-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
CNGCs break through—A rice cyclic nucleotide-gated channel paves the way for pollen tube growth
article has not abstract
Published in the journal: . PLoS Genet 13(11): e32767. doi:10.1371/journal.pgen.1007066
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1007066Summary
article has not abstract
Rice cyclic nucleotide-gated channel 13 is a novel player in pollen tube-pistil signaling
Lower plants generate mobile sperm cells that must reach their female counterparts by swimming. This requirement for water is a disadvantage for these plants as compared with angiosperms, for which the dry pollen attaches to a stigma and becomes hydrated, enabling the emerging pollen tube to grow in the protected environment of the pistil. After adhesion, hydration, and germination of the pollen at the stigmatic papilla cells, the pollen tube enters the stigma and grows in the intercellular space between papilla cells towards the style and transmission tract (TT). The TT contains a nutrient-rich extracellular matrix (ECM) and guides the pollen tube to the ovary. After penetrating the septum, the pollen tube grows through the funiculus and then enters the ovule though the micropyle to deliver the two nonflagellate sperm cells to the two female gametes, leading to double fertilization, a prerequisite to seed formation [1]. The 2017 study by Xu et al. [2] reveals, for the first time, the importance of a Ca2+ signal generated by rice cyclic nucleotide-gated channel 13 (OsCNGC13) in the pistil to induce programmed cell death (PCD), which facilitates proper pollen tube growth. Furthermore, they showed this step significantly affects the yield of rice grains.
The events during pollen grain–stigma interaction and pollen tube reception are relatively well studied [1, 3], while much less is known about the growth of the pollen tube through the style and TT tissue—particularly the signaling between the pollen tube and the pistil tissue(s). Intracellular signaling in the pollen tube during pollen tube growth has been studied extensively [4]. The role of Ca2+ is well established: in the pollen tube, a Ca2+ gradient is essential for pollen tube guidance [5]. External Ca2+ from the pistil must be taken up by the pollen tube and is required for its growth. Several potential Ca2+ channels that are expressed in the pollen tube have been identified in Arabidopsis. These include two glutamate receptor-like (GLR) channels (GLR1.2 and GLR3.7) [6] and six cyclic nucleotide-gated channels (CNGCs; CNGC7, 8, 9, 10, 16, and 18), of which CNGC18 has been shown to be a Ca2+-conducting channel that is essential for tip growth in pollen tubes [7] and pollen tube guidance (Fig 1) [5].
