-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
article has not abstract
Published in the journal: . PLoS Med 7(9): e32767. doi:10.1371/journal.pmed.1000326
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1000326Summary
article has not abstract
Summary Points
-
When Archie Cochrane reproached the medical profession for not having critical summaries of all randomised controlled trials, about 14 reports of trials were being published per day. There are now 75 trials, and 11 systematic reviews of trials, per day and a plateau in growth has not yet been reached.
-
Although trials, reviews, and health technology assessments have undoubtedly had major impacts, the staple of medical literature synthesis remains the non-systematic narrative review. Only a small minority of trial reports are being analysed in up-to-date systematic reviews. Given the constraints, Archie Cochrane's vision will not be achieved without some serious changes in course.
-
To meet the needs of patients, clinicians, and policymakers, unnecessary trials need to be reduced, and systematic reviews need to be prioritised. Streamlining and innovation in methods of systematic reviewing are necessary to enable valid answers to be found for most patient questions. Finally, clinicians and patients require open access to these important resources.
Thirty years ago, and a quarter of a century after randomised trials had become widely accepted, Archie Cochrane reproached the medical profession for not having managed to organise a “critical summary, by speciality or subspeciality, adapted periodically, of all relevant randomised controlled trials” [1]. Thirty years after Cochrane's reproach we feel it is timely to consider the extent to which health professionals, the public and policymakers could now use “critical summaries” of trials for their decision-making.
The Landscape
Keeping up with information in health care has never been easy. Even in 1753, when James Lind published his landmark review of what was then known about scurvy, he needed to point out that “… before the subject could be set in a clear and proper light, it was necessary to remove a great deal of rubbish” [2]. And 20 years later, Andrew Duncan launched a publication summarising research for clinicians, lamenting that critical information “…is scattered through a great number of volumes, many of which are so expensive, that they can be purchased for the libraries of public societies only, or of very wealthy individuals” [3]. We continue to live with these two problems—an overload of unfiltered information and lack of open access to information relevant to the well-being of patients.
A century later, the precursor of the US National Library of Medicine (NLM) began indexing the medical literature. Between 1865 and 2006, the index grew from 1,600 references to nearly 10 million [4]. Even with the assistance of electronic databases such as NLM's MEDLINE, the problem of having to trawl through and sift vast amounts of data has grown. As mountains of unsynthesised research evidence accumulate, we need to keep improving our methods for gathering, filtering, and synthesising it. Some of the key events in the story so far are shown on the timeline in Figure 1.
