-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Rise of FXR1: Escaping Cellular Senescence in Head and Neck Squamous Cell Carcinoma
article has not abstract
Published in the journal: . PLoS Genet 12(11): e32767. doi:10.1371/journal.pgen.1006344
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1006344Summary
article has not abstract
Cellular senescence is a key tumor-suppressing mechanism in response to numerous cellular threats including oxidative stress, telomere loss, and oncogene activation. It is essentially a permanent state of G1 cell cycle arrest in which cells remain viable and metabolically active. Recent studies indicate that senescence plays a pivotal role in suppression of tumorigenesis in vivo [1,2] and is frequently observed in different premalignant tumors such as lung adenomas, neurofibromas, and naevi [3,4]. Aside from its critical role in preventing cancer development, the senescence program also enhances the response to cancer therapy [5].
In order to become cancerous, cells must find ways to inactivate or bypass the senescence response. In fact, the viral oncoproteins E6 and E7 from human papillomavirus (HPV), which inhibit the tumor suppressors p53 and Rb, respectively, inactivate cellular senescence in response to oncogenic stress [6,7]. Thus, infection with HPV, an important risk factor for subsets of head and neck squamous cell carcinoma (HNSCC), could promote tumorigenesis by inhibiting cellular senescence. However, numerous HNSCCs are HPV-independent, thus underscoring the need to identify additional genetic alterations in HNSCC. In the September 2016 issue of PLOS Genetics, Majumber et al. reported that the Fragile X-related protein 1 (FXR1), an RNA-binding protein, suppresses the senescence response in two different HPV-negative HNSCC cell lines [8]. This further supports the requirement for bypassing senescence in both HPV-positive and -negative HNSCC and sheds light on the putative role of FXR1 in promoting HNSCC.
Bypass of Cellular Senescence by FXR1 and Transformation
FXR1 is a member of the Fragile X-related family of RNA-binding proteins, which also includes Fragile X Mental Retardation 1 (FMR1) and FXR2 and is frequently amplified in lung squamous cell carcinoma [9]. Majumber et al. have now revealed significant copy number amplification and mRNA overexpression of FXR1 in HNSCC by interrogating cancer genomics databases. Depletion of FXR1 in HNSCC cell lines caused G1 cell cycle arrest associated with features of cellular senescence, including senescence-associated β-galactosidase activity [10] and DNA damage foci [11]. The cellular response triggered by FXR1 depletion stimulated p53 levels and activity as indicated by concomitant elevation of p21, a classical p53-target gene mediating senescence [12]. Using a p53-mutated HNSCC cell line, the authors demonstrated p53-dependent up-regulation of p21 upon FXR1 knockdown. In addition, they confirmed the previously-described ability of FXR1 to directly destabilize p21 mRNA [13] in HNSCC, thereby revealing that FXR1 engages complementary p53-dependent and -independent mechanisms to control p21 levels in HNSCC. Further mining of genomics databases, as well as FISH analysis of tissue microarray and RNA quantification, revealed amplification of the telomerase RNA component TERC in HNSCC. The authors demonstrated that FXR1 is responsible for stabilizing TERC RNA and identified a G-rich region of TERC RNA that physically interacts with FXR1. Ultimately, the decreased TERC levels upon silencing of FXR1 interfered with telomerase activity, thus suggesting a role for FXR1 in acquisition of cell immortality, a critical prerequisite of neoplastic transformation [14]. The authors also demonstrated that depletion of p21 or ectopic expression of TERC allows bypass of senescence subsequent to loss of FXR1, further supporting their observation regarding FXR1-dependent regulation of p21 and TERC.
