. Only around 1% of hospital admissions have missing personal identification numbers [36]. Consequently, the register has been used in a variety of epidemiological investigations [35],[37].
Control Populations
For each disorder, ten general population individuals without the specific patient register diagnosis of the cases were matched individually to cases by birth year and gender.
We also conducted the following subanalyses. In the epilepsy group, we separately analysed cases first diagnosed as adults (i.e., aged 16 y and over) with those diagnosed earlier to examine whether there is a difference in risk between childhood-onset and adult-onset epilepsy. This was done because a previous study suggested an inverse relationship between childhood-onset epilepsy and juvenile delinquency [13]. We investigated categories of epilepsy and classified them into four types according to the diagnosis at second admission, as in previous work [38]: complex partial seizures (ICD-8 : 345.31; ICD-9 : 345M; ICD-10: G40.2), other partial seizures (ICD-8 : 345.30, 345.38, 345.39; ICD-10: G40.0, G40.1), generalised epilepsy (ICD-8 : 345.09, 345.10, 345.11; ICD-9 : 345J, 345K; ICD-10: G40.3), and other or unspecified epilepsy (ICD-8 : 345.18, 345.19, 345.29, 345.99; ICD-9 : 345L, 345P, 345Q, 345W, 345X; ICD-10: G40.4, G40.5, G40.6, G40.7, G40.8, G40.9, G41). As an index of severity, we compared those whose first treatment episode lasted for 15 d or more (90th percentile) with the others.
In the traumatic brain injury group, we conducted stratified analyses by age of onset, diagnostic subgroup, and severity. Specifically, we compared individuals with adult-onset traumatic brain injury (i.e., aged 16 and over at the onset of disease) with those with childhood-onset traumatic brain injury. We restricted subgroup analyses to ICD-10 diagnoses of traumatic brain injury (comparable subgroups are not found in ICD-8/9). For this, we subdivided those with traumatic brain injury into: (a) traumatic cerebral oedema (S06.1) and diffuse brain injury (S06.2), (b) focal brain injury (S06.3), and (c) epidural, traumatic subdural, or subarachnoid haemorrhage (S06.4–6). In addition, we compared rates of violent offending in individuals with diagnoses of concussion (ICD-8/9 : 850; ICD-10: S06.0)—a less severe form of brain injury—with traumatic brain injury.
Sibling Control Studies
For both diagnoses, we conducted additional analyses using unaffected full siblings of cases as controls. Using the Multi-Generation Register, we identified as cases individuals with epilepsy (n = 10,360) who also had full siblings without epilepsy, and those persons with traumatic brain injury (n = 11,499) who also had full siblings without traumatic brain injury. These individuals were compared with their unaffected full siblings (n = 17,448 full sibling controls compared to n = 10,360 individuals with epilepsy; n = 19,628 full sibling controls compared to n = 11,499 cases with traumatic brain injury). We conducted this additional analysis because the possibility of residual confounding was considered high, particularly in traumatic head injury [39], with impulsivity being a possible mechanism [40]. For these analyses, we adjusted by gender and age.
Outcome Measures
Data on all convictions for violent crime from 1 January 1973 to 31 December 2009 were retrieved for all individuals aged 15 y and older (15 y is the age of criminal responsibility in Sweden; antisocial behaviour under this age is not prosecuted or systematically registered). Consistent with other work in schizophrenia and severe mental illness, violent crime was defined as homicide, assault, robbery, arson, any sexual offense (rape, sexual coercion, child molestation, indecent exposure, and sexual harassment), or illegal threats or intimidation [36]. Attempted and aggravated forms of included offenses, where applicable according to the Swedish Criminal Code, were also included. Burglary, other property offenses, and traffic and drug offences were excluded. In individuals born from 1954 to 1994, this amounted to 217,134 (unique) persons with at least one violent conviction.
Conviction data were used because the Criminal Code in Sweden determines that individuals are convicted as guilty regardless of medical conditions (such as epileptic automatisms) or mental disorder (which may be comorbid with neurological conditions). Therefore, it includes also those who are found not guilty by reason of insanity (who would be acquitted in other jurisdictions), those receiving custodial or non-custodial sentences, and individuals transferred to psychiatric hospitals on sentencing. Furthermore, conviction data included those cases where the prosecutor decided to caution or fine. In addition, as plea-bargaining is not permitted in Sweden, conviction data accurately reflect the extent of officially resolved criminality. The Crime Register has excellent coverage; only 0.05% of crimes had incomplete personal identification numbers during 1988–2000 [36].
Socio-Demographic Covariates
Household income (divided into thirds) of the family of origin for those 15 y or younger at the time of the 1990 census was used as a proxy for income. Single marital status was defined as being unmarried. Immigrant status was defined as being born outside of Sweden. Missing data were not replaced by imputation or other methods.