Fig. 1. Policy and academic milestones in the development of trials and the science of reviewing trials. 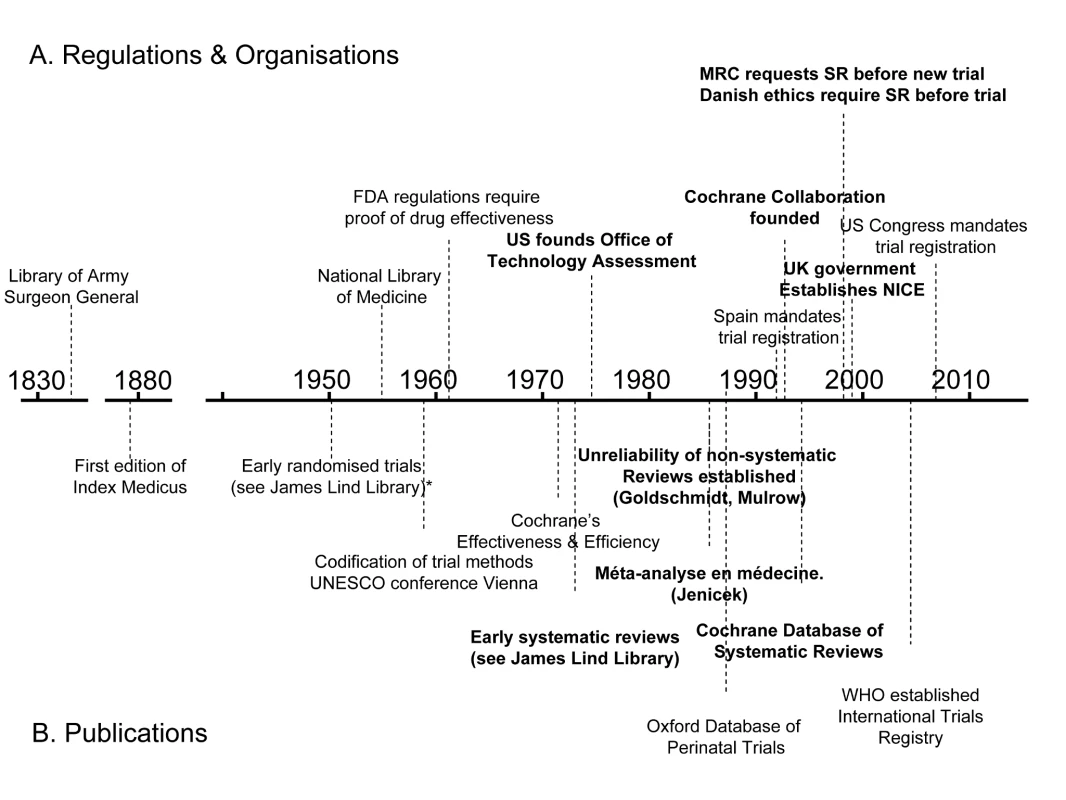
A legal regulatory framework overseen by the US Food and Drug Administration (FDA) requiring proof of efficacy of new drugs was introduced in 1962, and other countries followed suit. These developments made it inevitable that randomised trials would increasingly become an important component of the evidence base [5]. Government health technology assessment agencies were also established as policymakers sought to have more reliable evidence of the effects of other forms of health care interventions [6].
As the number of clinical trials grew, so too did the science of reviewing trials. Systematic reviews and meta-analyses endeavouring to make sense of multiple trials began to appear in a variety of health fields in the 1970s and 1980s (see Box 1). An important early example showed that postoperative radiotherapy after surgical treatment of breast cancer was associated with a previously unrecognised increased risk of death [7]. Another challenged beliefs about vitamin C and the common cold [8]. A third suggested a previously unrecognised advantage of some forms of fetal monitoring during labour in reducing neonatal seizures [9].
Box 1. Early Systematic Reviews of the Effects of Health Care Interventions
-
Stjernswärd J (1974) Decreased survival related to irradiation postoperatively in early breast cancer. Lancet 304 : 1285-1286.
-
Chalmers TC (1975) Effects of ascorbic acid on the common cold. An evaluation of the evidence. Am J Med 58 : 532-536.
-
Cochran WG, Diaconis P, Donner AP, Hoaglin DC, O'Connor NE, Peterson OL, Rosenoer VM (1977) Experiments in surgical treatments of duodenal ulcer. In: Bunker JP, Barnes BA, Mosteller F, eds. Costs, risks and benefits of surgery. Oxford: Oxford University Press. pp 176-197.
-
Smith ML, Glass GV (1977) Meta-analysis of psychotherapy outcome studies. Am Psychol 32 : 752-760.
-
Hemminki E, Starfield B (1978) Routine administration of iron and vitamins during pregnancy: Review of controlled clinical trials. Br J Obstet Gynaecol 85 : 404-410.
-
Hemminki E, Starfield B (1978) Prevention and treatment of premature labour by drugs: Review of controlled clinical trials. Br J Obstet Gynaecol 85 : 411-417.
-
Chalmers I (1979) Randomized controlled trials of fetal monitoring, 1973–1977. In: Thalhammer O, Baumgarten K, Pollak A, eds. Perinatal medicine. Stuttgart: Georg Thieme. pp 260-265.
-
Policy Research Incorporated (1979) Medical Practice Information Demonstration Project. Bipolar disorder, a state of the science report. Baltimore: Policy Research Incorporated.
-
Editorial (1980) Aspirin after myocardial infarction. Lancet 1 : 1172-1173. [Published anonymously but written by Richard Peto.]
-
Baum ML, Anish DS, Chalmers TC, Sacks HS, Smith H, Fagerstrom RM (1981) A survey of clinical trials of antibiotic prophylaxis in colon surgery: Evidence against further use of no-treatment controls. N Engl J Med 305 : 795-799.
-
Hampton JR (1982) Should every survivor of a heart attack be given a beta-blocker? Part I: Evidence from clinical trials. BMJ 285 : 33-36.