Fig. 1. Ca2+ channels in pollen tubes and pistils in Arabidopsis and rice. 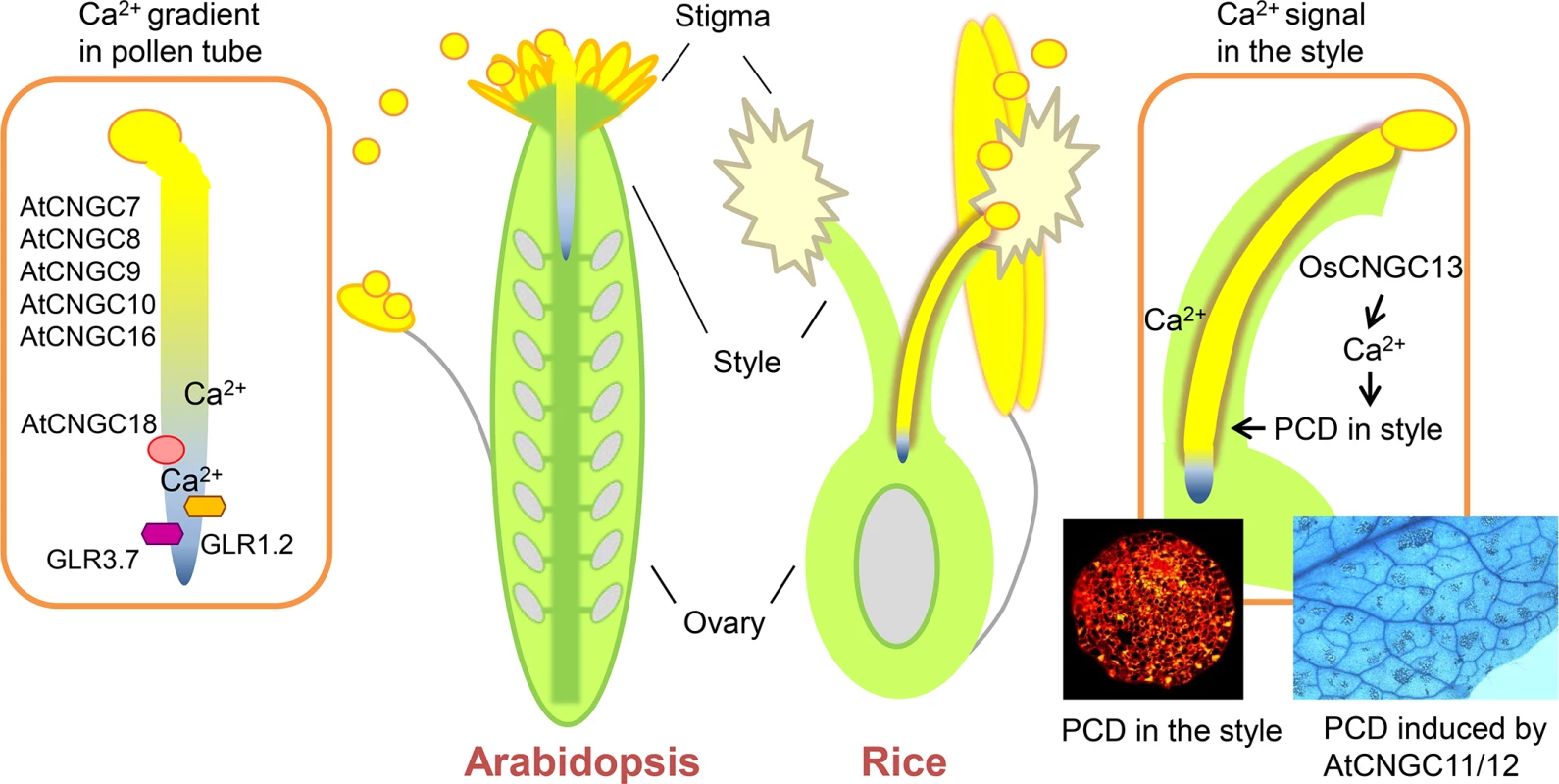
The Ca2+ gradient in pollen tubes is created by members of the CNGC and GLR families (left). Xu et al. [2] reveal a role of OsCNGC13 in the TT of the rice style, where a CNGC-mediated Ca2+ signal is required to induce cell death, which is necessary for the pollen tube to grow through the TT to reach the ovary (right). A role for CNGCs in PCD formation had been previously shown for AtCNGC11/12 [21]. CNGC, cyclic nucleotide-gated channel; GLR, glutamate receptor-like; PCD, programmed cell death; TT, transmission tract. Pollen tubes can grow in vitro; however, for the pollen tube to make its way through the style to the ovary, some communication with the sporophyte is necessary. But so far, few signaling components have been identified that drive the interaction between the TT and the pollen tube. On the pollen tube side, two membrane-localized leucine rich repeat (LRR) receptor kinases, LePRK1 and 2, have been identified that may interact with different ligands in different pistil tissues [8]. Other examples include the pollen tube-localized GLRs, GLR1.2 and GLR3.7. They are activated by D-serine, which is produced in the TT by the serine racemase SR1, which converts L-serine to its biologically active enantiomer [6]. The C2H2/C2HC zinc finger transcription factor No Transmitting Tract (NTT) has been identified as a key regulator of the ECM tissue; pollen tubes will terminate prematurely in ntt mutants [9]. Other factors in the TT that affect pollen tube growth are three HECATE transcription factors and the auxin response factors ARF6 and 8 [10].
Xu et al. show that the semi-seed-setting rate1-Dominant (sss1-D) rice mutant displays a low seed yield phenotype, caused by premature termination of pollen tube growth, which was connected to delayed PCD in the style. The causal mutation was mapped to OsCNGC13. An inversion of a DNA segment caused a truncation of this gene, resulting in the OsCNGC13-D protein with only 440 amino acids with five transmembrane domains but lacking the pore-forming region and the cytosolic C-terminal domain, which contains important regulatory domains such as cyclic nucleotide-binding domain (CNBD) and calmodulin binding domain(s) (CaMDB) [11]. The truncated protein does not act as a dominant negative because it did not bind to wild-type (WT) OsCNGC13 (although this aspect may need to be confirmed by additional experiments), but rather the expression of the truncated transcript seems to lead to a down-regulation of WT OsCNGC13, potentially through an RNA interference (RNAi) mechanism.