Analyses
Only violent convictions recorded after first diagnosis for traumatic brain injury and epilepsy were included. We estimated the association between having been diagnosed with either of these neurological disorders and violent offending with conditional logistic regression, as per related work using matched or sibling controls [35], using the clogit command in Stata, version 10 (StataCorp). The clogit command fits conditional (fixed effects) logistic regression models to matched case–control groups. Ten controls from the general population were selected for each case and matched by birth year and gender. In the sibling control study, all unaffected siblings were compared with their sibling with traumatic brain injury or epilepsy, and analyses were adjusted for age and gender. Among the general population and sibling controls, violent crime was counted only if it occurred after the date of diagnosis in the matched cases. We included three confounders (low income, single, and immigrant status) on theoretical grounds, based on related work in severe mental illness [35],[41], and also tested whether they were each independently associated with caseness and violent crime, respectively, in univariate analyses at the 5% level of significance [42]. In a further analysis, we additionally adjusted for comorbid substance abuse.
Power calculations (with an alpha of 0.05, and a power of 0.90) suggested that 2,385 cases and 23,850 controls were needed to determine a 1.5-fold difference in violence risk.
STROBE guidelines were followed (see Text S1 for details).
Results
Epilepsy
We identified 22,947 individuals with epilepsy and compared them with 224,006 age - and gender-matched general population controls (see Table 1 for baseline data). Of the cases, 973 had at least one violent conviction (4.2%) subsequent to diagnosis, compared with 5,504 controls (2.5%). We found a risk increase for violent crime in individuals with epilepsy after matching and adjustment for age, gender, and socio-demographic confounders (adjusted odds ratio [aOR] = 1.5, 95% CI: 1.4–1.7; Table 2), an absolute risk increase of 1.7% compared with age - and gender-matched population controls. This effect was attenuated after further adjustment for substance use (aOR = 1.2, 1.1–1.3). The rate for violent crime was significantly lower in those first diagnosed before age 16 y than in those first diagnosed at age 16 y or older. In addition, subtypes of epilepsy involving loss of consciousness (complex partial seizures and generalised epilepsy) were associated with lower rates of violent crime (Table 3).