-
Stampfer MJ, Goldhaber SZ, Yusuf S, Peto R, Hennekens CH (1982) Effect of intravenous streptokinase on acute myocardial infarction: Pooled results from randomized trials. N Engl J Med 307 : 1180-1182.
-
Sacks HS, Chalmers TC, Berk AA, Reitman D (1985) Should mild hypertension be treated? An attempted meta-analysis of the clinical trials. Mt Sinai J Med 52 : 265-270.
-
Yusuf S, Peto R, Lewis J, Collins R, Sleight P (1985) Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog Cardiovasc Dis 27 : 335-371.
By the mid-1980s, the need to minimise the likelihood of being misled by the effects of biases and the play of chance in reviews of research evidence was being made evident in articles [10]–[14] and textbooks [15]. In 1988, regularly updated electronic publication of systematic reviews and meta-analyses, along with bibliographies of randomised trials, began in the perinatal field [16],[17]. This provided a model for the inauguration of the international Cochrane Collaboration in 1993 to prepare, maintain, and disseminate systematic reviews of the effects of health care interventions.
Where Are We Now?
Despite this progress, the task keeps increasing in size and complexity. We still do not know exactly how many trials have been done. For a variety of reasons, a large proportion of trials have remained unpublished [18],[19]. Furthermore, many trials have been published in journals without being electronically indexed as trials, which makes them difficult to find. One of the first steps in being able to adequately review literature is that scientific contributions which predate digitalised information systems and trial indexing need to be “rediscovered and inserted into the memory system” [20]. Through the 1990s, to identify possible reports of controlled trials, the Cochrane Collaboration mobilised thousands of volunteers around the globe to comb the major databases, and to hand-search nondigitalised health literature, unpublished conference proceedings, and books. The result of this collaborative effort is the Cochrane Controlled Trials Register (CCTR) (now called the Cochrane Central Register of Controlled Trials).
The differences between the numbers of trial records in MEDLINE and CCTR (see Figure 2) have multiple causes. Both CCTR and MEDLINE often contain more than one record from a single study, and there are lags in adding new records to both databases. The NLM filters are probably not as efficient at excluding non-trials as are the methods used to compile CCTR. Furthermore, MEDLINE has more language restrictions than CCTR. In brief, there is still no single repository reliably showing the true number of randomised trials. Similar difficulties apply to trying to estimate the number of systematic reviews and health technology assessments (HTAs).
Fig. 2. The number of published trials, 1950 to 2007. 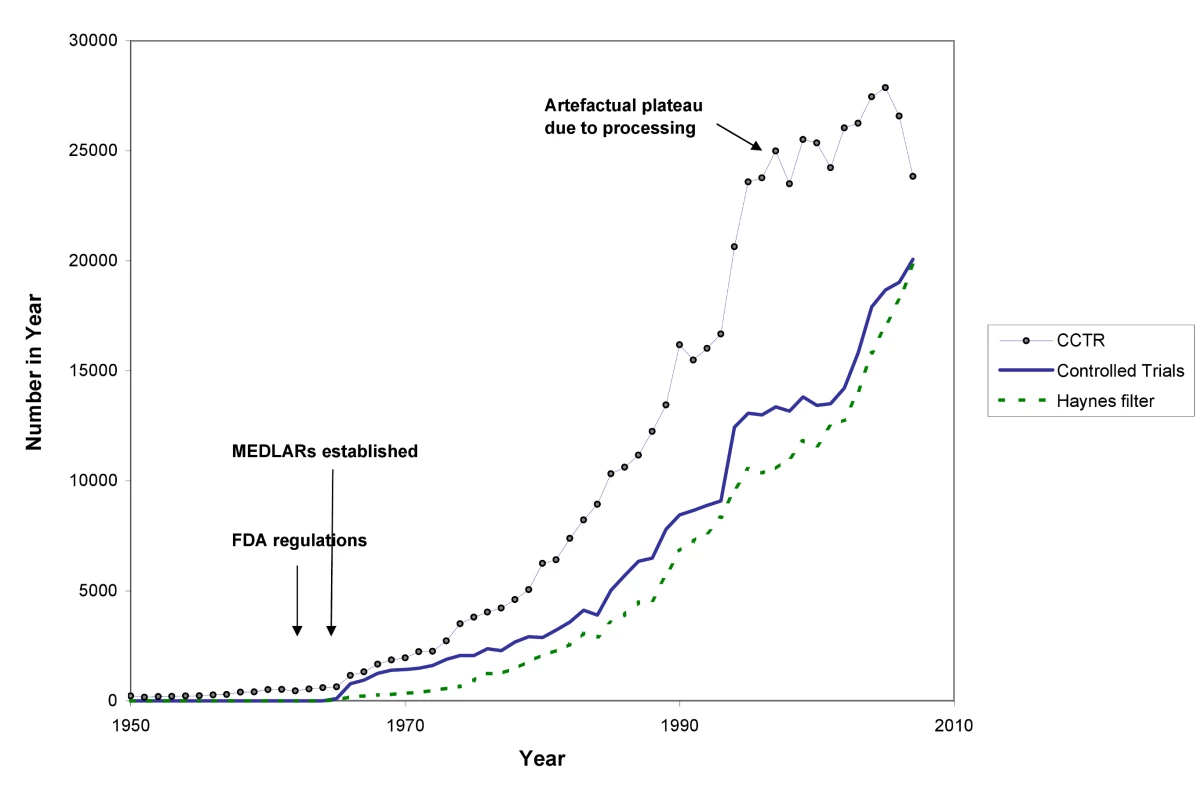