Only a handful of RNA-binding proteins have thus far been described for their role in modulating cellular senescence [15]. Majumber et al. now add FXR1 to this very short list by showing that this factor modulates p21 expression and stabilizes TERC RNA, in turn engaging different mechanisms to counteract cellular senescence and promote transformation (Fig 1). It would now be relevant to investigate whether the functionally related FMR1 and FXR2 also modulate cellular senescence. Also, further investigation of FXR1 amplification during HPV infection would provide additional clues as to whether FXR1 amplification and E6/E7 HPV proteins are mutually exclusive or cooperate to promote HNSCC. In addition, whether the requirement for FXR1 in cancer development is specific to HNSCC, or could apply to other tumour types exhibiting FXR1 amplification such as lung cancer [9], remains to be determined.
Fig. 1. FXR1 engages dual mechanisms to promote malignant transformation in HNSCC. 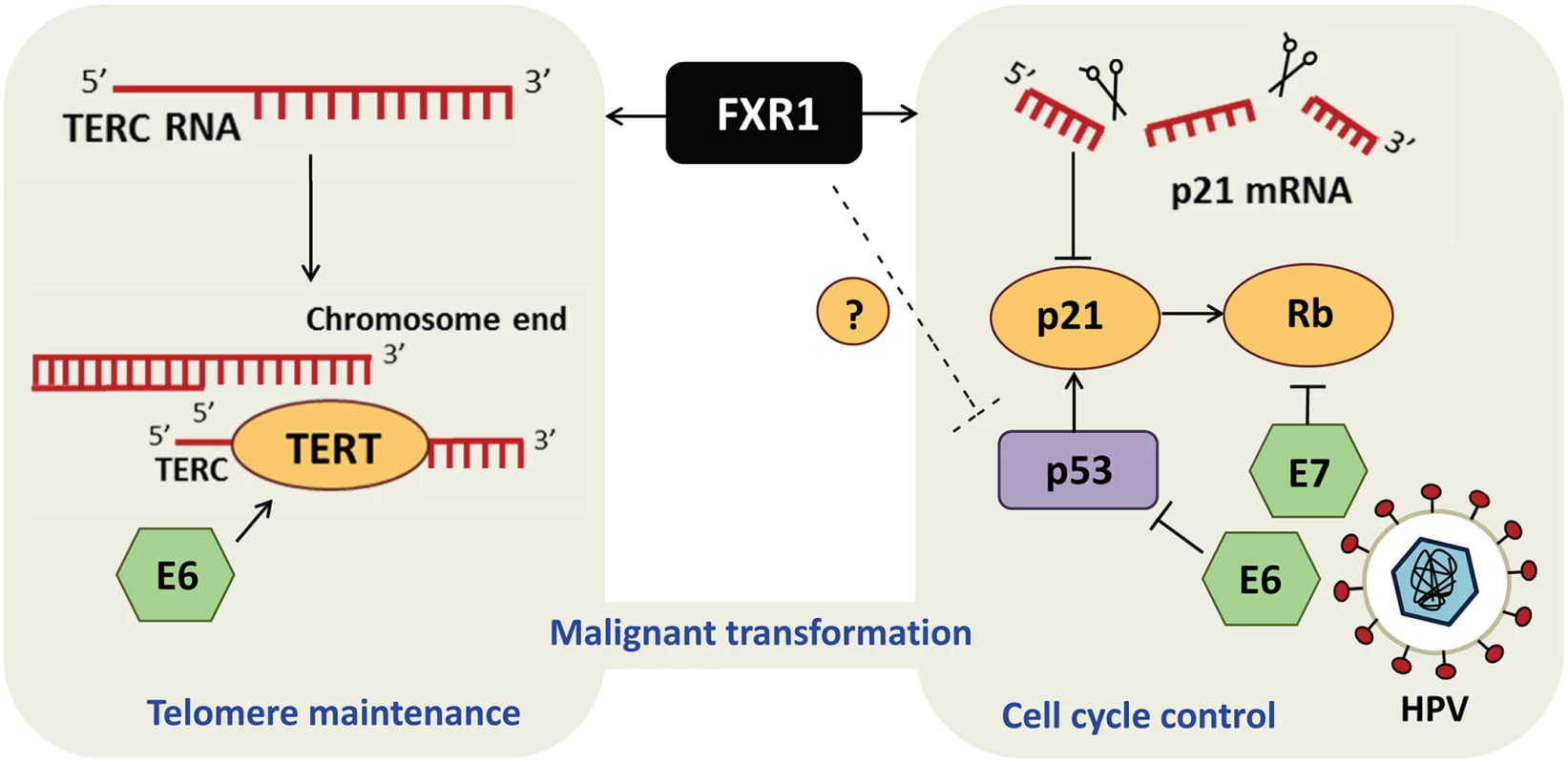
RNA-binding protein FXR1 regulates TERC RNA and p21 mRNA turnover to modulate telomerase activity (left panel) and cell cycle (right panel), respectively, to allow escape from senescence and stimulate transformation. Interestingly, the HPV oncoproteins E6 and E7, which contribute to subtypes of HNSCC, also modulate the p53/p21/Rb tumor suppressor pathways and telomerase activity, like FXR1. Modulating FXR1 Activity to Treat HNSCC and Facioscapulohumeral Muscular Dystrophy?