Tab. 1. Baseline socio-demographic information for individuals included in the study. 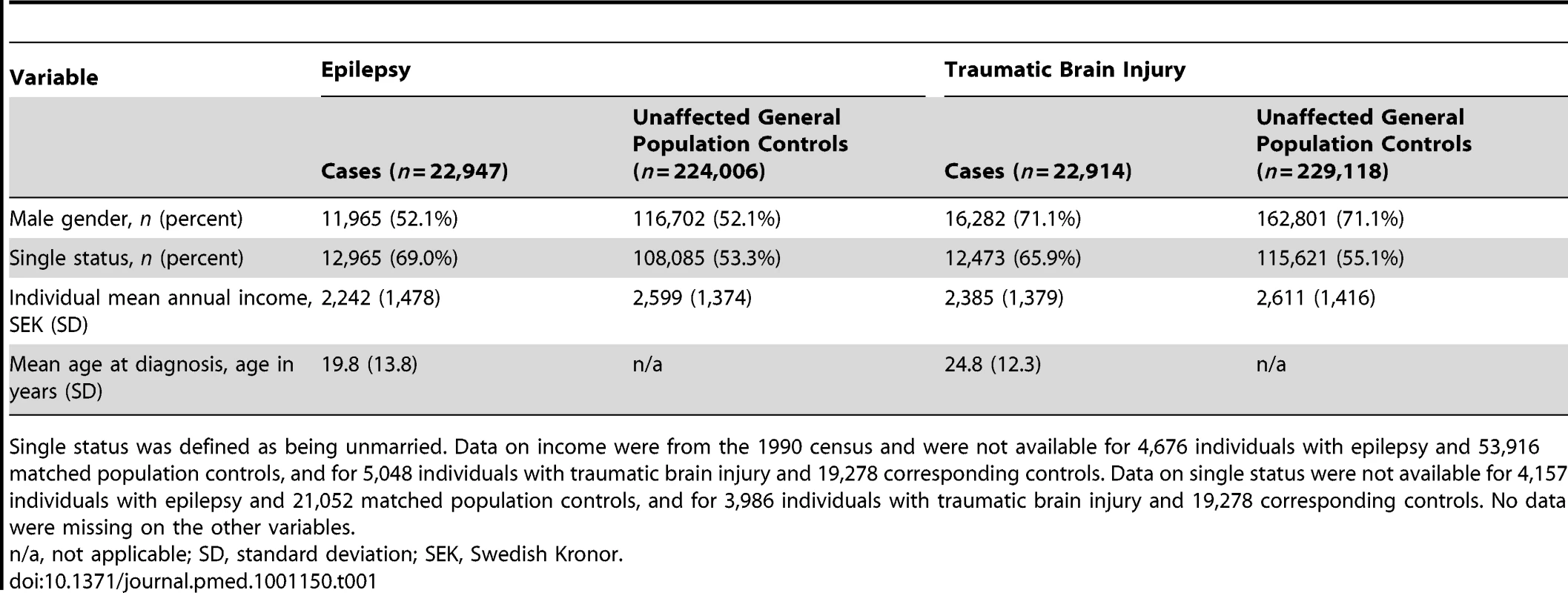
Single status was defined as being unmarried. Data on income were from the 1990 census and were not available for 4,676 individuals with epilepsy and 53,916 matched population controls, and for 5,048 individuals with traumatic brain injury and 19,278 corresponding controls. Data on single status were not available for 4,157 individuals with epilepsy and 21,052 matched population controls, and for 3,986 individuals with traumatic brain injury and 19,278 corresponding controls. No data were missing on the other variables. Tab. 2. Risk of violent crime in individuals after diagnosis with epilepsy or traumatic brain injury in Sweden (1973–2009) compared with general population controls. 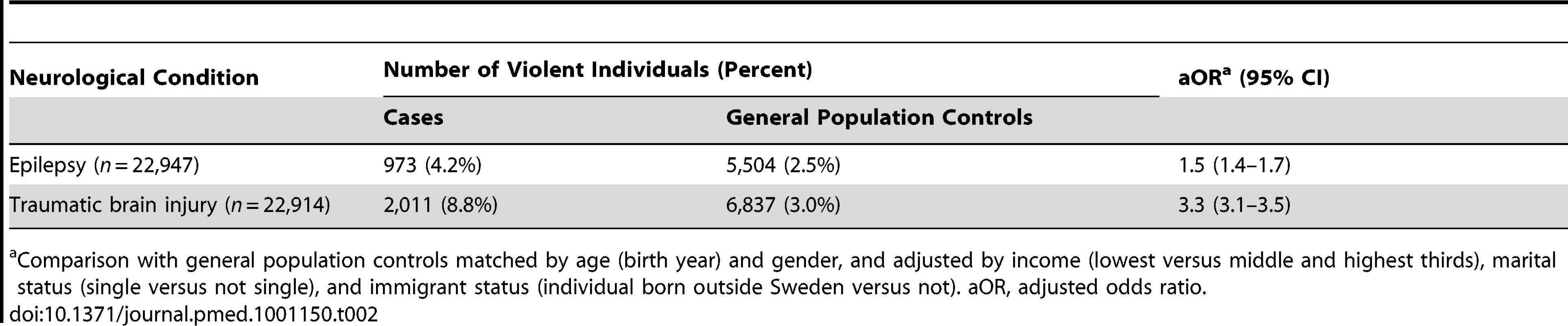
Comparison with general population controls matched by age (birth year) and gender, and adjusted by income (lowest versus middle and highest thirds), marital status (single versus not single), and immigrant status (individual born outside Sweden versus not). aOR, adjusted odds ratio. Tab. 3. Association between epilepsy and traumatic brain injury and subsequent violent crime in Sweden (1973–2009) stratified by age of first diagnosis, clinical subtype, and severity. 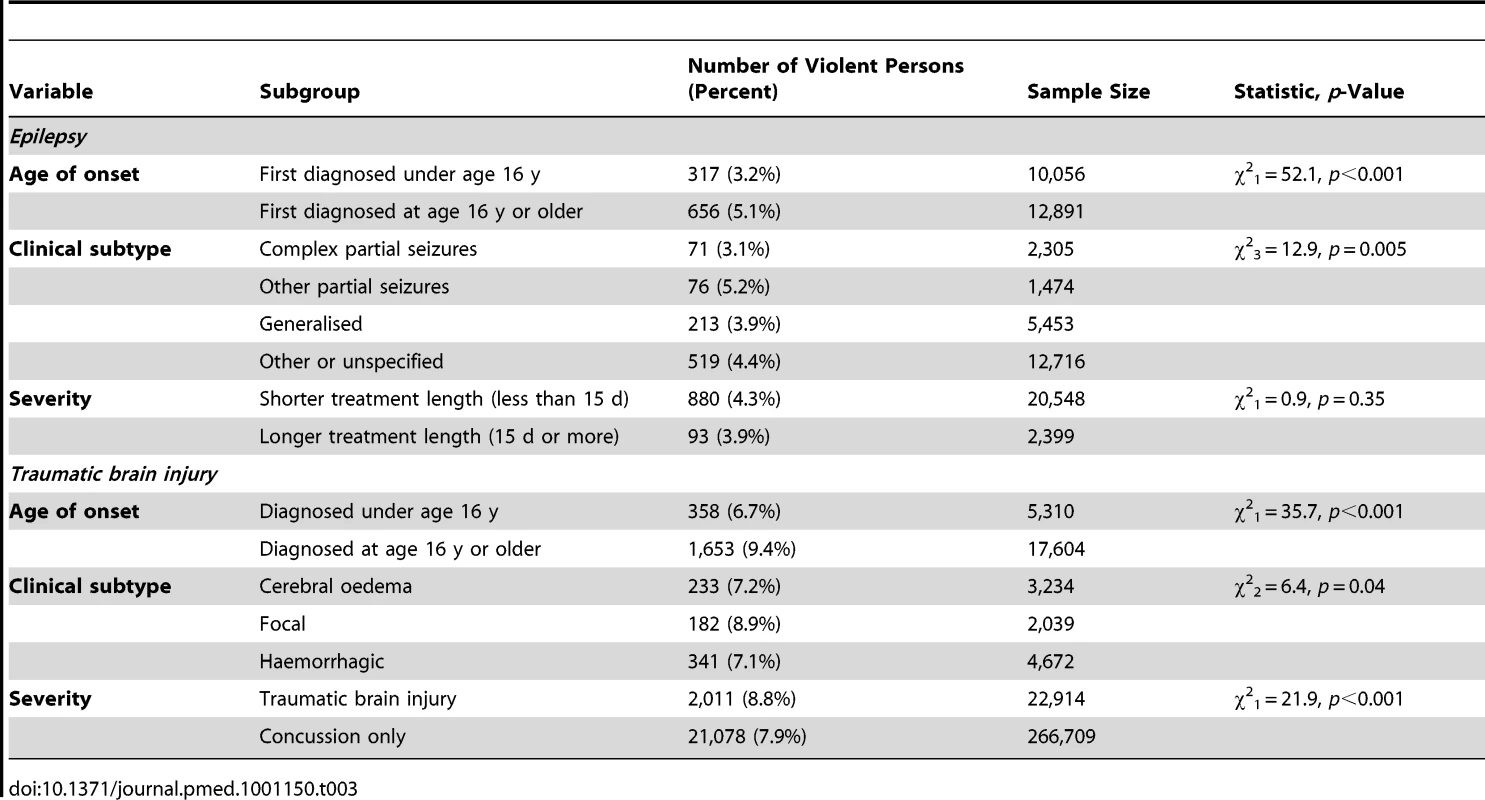
Traumatic Brain Injury
We compared 22,914 individuals with traumatic brain injury with 229,118 general population controls (Table 1 for baseline data), of whom 2,011 (8.8%) were violent after first diagnosis. Cases had a significantly higher risk of violent crime compared with general population controls after adjustment for age, gender, and socio-demographic confounders (aOR = 3.3, 3.1–3.5; Table 2) and further adjustment for substance abuse (aOR = 2.3, 2.2–2.5). This equated to an absolute risk increase of 5.8% in the traumatic brain injury group compared with controls.
We conducted three stratified analyses: age of injury, severity of injury, and subtypes of brain injury (Table 3). Those first diagnosed under age 16 y (versus 16 and older) and those diagnosed with concussion only (versus traumatic brain injury) had lower rates of violent crime, whereas individuals with focal injuries had higher rates than those with more diffuse or haemorrhage-related injuries.
Sibling Control Studies
