-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMouse Y-Encoded Transcription Factor Is Essential for Sperm Formation and Function in Assisted Fertilization
The mammalian Y chromosome was once thought to be a genetic wasteland with testis determinant Sry being the only gene of importance. We now know that there are many genes on this chromosome crucial for male reproduction but their specific roles are often undefined. Here, we investigated the function of the Y chromosome gene Zfy2 during a final step of male gamete formation. We demonstrated that Zfy2 is responsible for allowing sperm precursor cells, haploid round spermatids, to undergo transformation into spermatozoa, and that these sperm are capable of yielding live offspring when injected into the oocytes. Thus, we identified a novel role of the Zfy2 gene during spermatogenesis and fertilization. Considering that in human sperm formation is a prerequisite for male infertility treatment using assisted reproduction technologies, our finding bear translational significance.
Published in the journal: . PLoS Genet 11(12): e32767. doi:10.1371/journal.pgen.1005476
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005476Summary
The mammalian Y chromosome was once thought to be a genetic wasteland with testis determinant Sry being the only gene of importance. We now know that there are many genes on this chromosome crucial for male reproduction but their specific roles are often undefined. Here, we investigated the function of the Y chromosome gene Zfy2 during a final step of male gamete formation. We demonstrated that Zfy2 is responsible for allowing sperm precursor cells, haploid round spermatids, to undergo transformation into spermatozoa, and that these sperm are capable of yielding live offspring when injected into the oocytes. Thus, we identified a novel role of the Zfy2 gene during spermatogenesis and fertilization. Considering that in human sperm formation is a prerequisite for male infertility treatment using assisted reproduction technologies, our finding bear translational significance.
Introduction
Y chromosome has always been considered a symbol of maleness as it encodes testis determining gene Sry which acts in the developing gonads and induces the development of testes rather than ovaries [1–3]. Mammalian Y chromosomes encode a number of other genes most of which are thought to be involved in various aspects of male reproduction, and other playing roles of broadly expressed regulators of transcription, translation and protein stability [4]. In spite of these clearly important functions, the knowledge linking the roles of specific Y chromosome genes to specific reproductive processes remains limited.
We recently investigated spermatogenesis progression and germ cell function in male mice with significantly abrogated Y chromosome complements [5]. We have shown that males with the Y chromosome contribution provided by two transgenes, the testis determinant Sry and the spermatogonial proliferation factor Eif2s3y (Fig 1B, XEOSry and XEY*XSry) have meiotic and postmeiotic arrest, the rare spermatids present in the testes do not elongate, and sperm are not formed. When round spermatids from these males were injected into the oocytes, live mouse progeny were obtained. The success of round spermatid injection (ROSI) was low, with less than 10% of transplanted embryos developing to live offspring. Interestingly, when the Sry transgene was replaced with the Y chromosome derived sex reversal factor Sxrb, encoding for Sry, H2al2y, Prssly, Teyorf1, Rbmy gene cluster, and Zfy2/1 fusion gene (Fig 1A, Sxrb) the resulting males (Fig 1B, XESxrbO and XESxrbY*X) had more advanced spermatid development with clear elongation of these cells, occasional appearance of sperm, and increased ROSI efficiency.