The senescence response triggered upon FXR1 depletion has been elegantly described and characterized by Majumber et al. and highlights the potential interest of interfering with the RNA-binding functions of FXR1 to treat oral squamous cell carcinoma (OSCC), the most common oral cancer. Nevertheless, the precise contribution of FXR1 amplification in the complex sequence of events leading to OSCC, as well as the underlying molecular mechanisms, remain to be elucidated. This would be expected to reveal additional therapeutic avenues to limit FXR1 expression in HNSCC.
In addition to its amplification in lung, breast, and ovarian as well as head and neck cancers [8,9], FXR1 was shown to be down-regulated in other human pathologies such as facioscapulohumeral muscular dystrophy, an inherited myopathy [16]. FXR1 plays an important role in myogenesis in both mouse and Xenopus. FXR1 knockout mice die shortly after birth and display disruption of the cellular architecture of skeletal and cardiac muscle [17]. Majumber et al. demonstrated that loss of FXR1 not only leads to senescence in cancer cells, but also in normal cells such as mouse embryonic fibroblasts [8]. Considering that cellular senescence limits the muscle stem cell regenerative capacity [18], it is reasonable to envisage that the phenotype observed following FXR1 dysfunction in facioscapulohumeral muscular dystrophy could be caused by concomitant overstimulation of cellular senescence, thus leading to muscle tissue degeneration. This considerably extends the potential reach of the discovery made by Majumber et al.
Cell Fate Decision by FXR1: Cellular Senescence or Quiescence?
The work of Majumber et al. raises an interesting point regarding the potential contribution of FXR1 to cell fate decisions. Previous reports described a role for this protein in mediating translation during quiescence [19]. The recent discovery by Majumber et al. that FXR1 loss promotes senescence, while FXR1 activity participates in quiescence, might provide a hint about the molecular mechanisms involved in making cell cycle arrest fate decisions between senescence and quiescence.