We found evidence of familial confounding in the association between both epilepsy and traumatic brain injury and subsequent violent crime. In the epilepsy group, 418 (4.0%) of the 10,360 cases had violent offenses. This was not associated with an increased odds of violent crime compared to unaffected siblings (aOR = 1.1, 0.9–1.2), where 727 out of a possible 17,448 (4.2%) individuals had violent convictions.
In the traumatic brain injury group, there were 992 (8.6%) individuals with violent offences among the 11,499 cases. This corresponded to an increased odds of violent conviction compared to unaffected siblings (aOR = 2.0, 1.8–2.3), where 832 out of a possible 19,628 (4.2%) individuals had violent convictions.
Discussion
This population-based study examined the risk of violent crime in individuals after diagnosis with epilepsy or traumatic brain injury in Sweden over 35 y. We used longitudinal designs, adjusted for socio-demographic confounders, compared cases with both general population and unaffected sibling controls, and employed a reliable outcome (violent convictions) that allows for international comparisons. We also investigated rates of violence across diagnostic subtypes and among those with childhood-onset versus adult-onset diagnoses. With over 22,000 individuals each for the epilepsy and traumatic brain injury groups, the sample was, to our knowledge, more than 50 times larger than those used in previous related studies on epilepsy, and more than seven times larger than previous studies on brain injury [10]. Our main findings were that around 4% of individuals with epilepsy had violent convictions after first diagnosis, while approximately 9% of those with traumatic brain injury had violent convictions subsequent to diagnosis. Although this corresponded to a modest increase in the odds of violent crime in individuals with epilepsy compared to the general population, we found no risk increase in comparison with their unaffected siblings, which provided a powerful approach to adjust for familial confounding. This was in contrast to individuals with traumatic brain injury, for whom there was a 3-fold increase in the odds of violent crime compared with the general population, and there was a doubling of odds of violent crime in individuals with traumatic brain injury compared with their unaffected siblings. As these siblings shared half the genes and most of the early environment, this allowed us to partly account for personality traits that are associated with both violence and head injury or epilepsy.