Fig. 1. Mouse X and Y chromosomes, variant sex chromosomes, and mouse genotypes relevant to this study. 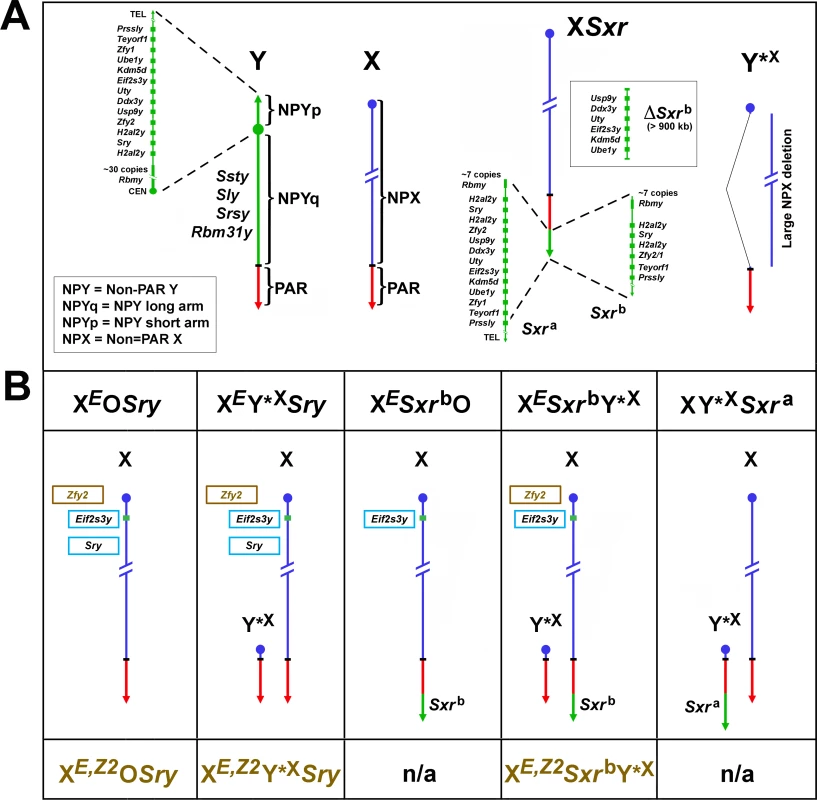
(A) The mouse Y chromosome contains ~90 Mb of male specific DNA and ~0.7 Mb constituting the pseudoautosomal region (PAR) situated at the end of the long arm. The PAR is the region of homology with the X that mediates pairing and recombination between the X and Y in normal males. The remaining non-pairing male specific part of Y (NPY) contains several genes and gene families. On the short arm (NPYp), there are single-copy genes: Prssly, Teyorf1, Uba1y, Smcy/Kdm5d, Eif2s3y, Uty, Dby/Ddx3y, Usp9y, Sry, duplicated gene Zfy (Zfy1 and 2), duplicated gene H2al2y, and a multi-copy gene Rbmy. The non-pairing region of the long arm (NPYq), representing ~90% of all NPY, contains mostly repetitive sequences, and encodes multiple copies of 5 distinct genes that are expressed in spermatids: Ssty1 and Ssty2, Sly, Srsy, Rbm31y [6]. Y*X is an X chromosome derivative encoding PAR, X centromere and near centromeric region. Sxra is a sex reversal variant Tp(Y)1CtSxr-a encoding almost intact NPYp complement but with Rbmy gene family reduced. Sxrb is a Sxra derivative with a 1.3 Mb deletion that has removed the majority of the NPYp gene complement and created a Zfy2/1 fusion gene. (B) The mice used in this study and their Y chromosome contribution. The X chromosome located Eif2s3y and autosomally located Sry transgenes, are shown in light blue frames. The Zfy2 transgene, shown in brown frame, is located on the X chromosome in the Hrpt locus in close proximity to the Eif2s3y transgene. The genotype designations without the Zfy2 transgene are shown above the diagrammatic representation of sex chromosomes and with the Zfy2 transgene below them (brown font). Sxra and Sxrb gene content is shown in A. n/a = mice with transgenic Zfy2 addition were either not produced or not examined in this study. These findings indicated that a gene/s encoded within Sxrb plays a role in spermiogenesis progression and germ cell function. Here, we identify Zfy2 as the gene responsible. We present the evidence that the Y chromosome gene Zfy2 promotes sperm morphogenesis, improves ROSI success, and is necessary for a formation of sperm capable of yielding live offspring after intracytoplasmic injection into the oocytes.
Results
Sperm from XESxrbO and XESxrbY*X males are not functional in assisted fertilization
The presence of Sxrb enables spermatid elongation in XESxrbO and XESxrbY*X males, with occasional development of mature testicular sperm [5,7] (S1 Fig). To test for the ability of these testicular sperm to participate in successful assisted fertilization and embryo development, we performed intracytoplasmic sperm injection (ICSI). No live offspring were obtained from XESxrbO males (n = 4 males, 0/94 fetuses from embryos transferred), while ICSI with sperm from XESxrbY*X males yielded a single fetus (n = 5 males, 1/84 fetuses from embryos transferred). Thus, sperm from XESxrbO and XESxrbY*X males are virtually not successful in assisted fertilization.