Zdroje
1. Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005; 436 : 660–665. doi: 10.1038/nature03841 16079837
2. Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005; 436 : 725–730. doi: 10.1038/nature03918 16079851
3. Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature 2005; 436 : 642. doi: 10.1038/436642a 16079833
4. Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005; 436 : 720–724. doi: 10.1038/nature03890 16079850
5. Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002; 109 : 335–346. 12015983
6. Hall AH, Alexander KA. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J Virol 2003; 77 : 6066–6069. doi: 10.1128/JVI.77.10.6066-6069.2003 12719599
7. Mallette FA, Goumard S, Gaumont-Leclerc MF, Moiseeva O, Ferbeyre G. Human fibroblasts require the Rb family of tumor suppressors, but not p53, for PML-induced senescence. Oncogene 2004; 23 : 91–99. doi: 10.1038/sj.onc.1206886 14712214
8. Majumber M, House R, Palanisamy N, Qie S, Day TA, Neskey D, et al. RNA-binding protein FXR1 regulates p21 and TERC RNA to bypass p53-mediated cellular senescence in OSCC. PLoS Genet. 2016; 12: e1006306. doi: 10.1371/journal.pgen.1006306 27606879
9. Qian J, Hassanein M, Hoeksema MD, Harris BK, Zou Y, Chen H, et al. The RNA binding protein FXR1 is a new driver in the 3q26-29 amplicon and predicts poor prognosis in human cancers. Proc Natl Acad Sci U S A 2015; 112 : 3469–3474. doi: 10.1073/pnas.1421975112 25733852
10. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92 : 9363–9367. 7568133
11. Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev 2007; 21 : 43–48. doi: 10.1101/gad.1487307 17210786
12. Chan HM, Narita M, Lowe SW, Livingston DM. The p400 E1A-associated protein is a novel component of the p53 —> p21 senescence pathway. Genes Dev 2005; 19 : 196–201. doi: 10.1101/gad.1280205 15655109
13. Davidovic L, Durand N, Khalfallah O, Tabet R, Barbry P, Mari B, et al. A novel role for the RNA-binding protein FXR1P in myoblasts cell-cycle progression by modulating p21/Cdkn1a/Cip1/Waf1 mRNA stability. PLoS Genet 2013; 9: e1003367. doi: 10.1371/journal.pgen.1003367 23555284
14. Hanahan DWeinberg RA, Hallmarks of cancer: the next generation. Cell 2011; 144 : 646–674. doi: 10.1016/j.cell.2011.02.013 21376230
15. Wang W. Regulatory RNA-binding proteins in senescence. Ageing Res Rev 2012; 11 : 485–490. doi: 10.1016/j.arr.2012.02.006 22414963
16. Davidovic L, Sacconi S, Bechara EG, Delplace S, Allegra M, Desnuelle C, et al. Alteration of expression of muscle specific isoforms of the fragile X related protein 1 (FXR1P) in facioscapulohumeral muscular dystrophy patients. J Med Genet 2008; 45 : 679–685. doi: 10.1136/jmg.2008.060541 18628314
17. Mientjes EJ, Willemsen R, Kirkpatrick LL, Nieuwenhuizen IM, Hoogeveen-Westerveld M, Verweij M, et al. Fxr1 knockout mice show a striated muscle phenotype: implications for Fxr1p function in vivo. Hum Mol Genet 2004; 13 : 1291–1302. doi: 10.1093/hmg/ddh150 15128702
18. Blanc RS, Vogel G, Chen T, Crist C, Richard S. PRMT7 preserves satellite cell regenerative capacity. Cell Rep 2016; 14 : 1528–1539. doi: 10.1016/j.celrep.2016.01.022 26854227
19. Bukhari SI, Truesdell SS, Lee S, Kollu S, Classon A, Boukhali M, et al. A specialized mechanism of translation mediated by FXR1a-associated microRNP in cellular quiescence. Mol Cell 2016; 61 : 760–773. doi: 10.1016/j.molcel.2016.02.013 26942679
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2016 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Suggestions from Geroscience for the Genetics of Age-Related Diseases
- The Mighty Fruit Fly Moves into Outbred Genetics
- The Rise of FXR1: Escaping Cellular Senescence in Head and Neck Squamous Cell Carcinoma
- A Helping Hand: RNA-Binding Proteins Guide Gene-Binding Choices by Cohesin Complexes
- The Genetic Architecture of Quantitative Traits Cannot Be Inferred from Variance Component Analysis
- Ancient Out-of-Africa Mitochondrial DNA Variants Associate with Distinct Mitochondrial Gene Expression Patterns
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Suggestions from Geroscience for the Genetics of Age-Related Diseases
- The Mighty Fruit Fly Moves into Outbred Genetics
- Ancient Out-of-Africa Mitochondrial DNA Variants Associate with Distinct Mitochondrial Gene Expression Patterns
- A Helping Hand: RNA-Binding Proteins Guide Gene-Binding Choices by Cohesin Complexes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