For epilepsy, the findings of an absolute rate of violent crime of 4% and the lack of any association in the sibling control study should be seen in the context of expert opinion in the field that states that the link is strong [3],[8]; these findings are also potentially important with respect to the fact that epilepsy remains heavily stigmatised [20],[43],[44]. Previous views may have been influenced by high-profile criminal cases of individuals with epilepsy who committed homicide [45],[46], and reports of high prevalences of epilepsy in prisoners [11],[12], the latter that have not been subsequently confirmed [47]. Our finding on relative risks counter a recent systematic review that found a slightly protective risk for epilepsy, but this review was based on three investigations, all of which were in selected samples [10]. The finding that certain subtypes of epilepsy (including simple partial seizures and temporal lobe epilepsy) are associated with higher rates of violent crime may assist in clarifying mechanisms and potential treatments, and suggests that these patients could be assessed for violence risk if these findings on subtypes are validated. Frontal lobe seizures, associated with violence in some cases [48], could be one mechanism to explain the excess in simple partial seizures. Interestingly, these subgroup differences are consistent with the finding for traumatic brain injury, as discussed below, that focal, in contrast to generalised, brain injuries were linked with higher violence risk.
The increased risk of violent crime in individuals with traumatic brain injury compared with general population controls is consistent with clinical studies [39],[49]–[52] and a recent systematic review [10]. However, the latter review identified no population-based or unselected investigations, and synthesised information based on around 2,500 individuals with head injury. This previous review reported a risk increase for brain injury as a pooled odds ratio of 1.7. The current report findings nearly double this risk estimate, even after adjustment for socio-demographic confounders. As there is likely to be residual confounding in such comparisons, we used unaffected siblings as controls, and found a moderated but still significant association with violent crime. Therefore, although there are plausible aetiological hypotheses that propose mechanisms for violence in individuals with traumatic brain injury, including damage to the frontal and prefrontal cortices [14],[53], this study suggests that shared familial factors explain some of this association. Familial confounding may occur through genetic susceptibility, early environmental effects, or both. Such effects may involve personality traits (such as impulsivity, risk taking, and propensity to substance abuse [54]) and handling of interpersonal situations that increase the risk of head injury and are also associated with violence.
Despite evidence of familial confounding, we found support for a direct effect in brain injury leading to violence, in that focal brain injuries were associated with the highest risk, although the diagnostic information available in the hospital registers did not specify the location of the injury. Regarding the finding that younger age was related with less likelihood of subsequent violent crime, one explanation might be that earlier injuries are associated with better outcomes because of neuroplasticity or more effective treatments [55], or that a later onset of injury is more strongly correlated with an antisocial lifestyle, sensation seeking, and risk taking. Further research is necessary to identify the specific mechanisms underlying the age effect and familial confounding, and may contribute to the development of preventive strategies. Examining the role of repetitive brain injuries on risk of violence is another area where further research is necessary. Other work has found some evidence that cumulative mild injuries might lead to a longer period of future antisociality [56] and increased risk of repeat offending in prisoners [17],[57].
From a public health and policy perspective, these findings point in different directions for health services, the criminal justice system, and patient charities. On the one hand, the lack of an association with violent crime in epilepsy cases after adjustment for familial factors may be valuable for patient charities and other stakeholders in addressing one of the potential causes of the stigma associated with this condition. Health services may consider violence risk assessment and management worthwhile in certain high-risk groups of individuals with epilepsy, particularly if they have violent histories. For traumatic brain injury, absolute and relative risks more clearly suggest that there are certain groups of patients who would benefit from violence risk assessment. As current guidelines for the assessment of brain injury make no recommendations in relation to the assessment or investigation of violence risk [58], our findings suggest that these may need review, at least for some groups of patients with traumatic head injury, particularly if they abuse illegal drugs or alcohol. In prisoners with traumatic head injury, improved screening, assessment, and management may improve repeat offending rates [17]. An additional group that may benefit from more detailed assessment and treatment are head injured juvenile delinquents [59]. The odds ratio of violent crime reported in this study for head injury (3.3) is similar to those reported for schizophrenia (where violence risk assessment should be routinely considered [60]) and bipolar disorder [41], but less than the odds ratio of 7–9 reported for substance abuse [1].