Thus, the dominant nature of sss1-D can be explained by haploinsufficiency of WT OsCNGC13. The levels of WT transcript are decreased in heterozygous plants as well as in OsCNGC13-D–overexpressing plants, while knocking out OsCNGC13 phenocopies the sss1-D mutant phenotype.
Increased expression of OsCNGC13 was detected in WT pistils 30 minutes after pollination. Patch-clamp whole cell recordings in HEK293 cells detected inward Ca2+ but not K+ currents. Furthermore, an increase in [Ca2+]cyt at the bottom of the style after pollination was shown, which was absent in sss1-D plants. Taken together, they concluded that a Ca2+ signal in the pistil mediated by OsONGC13 is required for normal pollen tube growth.
CNGC-mediated PCD in pollen tube growth and immunity
What makes this finding relevant for the broader CNGC research field is the connection between Ca2+ influx and PCD. At 30 minutes after pollination, TUNEL staining revealed small patches of dying cells in the style that widen the intercellular space, allowing the pollen tube to grow through the ECM (Fig 1). Prior to this study, several CNGCs had been connected to PCD and plant immunity, particularly AtCNGC11 and 12 (Fig 1) of group I and AtCNGC2 and 4 of group IVb [12]. OsCNGC13 is the rice ortholog of AtCNGC19, which belongs to group IVa. This is the first report to connect a group IVa channel to PCD.
So far, the role of AtCNGC19 has not been well established. It has been connected to NaCl stress responses [13], and gene expression studies show that it is up-regulated after pathogen treatment(s). Furthermore, AtCNGC19 KO lines also exhibit enhanced susceptibility against the necrotrophic pathogen Botrytis cinerea [12]. Interestingly, OsCNGC13 is also induced by a pathogen, Pseudomonas fuscovaginae [14], suggesting a role in immunity as well. This may indicate that the biological function of IVa CNGCs is conserved among different plant species, thus it will be interesting to know whether AtCNGC19 and 20 are also involved in PCD and/or pollen tube growth.
What can we learn regarding function and regulation of CNGCs?
Group IV CNGCs are the most divergent group of CNGCs, with two subclasses, IVa and IVb. The closest paralog to AtCNGC19—which is the orthlog of OsCNGC13—and sole other IVa group member is AtCNGC20, which is the ortholog of OsCNGC12 [14]. Loss-of-function mutants of AtCNGC2 or 4, which make up group IVb, show a clear morphological phenotype and display constitutive cell death [15]. The barley lesion mimic phenotype of the nec1 mutant is also caused by a mutation of the barley ortholog of AtCNGC4, indicating functional conservation in monocots [16]. Interestingly, AtCNGC2 has been connected to reduced fertility. Pollen tubes in cngc2 mutant pistils exhibited large rates of premature pollen tube termination prior to reaching the ovules, which was even more pronounced under increased Ca2+ concentrations [17].
The group IVa CNGCs differ from other CNGCs in two aspects. First, they possess a substantially larger N-terminal cytosolic domain (172–204 amino acids versus 40–100 for other CNGCs). Interestingly, a CaMBD is predicted for the AtCNGC19/OsCNGC13 N - termini (http://calcium.uhnres.utoronto.ca/ctdb/pub_pages/search/index.htm), although no CaM binding has been shown yet. Since they also possess a C-terminal isoleucine glutamine (IQ) domain (a type of CaMBD) [18], they may have a similarly complex regulation by calmodulin, as previously shown for AtCNGC12 [11].
Second, class IVa CNGCs have 10 additional amino acids in their CNBD between the phosphate binding cassette (PBC) and the hinge motifs, suggesting that this group is regulated differently than the other CNGCs [19].
In summary, the 2017 study by Xu et al. [2] connects [Ca2+]cyt accumulation, ECM composition, and PCD in the style after pollination, paving the way for further research into how signal transduction in the style allows the pollen tube to penetrate the style and TT. Furthermore, it demonstrates a function for another member of the CNGC family. Most of the family members (20 in Arabidopsis, 16 in rice) have not been functionally characterized, though there has been significant progress recently [20]. Future research challenges include uncovering the regulation of CNGC channel function by cyclic nucleotides (or other ligands) and the Ca2+ sensor protein, calmodulin, as well as determining whether CNGCs form homo - or hetero-tetrameric channels.