CCTR is the Cochrane Controlled Trials Registry; Haynes filter uses the “narrow” version of the Therapy filter in PubMed:ClinicalQueries; see Text S1. In Figures 2 and 3 we use a variety of data sources to estimate the numbers of trials and systematic reviews published from 1950 to the end of 2007 (see Text S1). The number of trials continues to rise: although the data from CCTR suggest some fluctuation in trial numbers in recent years, this may be misleading because the Cochrane Collaboration virtually halted additions to CCTR as it undertook a review and internal restructuring that lasted a couple of years.
Even though these figures must be seen as more illustrative than precise, multiple data sources tell the same story: astonishing growth has occurred in the number of reports of clinical trials since the middle of the 20th century, and in reports of systematic reviews since the 1980s—and a plateau in growth has not yet been reached. With a median of perhaps 80 participants per trial, the number of people being enrolled in trials is likely to be more than 2,000,000 per year [21]. Prospective trial registration establishes a new genre of evidence repository: trials are registered in these databases at inception, theoretically enabling an overview of all published and unpublished trials.
In 2004, the International Committee of Medical Journal Editors (ICMJE, http://www.icmje.org/) announced that their journals would no longer publish trials that had not been prospectively registered [22]. Before this announcement, an average of 30 trials a week were being prospectively registered around the world. Once the journal editors' deadline came into force, more than 200 ongoing trials per week were being registered [23]. In 2007, the US Congress made detailed prospective trial registration legally mandatory [24]. As WHO's international clinical trials platform develops, it will become possible to generate a more realistic picture of how many trials are being done. This registry draws together standardised core data from all the trial registries meeting specified quality criteria. Registering full protocols and reporting trial results in these registries are the next frontiers.
How Close Are We to Archie Cochrane's Goal?
In 1986 and 1987, Goldschmidt and Mulrow showed how great the potential is for error in reviews of health literature that were not conducted systematically [9],[10]. Looking at data such as those in Figure 3 could provide the comforting illusion that systematic reviews have displaced other less reliable forms of information. However, as Figure 4 shows, this is far from the case. The growth has been even more remarkable in non-systematic (“narrative”) reviews and case reports. Journal publishing of non-systematic reviews, and the emergence of many journals whose sole product is non-systematic reviews, has far outstripped the growth of systematic reviews and HTAs, as impressive as the latter has been. And the number of case reports—which can also provide important new information such as adverse effects—is far higher than the number of trials or systematic reviews. Trials, systematic reviews, and HTAs have undoubtedly had major impacts, including on clinical guidelines: they are more likely to be cited and read than other study types [25]. However, the staple of medical literature synthesis remains the non-systematic narrative review.