Limitations
Study weaknesses include our reliance on patient registers for case ascertainment. This meant that the sample was selected towards more severe cases of epilepsy and traumatic brain injury. This could have led to an underestimation of the association with violent crime if individuals with more severe disease are more likely to be physically disabled and thus less likely to commit violent crime (although the use of outpatient information should have moderated against this). However, it is also possible that the more severe presentations of these disorders are more prone to violence, and hence we may have overestimated the risk. We found some support for this in persons with traumatic brain injury, who had higher rates of violent crime than those with concussion diagnoses alone. The fact that we may have oversampled the more severe cases may be more relevant in epilepsy, as we selected only individuals with two or more hospital diagnoses of epilepsy in order to improve diagnostic specificity, but the reported finding that those with longer treatment episodes were not at higher risk of violent crime argues against this potential bias. Nevertheless, as we are not certain what proportion of individuals with epilepsy are hospitalised over a 30-y period, our results may be less generalisable to individuals with epilepsy who are not inpatients or outpatients at some point in their illness. Another limitation was the lack of data on the extent and character of treatment for these conditions. It is possible that treatment effects mediated some of the differences found here, particularly the mood-stabilising effects of anticonvulsants prescribed to epilepsy patients, although a recent review found no clear evidence that such medications reduce violence [61]. In the analyses, we adjusted for substance abuse but did not examine comorbidity for other psychiatric illnesses, as was done in one Danish population study [62], since the validity of outpatient data for less severe mental illnesses, such as depression and anxiety disorders, is uncertain. Nevertheless, future work could examine whether the risk differs by comorbidity. Although we relied on conviction data, other work has shown that the degree of underestimation of violence is similar in psychiatric patients and controls compared with self-report measures, and hence the risk estimates were unlikely to be affected [63]. This has also been found for studies investigating violence risk in individuals with schizophrenia [1]. We have no reason to think that this would be different for these two neurological conditions. Overall rates of violent crime and their resolution are mostly similar across western Europe, suggesting some generalisability of our findings [64]. Comparisons with the US are more difficult because of differences in legal and judicial systems, but police-recorded assault rates for the time period 1981–1999 were 3.7 per 1,000 individuals in the US and 4.1 per 1,000 individuals in Sweden [65].
In conclusion, by using Swedish population-based registers over 35 y, we reported risks for violent crime in individuals with epilepsy and traumatic brain injury that contrasted with each other, and appeared to differ within each diagnosis by subtype, severity, and age at diagnosis. The implications of these findings are likely to vary for clinical services, the criminal justice system, and patient charities.
Supporting Information
Zdroje
1. FazelSGulatiGLinsellLGeddesJRGrannM 2009 Schizophrenia and violence: systematic review and meta-analysis. PLoS Med 6 e1000120 doi:10.1371/journal.pmed.1000120
2. WallaceCMullenPBurgessPPalmerSRuschenaD 1998 Serious criminal offending and mental disorder. Br J Psychiatry 172 477 484
3. ElliottFA 1992 Violence. The neurologic contribution: an overview. Arch Neurol 49 595 603
4. FilleyCPriceBNellVAntoinetteTMorganA 2001 Toward an understanding of violence: neurobehavioral aspects of unwarranted physical aggression—Aspen Neurobehavioral Conference Consensus Statement. Neuropsychiatry Neuropsychol Behav Neurol 14 1 14
5. MarkusRRonnieDM 2008 Epileptic automatisms in the criminal courts: 13 cases tried in England and Wales between 1975 and 2001. Epilepsia 49 138 145
6. TooneB 2009 Epilepsy. GelderMAndreasenNLopez-IborJGeddesJ The new Oxford textbook of psychiatry, 2nd ed Oxford Oxford University Press 1076 1080
7. TardiffK 2003 Violence. HalesRYudofskyS Textbook of clinical psychiatry, 4th ed Washington (District of Columbia) American Psychiatric Publishing
8. LambertMSchmitzERingHTrimbleMR 2003 Neuropsychiatric aspects of epilepsy. SchifferRRaoSFogelB Neuropsychiatry, 2nd ed Philadelphia Lippincott, Williams & Williams
9. MaudsleyH 1870 Body and mind London Macmillan
10. FazelSPhilipsonJGardinerLMerrittRGrannM 2009 Neurological disorders and violence: a systematic review and meta-analysis with a focus on epilepsy and traumatic brain injury. J Neurol 256 1591 1602
11. WhitmanSColemanTPatmonCDesaiBCohenR 1984 Epilepsy in prisons: elevated prevalence and no relationship to violence. Neurology 34 775 782