A possible reason for the lack of live offspring from these sperm could be sperm diploidy. We have previously shown that the great majority (86%) of round spermatids from XESxrbO males were diploid while the opposite was true for XESxrbY*X males, in which haploid round spermatids predominated (71%) [5]. To test whether testicular sperm from these males carried doubled DNA content we performed zygotic chromosome analysis after sperm injection (Table 1, S2 Fig). This analysis demonstrated that only about one-fourth of the embryos obtained after ICSI with sperm from XESxrbO males were diploid (26%, 12/46, Table 1); the remaining zygotes were triploid and thus presumably derived from diploid sperm. Zygotes obtained after ICSI with sperm from XESxrbY*X males were predominantly diploid (64%, 9/14, Table 1). These data support that while sperm diploidy might be responsible for the lack of ICSI success with XESxrbO, it is not likely the case with XESxrbY*X males. We have shown earlier that testicular sperm from males carrying the Y chromosome derived sex reversal factor Sxra (Fig 1A & 1B, Sxra, XY*XSxra) are haploid [8]. Thus, functional deficiency of sperm from XESxrbY*X males is likely due to lack of one or more Y chromosome genes that are present in XY*XSxra and not in XESxrbY*X.
Tab. 1. Zygotic chromosome analysis after ICSI with sperm from XESxrbO and XESxrbY*X males. 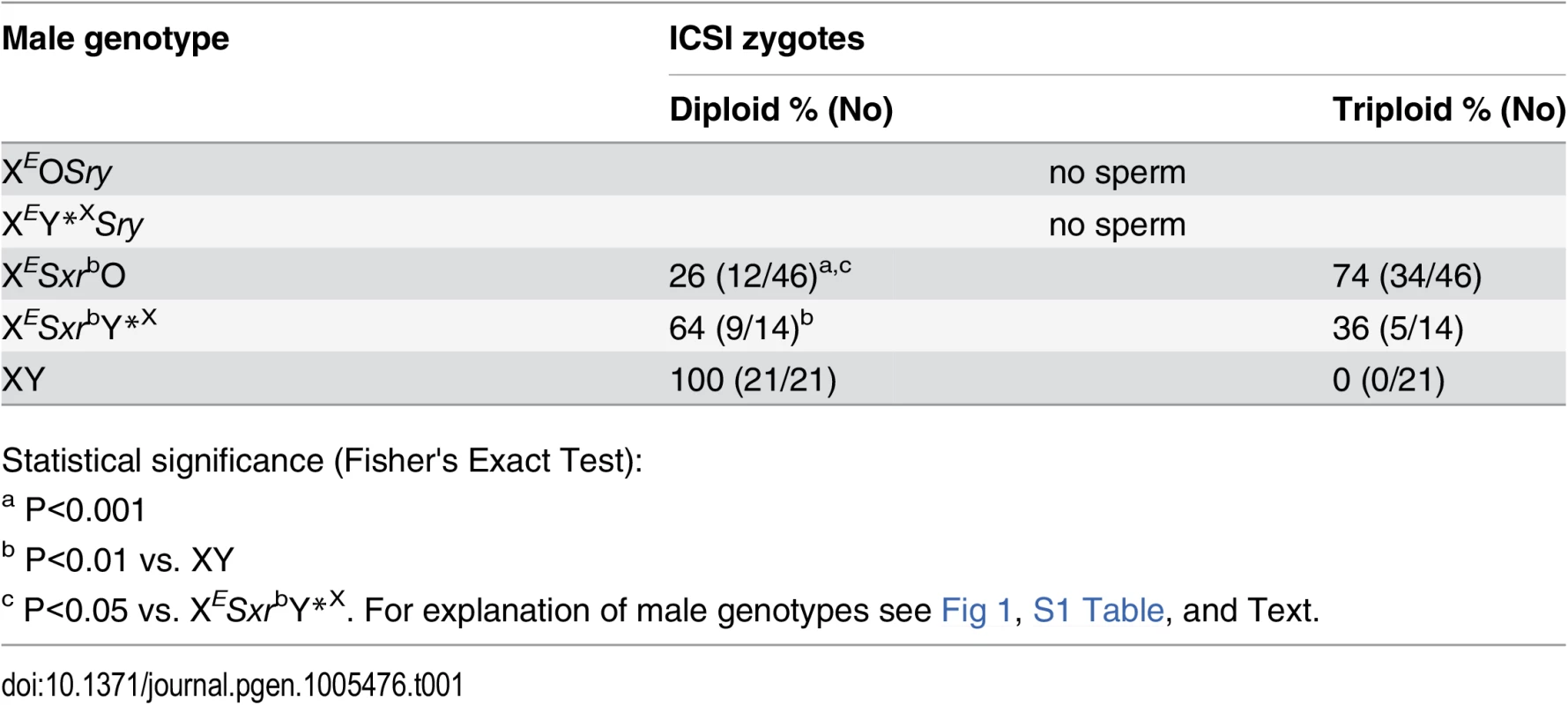
Statistical significance (Fisher's Exact Test): Overall, the data demonstrate that testicular sperm from XESxrbO and XESxrbY*X males are not functional in assisted fertilization and that this may reflect lack of certain Y gene/s.