Fig. 3. The number of systematic reviews in health care, 1990 to 2007. 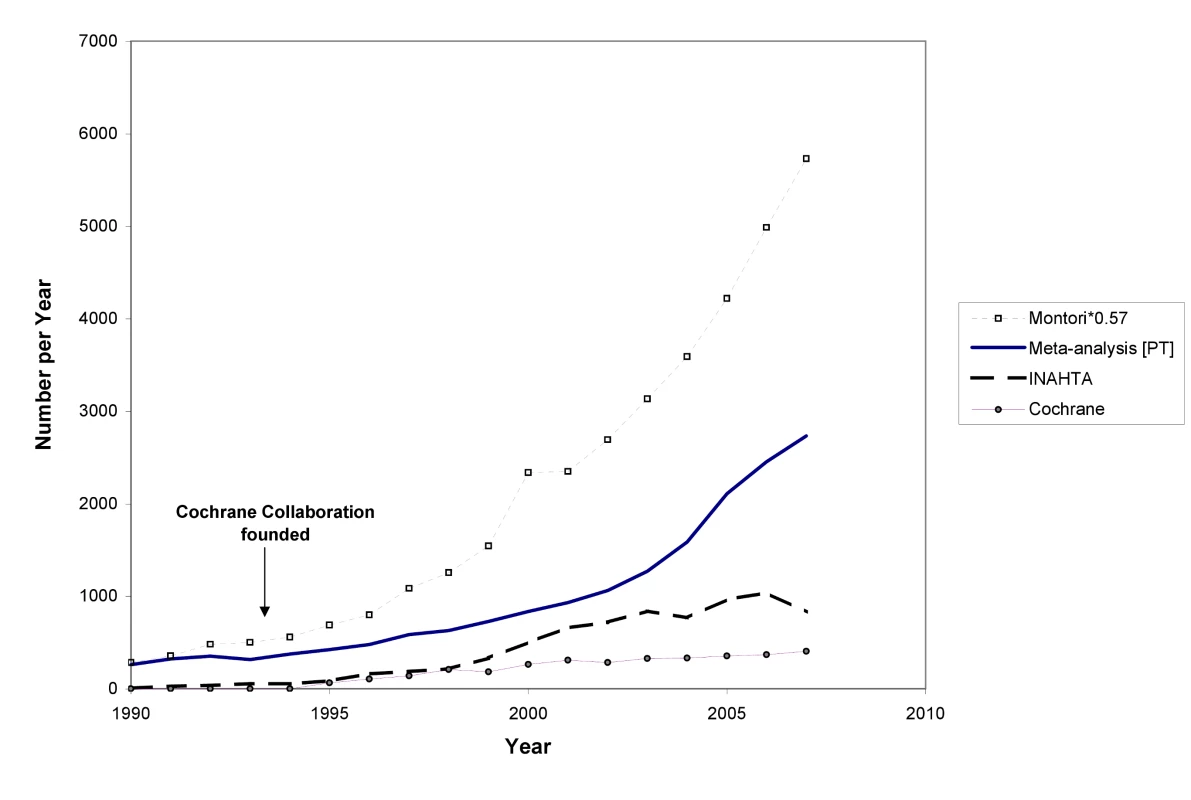
INAHTA is International Network of Agencies for Health Technology Assessment; the Montori systematic review filter is detailed in Text S1. Fig. 4. The rise in non-systematic reviews, case reports, trials, and systematic reviews, 1950 to 2007 (as identified in MEDLINE). 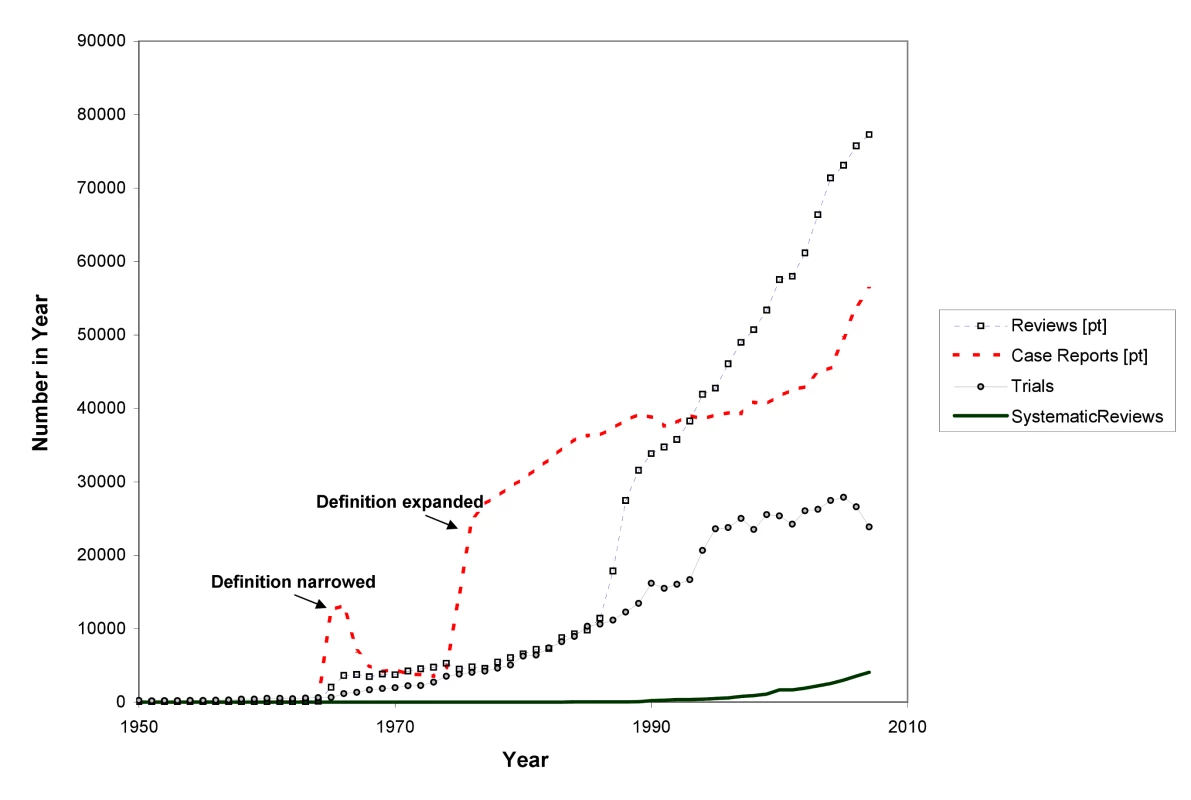
Furthermore, we are a long way from having all relevant trials incorporated into good systematic reviews. The workload involved in producing reviews is increasing, and the bulk of systematic reviews are now many years out of date [26]. The median number of trials contained within individual systematic reviews has been variously estimated at between six and 16 (Cochrane reviews now include an average of over 12 trials per review [27],[28]; M Clarke, personal communication), but many reviews have covered much the same territory. Thus, in the 30 years since systematic reviews began in earnest, with around 15 years of intensified and large-scale reviewing effort, only a minority of trials have been assessed in systematic reviews. Given the triple constraint posed by the growth in trials, the increasing complexity of review methods, and current resources, Archie Cochrane's vision will not be achieved without some serious changes in course—in particular, with a greater concentration on Cochrane's use of the word “relevant”.
Where to Now?