12. GunnJBonnJ 1971 Criminality and violence in epileptic prisoners. Br J Psychiatry 118 337 343
13. RantakallioPKoiranenMMottonenJ 1992 Association of perinatal events, epilepsy, and central nervous system trauma with juvenile delinquency. Arch Dis Child 67 1459 1461
14. DamasioHGrabowskiTFrankRGalaburdaADamasioA 1994 The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 264 1102 1105
15. GrafmanJSchwabKWardenDPridgenABrownHR 1996 Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 46 1231 1238
16. BlakePYPincusJHBucknerC 1995 Neurologic abnormalities in murderers. Neurology 45 1641 1647
17. WilliamsWHMewseAJTonksJMillsSBurgessCN 2010 Traumatic brain injury in a prison population: prevalence and risk for re-offending. Brain Inj 24 1184 1188
18. TurkstraLJonesDTolerH 2003 Brain injury and violent crime. Brain Inj 17 39 47
19. AndersonVSpencer-SmithMLeventerRColemanLAndersonP 2009 Childhood brain insult: can age at insult help us predict outcome? Brain 132 45 56
20. AmorosoCZwiASomervilleEGroveN 2006 Epilepsy and stigma. Lancet 367 1143 1144
21. SimpsonGMohrRRedmanA 2000 Cultural variations in the understanding of traumatic brain injury and brain injury rehabilitation. Brain Inj 14 125 140
22. TagliaferriFCompagnoneCKorsicMServadeiFKrausJ 2006 A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 148 255 268
23. ForsgrenLBeghiEÕunASillanpääM 2005 The epidemiology of epilepsy in Europe—a systematic review. Eur J Neurol 12 245 253
24. ButlerTKariminiaALevyMMurphyM 2004 The self-reported health status of prisoners in New South Wales. Aust N Z J Public Health 28 344 350
25. BrunsJHauserW 2003 The epidemiology of traumatic brain injury: a review. Epilepsia 44 2 10
26. Statistics Sweden 2005 Flergenerationsregistret 2004: En beskrivning av innehåll och kvalitet. [The Multi-Generation Register 2004: a description of content and quality.] Örebro (Sweden) Statistics Sweden
27. Swedish National Board of Health and Welfare 2011 Patientregistret. [Patient Register.] Available: http://www.socialstyrelsen.se/register/halsodataregister/patientregistret. Accessed 21 November 2011
28. HammarNAlfredssonLRosenMSpetzCLKahanT 2001 A national record linkage to study acute myocardial infarction incidence and case fatality in Sweden. Int J Epidemiol 30 Suppl 1 S30 S34
29. GedeborgREngquistHBerglundLMichaelssonK 2008 Identification of incident injuries in Hospital Discharge Registers. Epidemiol 19 860 867
30. LindbladURåstamLRanstamJPetersonM 1993 Validity of register data on acute myocardial infarction and acute stroke: the Skaraborg Hypertension Project. Scand J Soc Med 21 3 9
31. JiangGde Pedro-CuestaJFredriksonS 1995 Guillain-Barré syndrome in south-west Stockholm, 1973–1991, 1. Quality of registered hospital diagnoses and incidence. Acta Neurol Scand 91 109 117
32. EkholmBEkholmAAdolfssonRVaresMÖsbyU 2005 Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry 59 457 464
33. DalmanCBomsJCullbergJAllebeckP 2002 Young cases of schizophrenia identified in a national inpatient register. Are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol 37 527 531
34. LudvigssonJAnderssonEEkbomAFeychtingMKimJ-L 2011 External review and validation of the Swedish national inpatient register. BMC Public Health 11 450
35. FazelSLångströmNHjernAGrannMLichtensteinP 2009 Schizophrenia, substance abuse, and violent crime. JAMA 301 2016 2023
36. FazelSGrannM 2006 The population impact of severe mental illness on violent crime. Am J Psychiatry 163 1397 1403
37. HjernALindbladFVinnerljungB 2002 Suicide, psychiatric illness, and social maladjustment in intercountry adoptees in Sweden: a cohort study. Lancet 360 443 448
38. QinPXuHLaursenTMVestergaardMMortensenPB 2005 Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ 331 23
39. GreveKWSherwinEStanfordMSMathiasCLoveJ 2001 Personality and neurocognitive correlates of impulsive aggression in long-term survivors of severe traumatic brain injury. Brain Inj 15 255 262
40. DyerKFWBellRMcCannJRauchR 2006 Aggression after traumatic brain injury: analysing socially desirable responses and the nature of aggressive traits. Brain Inj 20 1163 1173
41. FazelSLichtensteinPGrannMGoodwinGMLangstromN 2010 Bipolar disorder and violent crime: new evidence from population-based longitudinal studies and systematic review. Arch Gen Psychiatry 67 931 938
42. Klein-GeltinkaJRochonbPDyeraSLaxerbMAndersonG 2007 Readers should systematically assess methods used to identify, measure and analyze confounding in observational cohort studies. J Clin Epidemiol 60 766 772
43. KaleR 1997 Bringing epilepsy out of the shadows. BMJ 315 2 3
44. de BoerHM 2002 “Out of the shadows”: a global campaign against epilepsy. Epilepsia 43 7 8
45. LivingstonS 1964 Epilepsy and murder. JAMA 188 172
46. TreimanD 1986 Epilepsy and violence: medical and legal issues. Epilepsia 27 S77 S104
47. FazelSVassosEDaneshJ 2002 Prevalence of epilepsy in prisoners: systematic review. BMJ 324 1495
48. ShihJJLeslieMazwiTFalcaoGVan GerpenJ 2009 Directed aggressive behavior in frontal lobe epilepsy: a video-EEG and ictal SPECT case study. Neurology 73 1804 1806