Addition of Zfy2 to XEY*XSry males enables spermatid elongation
We next investigated which of the Sxrb genes is responsible for spermatid elongation. The gene content of Sxrb is represented by few copies of Rbmy, two copies of H2al2y, one copy of Sry, Prssly and Teyorf1, and a Zfy2/1 fusion gene spanning the Sxrb deletion breakpoint (Fig 1A). Rbmy appears early during spermatogenesis and is not expressed, and certainly not translated, after the zygotene stage [9]. H2al2y has been shown to be expressed late during spermiogenesis [10,11] and Sry transcripts in adult gonads are thought to be aberrant and not translatable [12,13]. Prssly and Teyorf1 are newly discovered genes [6] whose expression has not been characterized; we were not aware of these genes existence when the study was initiated. Based on the known expression pattern, we excluded Rbmy, H2al2y, and Sry as the candidates for ensuring sperm head and tail morphogenesis in males with Sxrb, and focused our attention on the Zfy2/1 fusion gene.
Postnatal expression of Zfy1 and Zfy2 is restricted to spermatogenic cells [14–16]. Both genes are first expressed in the testis around the time when cells enter meiosis. They then undergo transcriptional silencing as the cells enter pachynema. The expression is reactivated in secondary spermatocytes and continues postmeiotically [11,17]. In round spermatids there is a clear predominance of Zfy2 transcripts in Y-bearing round spermatids; this strong expression appears because of the activity of 'acquired' strong Cypt-derived spermatid-specific promoter driving Zfy2 expression [17]. The CYPT exon of Zfy2, encoding the Cypt1 promoter is thought to be derived from the Cypt1 gene [14,17], which belongs to the CYPT spermatid-specific gene family [18]. The expression of Zfy1, which lacks the Cypt promoter, is limited at the round spermatid stage. The Zfy2/1 fusion gene is driven by the Cypt promoter and is strongly expressed in spermatids. Our expectation therefore was that Zfy2, and not Zfy1, would mimic the effect of Sxrb.
To assess Zfy2 role in spermatogenesis progression we investigated testis histology in XEY*XSry males transgenic for Zfy2 (Fig 2 and S3 Fig). These males are subsequently called XE,Z2Y*XSry (Fig 1B, S1 Table). While in XEY*XSry males spermatid development did not progress beyond the round spermatid stage, in XE,Z2Y*XSry males spermatids elongated (Fig 2A). The elongated, condensed spermatids were more frequently observed in XE,Z2Y*XSry than in XESxrbY*X males; in the latter genotype elongation often ceased earlier (at step 12–13) and the spermatid nuclei were less compacted, with lighter staining pattern. Quantitative analysis of spermatogenesis progression (Fig 2B) demonstrated that XE,Z2Y*XSry had more round spermatids than XEY*XSry (~2.8-fold increase), reaching a level similar to that observed with XESxrbY*X males, but less than in wild-type controls. The number of elongating/elongated spermatids in XE,Z2Y*XSry and XESxrbY*X was not significantly different, and ~10-fold lower than in wild-type controls. In the quantitative analysis of testis sections we did not distinguish between the elongating and elongated spermatids because the abnormal morphology of developing spermatids, which ultimately resulted in severely morphologically abnormal headshape of testicular and epididymal sperm, made such distinction impossible.