Zdroje
1. Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant 2013; 6 : 1018–1036. doi: 10.1093/mp/sst061 23571489
2. Xu Y, Yang J, Wang Y, Wang J, Yu Y, Long Y,et al. OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLoS Genet. 2017 13(7):e1006906. doi: 10.1371/journal.pgen.1006906 28708858
3. Doucet J, Lee HK, Goring DR. Pollen acceptance or rejection: a tale of two pathways. Trends Plant Sci. 2016; 21 : 1058–1067. doi: 10.1016/j.tplants.2016.09.004 27773670
4. Guan Y, Guo J, Li H, Yang Z. Signaling in pollen tube growth: crosstalk, feedback, and missing links. Mol Plant 2013; 6 : 1053–1064. doi: 10.1093/mp/sst070 23873928
5. Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, et al. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc Natl Acad Sci USA 2016; 113 : 3096–3101. doi: 10.1073/pnas.1524629113 26929345
6. Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, et al. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011; 332 : 434–437. doi: 10.1126/science.1201101 21415319
7. Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, et al. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci U S A. 2007; 104 : 14531–14536. doi: 10.1073/pnas.0701781104 17726111
8. Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S. LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J. 2004; 39 : 343–53. doi: 10.1111/j.1365-313X.2004.02139.x 15255864
9. Crawford BC, Ditta G, Yanofsky MF. The NTT gene is required for transmitting - tract development in carpels of Arabidopsis thaliana. Curr Biol 2007, 17 : 1101–1108. doi: 10.1016/j.cub.2007.05.079 17600712
10. Palanivelu R, Tsukamoto T. Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. WIREs Dev. Biol. 2012; 1 : 96–113.
11. DeFalco TA, Marshall CB, Munro K, Kang HG, Moeder W, Ikura M, et al. Multiple calmodulin-binding sites positively and negatively regulate Arabidopsis cyclic nucleotide-gated channel12. Plant Cell 2016; 28 : 1738–1751. doi: 10.1105/tpc.15.00870 27335451
12. Moeder W, Urquhart W, Ung H, Yoshioka K. The role of cyclic nucleotide-gated ion channels in plant immunity. Mol. Plant 2011; 4 : 442–452. doi: 10.1093/mp/ssr018 21459831
13. Kugler A, Kögler B, Palme A, Wolff P, Dietrich P Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol. 2009; 9 : 140. doi: 10.1186/1471-2229-9-140 19943938
14. Nawaz Z, Kakar KU, Saand MA, Shu QY. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genomics 2014; 15 : 853. doi: 10.1186/1471-2164-15-853 25280591
15. Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Bent AF. The Arabidopsis dnd1 "defense, no death" gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA. 2000; 97 : 9323–9328. doi: 10.1073/pnas.150005697 10900264
16. Rostoks N, Schmierer D, Mudie S, Drader T, Brueggeman R, Caldwell DG, et al. Barley necrotic locus nec1 encodes the cyclic nucleotide - gated ion channel 4 homologous to the Arabidopsis HLM1. Mol. Genet. Genomics 2006; 275 : 159–168. doi: 10.1007/s00438-005-0073-9 16341885
17. Chaiwongsar S, Strohm AK, Roe JR, Godiwalla RY, Chan CWM. A cyclic nucleotide-gated channel is necessary for optimum fertility in high-calcium environments. New Phytol. 2009; 183 : 76–87. doi: 10.1111/j.1469-8137.2009.02833.x 19368669
18. Fischer C, DeFalco T, Karia P, Snedden W, Moeder W, Yoshioka K, et al. Calmodulin as a Ca2+ sensing subunit ofArabidopsis cyclic nucleotide-gated channel complexes. Plant Cell Physiol. 2013; 54 : 573–584. doi: 10.1093/pcp/pct021 23385145
19. Zelman AK, Dawe A, Gehring C, Berkowitz GA. Evolutionary and structural perspectives of cyclic nucleotide-gated cation channels. Front. Plant Sci. 2012; 3 : 95. doi: 10.3389/fpls.2012.00095 22661976
20. DeFalco TA, Moeder W, Yoshioka K. Opening the gates: insights into cyclic nucleotide-gated channel-mediated signaling. Trends Plant Sci 2016; 21 : 903–906. doi: 10.1016/j.tplants.2016.08.011 27623305
21. Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, Berkowitz G, et al. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell. 2006;18 : 747–763. doi: 10.1105/tpc.105.038786 16461580
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2017 Číslo 11- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
Nejčtenější v tomto čísle- How convincing is a matching Y-chromosome profile?
- CNGCs break through—A rice cyclic nucleotide-gated channel paves the way for pollen tube growth
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