First, we need to prioritise effectively and reduce avoidable waste in the production and reporting of research evidence [29]. This has implications for trials as well as systematic reviews. Some funders and others will now not consider supporting a trial unless a systematic review has shown the trial to be necessary [30]. It is essential that this requirement be more widely adopted. And it is essential that reviews address questions that are relevant to patients, clinicians and policymakers.
Second, we may need to choose between elaborate reviews of a quarter of the questions clinicians and patients have or “leaner” reviews of most of what we want to know. The methodological standards for systematic reviewing have been increasing over time [28], and the evolution of standards in the Cochrane Collaboration and in HTA has been remarkable. The increase in steps and reporting required is reflected in the length of reviews. Early Cochrane reviews could typically be printed out in 10 or 20 pages, even when they incorporated several trials. Today, it is not unusual for a review by a health technology agency to run to several hundred pages. Often the reviews are longer than the combined length of the reports of all the included trials.
A contributing factor here is the increasing expectation for reviews to include study types other than randomised trials. This will often be essential for detecting less common adverse effects. However, the inclusion of all study types to answer all questions about the effects of treatments would not necessarily provide better quality information in every instance – while it would unquestionably increase the time and resource requirement for reviews. While it is vital that reviews are scientifically defensible, burdening those preparing them with excessive requirements could result in having valid answers to relatively few questions.