Fig. 2. Histology analysis. 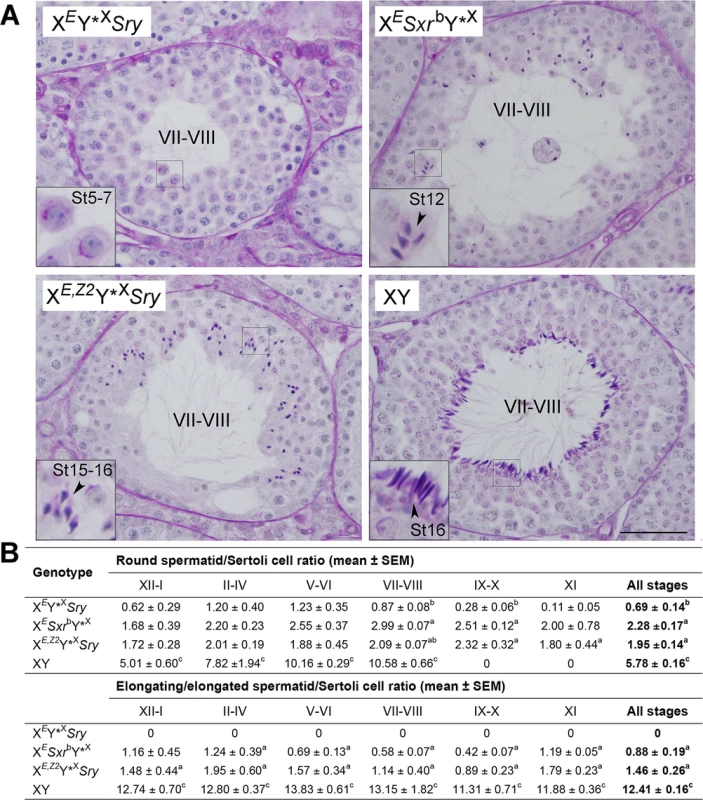
(A) Exemplary tubules of stage VII-VIII testis sections. XEY*XSry males have meiotic and post-meiotic arrest that only occasionally allow formation of round spermatids that do not develop beyond step 7 of spermatid development. In XESxrbY*X spermatid elongation is observed but usually ceases at step 11–12, with few occurrences of more advanced stages. In XE,Z2Y*XSry males spermatogenesis is progressing with good spermatid elongation and many spermatids developing to step 15–16; these elongated spermatids are morphologically abnormal, which is expected from males lacking NPYq genes [19]. Tubule stages are shown in Roman numerals and steps of spermatid development (St) in Arabic numerals. Bar = 50 μm; insets = x3 magnification. See also S3 Fig emphasizing spermatid at step 7–8. (B) Quantitative analysis of spermatogenesis progression. For each male 10 tubules were examined per stage and the numbers of round spermatid (steps 1–8), elongating/ed spermatid (steps 9–16), and Sertoli cells were counted. The data are expressed as spermatid/Sertoli cell ratios. In wild-type males no round spermatids are present in stages IX-XI so those observed in males with limited Y gene complement represent 'delayed spermatids'. Statistical significance (t-test): a different than XEY*XSry; b different than XESxrbY*X; c different than all other. Three males per genotypes were included in the analysis. For explanation of male genotypes see Fig 1, S1 Table, and text. Sperm from XEY*XSry males have headshape defects
Sperm from XE,Z2Y*XSry males were also observed in live epididymal and testicular cell suspension, with and on silver stained testicular cell spreads (S4 Fig). The epididymal sperm were extremely rare; only few immotile sperm were noted in 4 out of 5 males. The headshape of both testicular and epididymal sperm was abnormal, as expected from males lacking Y chromosome long arm [19]. To characterize structural sperm defects in more detail we performed the analysis of sperm headshape on silver stained testicular cell spreads (Fig 3). Only sperm with fully developed tails were included in this analysis. In XY males, the great majority of testicular sperm were normal (84%, 31/37), with remaining having slight headshape defects, comparable to those noted earlier in epididymal sperm [19]. In XY*XSxra, XESxrbY*X and XE,Z2Y*XSry males all sperm were morphologically abnormal. We divided the observable headshape defects into 7 categories (A-G) (Fig 3B) and quantified their incidence (Fig 3A). In XE,Z2Y*XSry males sperm heads were either oval or rounded in shape, with no hint of a hooked tip, and frequently highly condensed (categories D and E, 61%), or were elongated with no curvature reminiscent of crescent shape typical for mouse sperm, and occasional hint of a hooked tip (category B, 39%). Sperm in XY*XSxra males, which similarly as XE,Z2Y*XSry lack Y chromosome long arm, had better developed heads than in XE,Z2Y*XSry males, with predominance of sperm with clear head elongation with or without curvature, and with and without a marked hooked tip (category A and B, 66%), suggesting that presence of additional Y genes within Sxra facilitates head restructuring. In XESxrbY*X males the great majority of sperm were scored as elongated but poorly condensed (category G, 74%; this category was specific for this genotype). The tail development in all examined genotypes was normal (see S4 Fig for images of whole testicular sperm from XE,Z2Y*XSry males).