49. KreutzerJMarwitzJWitolA 1995 Interrelationships between crime, substance abuse, and aggressive behaviours among persons with traumatic brain injury. Brain Inj 9 757 768
50. TatenoAJorgeRERobinsonRG 2003 Clinical correlates of aggressive behaviour after traumatic brain injury. J Neuropsychiatry Clin Neurosci 15 155 160
51. WoodRLLiossiC 2006 Neuropsychological and neurobehavioral correlates of aggression following traumatic brain injury. J Neuropsychiatry Clin Neurosci 18 333 341
52. BaguleyICooperJFelminghamK 2006 Aggressive behavior following traumatic brain injury: how common is common? J Head Trauma Rehabil 21 45 56
53. DamasioAR 2000 A neural basis for sociopathy. Arch Gen Psychiatry 57 128 129
54. GrahamDPCardonAL 2008 An update on substance use and treatment following traumatic brain injury. Ann N Y Acad Sci 1141 148 162
55. SteinSCDoolinEJ 1995 Management of minor closed head injury in children and adolescents. Pediatric Surg Int 10 465 471
56. RaineAMoffittTECaspiALoeberRStouthamer-LoeberM 2005 Neurocognitive impairments in boys on the life-course persistent antisocial path. J Abnorm Psychol 114 38 49
57. WilliamsHCordanGMewseAJTonksJBurgessC 2010 Self-reported traumatic brain injury in male young offenders: a risk factor for re-offending, poor mental health and violence? Neuropsychol Rehab 20 801 812
58. National Institute for Health and Clinical Excellence 2007 Head injury: triage, assessment, investigation and early management of head injury in infants, children and adults London National Institute for Health and Clinical Excellence
59. Leon-CarrionJRamosF 2003 Blows to the head during development can predispose to violent criminal behaviour: rehabilitation of consequences of head injury is a measure for crime prevention. Brain Inj 17 207 216
60. American Psychiatric Association 2004 Practice guideline for the treatment of patients with schizophrenia Arlington (Virginia) American Psychiatric Association
61. HubandNFerriterMNathanRJonesH 2010 Antiepileptic drugs for treating recurrent aggression. Cochrane Database Syst Rev 2 CD003499 doi:10.1002/14651858.CD003499.pub3
62. ChristensenJVestergaardMMortensenPBSideniusPAgerboE 2007 Epilepsy and risk of suicide: a population-based case-control study. Lancet Neurol 6 693 698
63. ArseneaultLMoffittTCaspiATaylorPSilvaP 2000 Mental disorders and violence in a total birth cohort: results from the Dunedin study. Arch Gen Psychiatry 57 979 986
64. DolménL 2001 Brottsligheten i olika länder. [Criminality in different countries.] Stockholm National Council for Crime Prevention
65. FarringtonDLanganPTonryM 2004 (2004) Cross-national studies in crime and justice Washington (District of Columbia) US Department of Justice, Bureau of Justice Statistics
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 12- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- The Toxic Effects of Cigarette Additives. Philip Morris' Project Mix Reconsidered: An Analysis of Documents Released through Litigation
- The Role of Group Dynamics in Scientific Inconsistencies: A Case Study of a Research Consortium
- Violent Crime, Epilepsy, and Traumatic Brain Injury
- Disclosure of Investigators' Recruitment Performance in Multicenter Clinical Trials: A Further Step for Research Transparency
- Shift Work as a Risk Factor for Future Type 2 Diabetes: Evidence, Mechanisms, Implications, and Future Research Directions
- Estimating the Burden of Malaria: The Need for Improved Surveillance
- Rotating Night Shift Work and Risk of Type 2 Diabetes: Two Prospective Cohort Studies in Women
- Measuring the Population Burden of Injuries—Implications for Global and National Estimates: A Multi-centre Prospective UK Longitudinal Study
- Cognitive Performance in Late Adolescence and the Subsequent Risk of Subdural Hematoma: An Observational Study of a Prospective Nationwide Cohort
- Sex-Specific Immunization for Sexually Transmitted Infections Such as Human Papillomavirus: Insights from Mathematical Models
- A Comprehensive Framework for Human Resources for Health System Development in Fragile and Post-Conflict States
- Absorbable Versus Silk Sutures for Surgical Treatment of Trachomatous Trichiasis in Ethiopia: A Randomised Controlled Trial
- Risk of Violent Crime in Individuals with Epilepsy and Traumatic Brain Injury: A 35-Year Swedish Population Study
- Poor Diet in Shift Workers: A New Occupational Health Hazard?
- The Primacy of Public Health Considerations in Defining Poor Quality Medicines
- Worldwide Incidence of Malaria in 2009: Estimates, Time Trends, and a Critique of Methods
- Surgery Versus Epilation for the Treatment of Minor Trichiasis in Ethiopia: A Randomised Controlled Noninferiority Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Violent Crime, Epilepsy, and Traumatic Brain Injury
- Rotating Night Shift Work and Risk of Type 2 Diabetes: Two Prospective Cohort Studies in Women
- Surgery Versus Epilation for the Treatment of Minor Trichiasis in Ethiopia: A Randomised Controlled Noninferiority Trial
- Worldwide Incidence of Malaria in 2009: Estimates, Time Trends, and a Critique of Methods
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