In particular, we need leaner and more efficient methods of staying up-to-date with the evidence. Using current methods, the Cochrane Collaboration has not been able to keep even half of its reviews up-to-date [31], and other organisations are in a similar predicament [32]. We need to develop innovative methods to reduce the labour of updating, and provide what clinicians and patients need: an assurance that a conclusion is not out of date, even if not every later trial is included within every analysis. It is also the responsibility of reviewer authors and journal editors to ensure that every new systematic review places itself clearly in context of other systematic reviews and HTAs. It will be to little avail to the average clinician, patient, and information provider, however, if the resulting knowledge is not comprehensible and openly accessible.
Finally, although more funding for evaluative clinical research internationally remains a priority, more international collaboration could result in better use being made of resources for systematic reviewing and HTAs. While multiple reviews on topics can provide a rounded picture of an area as well as a de facto form of updating when the reviews are conducted several years apart, there is also considerable duplication of review effort.
In November 2009, an international meeting in Cologne formed a new collaboration called “KEEP Up,” which will aim to harmonise updating standards and aggregate updating results. This should reduce the workload and enable organisations to be alerted when there are important shifts in evidence. Initiated and coordinated by the German Institute for Quality and Efficiency in Health Care (IQWiG) and involving key systematic reviewing and guidelines organisations such as the Cochrane Collaboration, Duodecim, the Scottish Intercollegiate Guidelines Network (SIGN), and the National Institute for Health and Clinical Excellence (NICE), this effort will provide a platform for tackling practical and methodological issues involved in keeping up-to-date.
There is nevertheless a risk that the increasing burdens placed on the methods of systematic reviewing could make the goal of keeping up-to-date with the knowledge won from trials recede ever more quickly into the distance. Perhaps one of the first questions we should ask whenever an additional process or more demanding methodology for systematic reviewing is proposed is this: Will this development serve or hinder our ability to better understand and communicate enough results from trials? In 1979, when Archie Cochrane argued that we needed critical summaries to keep up with the crucial knowledge those trials were generating, there were perhaps 14 trials a day being published. Thirty years later, it would be just as hard to keep up with the systematic reviews. Every day there are now 11 systematic reviews and 75 trials, and there are no signs of this slowing down: but there are still only 24 hours in a day.
Supporting Information
Zdroje
1. CochraneAL
1979 1931–1971: a critical review, with particular reference to the medical profession. Medicines for the Year 2000 London Office of Health Economics 1 11
2. LindJ
1753 A treatise of the scurvy. In three parts. Containing an inquiry into the nature, causes and cure, of that disease. Together with a critical and chronological view of what has been published on the subject. Edinburgh: Printed by Sands, Murray and Cochran for A Kincaid and A Donaldson. Accessed: 26 April 2009 http://www.jameslindlibrary.org/trial_records/17th_18th_Century/lind/lind_kp.html
3. DuncanA
1773 Introduction. Medical and Philosophical Commentaries. Volume First, Part I. London: J Murray 6 7
4. CummingsMM
1981 The National Library of Medicine.
WarrenKS
Coping with the biomedical literature: A primer for the scientist and the clinician New York Praeger 161 173
5. BarronBA
BukantzSC
1967 The evaluation of new drugs: current Food and Drug Administration regulations and statistical aspects of clinical trials. Arch Intern Med 119 547 556
6. BantaD
2003 The development of health technology assessment. Health Policy 63 121 132
7. StjernswärdJ
1974 Decreased survival related to irradiation postoperatively in early breast cancer. Lancet 304 1285 1286
8. ChalmersTC
1975 Effects of ascorbic acid on the common cold. An evaluation of the evidence. Amer J Med 58 532 536
9. ChalmersI
1979 Randomized controlled trials of fetal monitoring 1973–1977.
ThalhammerO
BaumgartenK
PollakA
Perinatal Medicine Stuttgart Georg Thieme 260 265
10. GoldschmidtPG
1986 Information synthesis: a practical guide. HSR: Health Services Research 21 215 236
11. MulrowCD
1987 The medical review article: state of the science. Ann Intern Med 106 485 488
12. L'AbbéKA
DetskyAS
O'RourkeK
1987 Meta-analysis in clinical research. Ann Int Med 107 224 232
13. SacksHS
BerrierJ
ReitmanD
Ancona-BerkVA
ChalmersTC
1987 Meta-analysis of randomized controlled trials. New Engl J Med 316 450 455
14. OxmanAD
GuyattGH
1988 Guidelines for reading literature reviews. Can Med Assoc J 138 697 703
15. JenicekM
1987 Méta-analyse en médecine. Évaluation et synthèse de l'information clinique et épidémiologique. St. Hyacinthe and Paris EDISEM and Maloine Éditeurs
16. ChalmersI
1991 The work of the National Perinatal Epidemiology Unit. One example of technology assessment in perinatal care. Int J Technol Assess Health Care 7 430 459
17. StarrM
ChalmersI
ClarkeM
OxmanAD
2009 The origins, evolution and future of The Cochrane Database of Systematic Reviews (CDSR). Int J Technol Assess Health Care 25 Suppl 1 182 195
18. HopewellS
LoudonK
ClarkeMJ
OxmanAD
DickersinK
2009 Publication bias in clinical trials due to statistical significance or direction of results. Cochrane Database Syst Rev Issue 1
19. LeeK
BacchettiP
SimI
2008 Publication of clinical trials supporting successful new drug applications: a literature analysis. PLoS Med 5 e191 doi:10.1371/journal.pmed.0050191
20. KassEH
1981 Reviewing reviews.
WarrenKS
Coping with the biomedical literature: a primer for the scientist and the clinician New York Praeger 79 91
21. ChanAW
AltmanDG
2005 Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 365 1159 1162
22. International Committee of Medical Journal Editors (ICMJE). Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Accessed on 24 April 2009 at: http://www.icmje.org/clin_trial.pdf
23. ZarinDA
IdeNC
TseT
HarlanWR
WestJC
2007 Issues in the registration of clinical trials. JAMA 297 2112 2120
24. One Hundred Tenth Congress of the United States of America. Food and Drug Administration Amendments Act of 2007 Accessed 17 May 2008 at: http://www.fda.gov/oc/initiatives/&HR3580.pdf
25. DijkersMPJM
The TaskGuidelines 2009 The value of “traditional” reviews in the era of systematic reviewing. Am J Phys Med Rehab 88 423 430
26. ShojaniaKG
SampsonM
AnsariMT
CoucetteS
MoherD
2007 How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med 147 224 233
27. MallettS
ClarkeM
2002 The typical Cochrane review. How many trials? How many participants? Int J Technol Assess Health Care 18 820 823
28. MoherD
TetzlaffJ
TriccoAC
SampsonM
AltmanDG
2007 Epidemiology and reporting characteristics of systematic reviews. PLoS Med 4 e78 doi:10.1371/journal.pmed.0040078
29. ChalmersI
GlasziouP
2009 Avoidable waste in the production and reporting of research evidence. Lancet 374 86 89
30. Danish Research Ethics Committee System 1997 Recommendation No. 20 Controlled clinical trials - the influence of existing and newly acquired scientific results on the research ethical evaluation. Copenhagen: Danish Research Ethics Committee System
31. KochGG
2006 No improvement – still less than half of the Cochrane reviews are up to date. XIV Cochrane Colloquium, Dublin, Ireland
32. GarrittyC
TsertsvadzeA
TriccoAC
SampsonM
MoherD
2010 Updating systematic reviews: an international survey. PLoS ONE 5 e9914 doi:10.1371/journal.pone.0009914
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Magnosolv a jeho využití v neurologii
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Examining the “Urban Advantage” in Maternal Health Care in Developing Countries
- Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: Raising the Bar
- Does It Matter Who Writes Medical News Stories?
- Drug Companies Should Be Held More Accountable for Their Human Rights Responsibilities
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Merck Perspective
- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis: A Multicenter, Randomized, Controlled Trial
- Combined Impact of Lifestyle-Related Factors on Total and Cause-Specific Mortality among Chinese Women: Prospective Cohort Study
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Perspective of the Former United Nations Special Rapporteur (2002-2008)
- AIDS Vaccine for Asia Network (AVAN): Expanding the Regional Role in Developing HIV Vaccines
- Are Drug Companies Living Up to Their Human Rights Responsibilities? Moving Toward Assessment
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
- Major Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: A Population-Based Cohort Study in Ontario
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Cost-Effectiveness of Pooled Nucleic Acid Amplification Testing for Acute HIV Infection after Third-Generation HIV Antibody Screening and Rapid Testing in the United States: A Comparison of Three Public Health Settings
- Community Case Management of Fever Due to Malaria and Pneumonia in Children Under Five in Zambia: A Cluster Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