Fig. 3. Sperm headshape analysis. 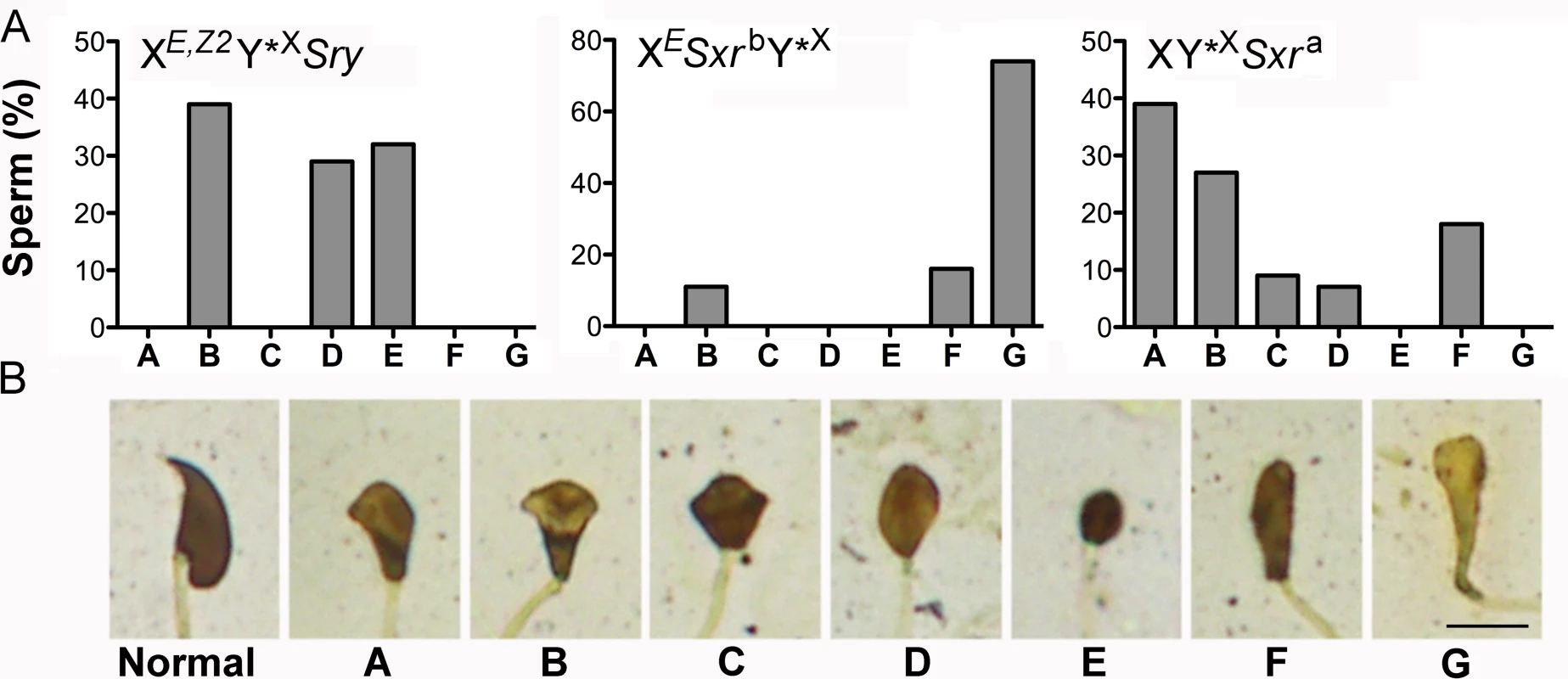
(A) The distribution of specific headshape defect categories among testicular sperm from XE,Z2Y*XSry (n = 41 sperm from 3 males), XESxrbY*X (n = 19 sperm from 2 males), and XY*XSxra (44 sperm from 3 males) males. (B) The categories of headshape defects. Normal: represents a normal shape of testicular sperm head. A ("dolphin"): sperm head is elongated and has some curvature reminiscent of crescent shape typical for mouse sperm, small hooked tip can be differentiated. B ("mushroom"): sperm head is elongated but the curvature is not present, hint of a hooked tip can sometimes be observed. C ("cupcake"): sperm head is no longer elongated, the caudal side is wider than in A and B categories, and opens up to a wide dorsal side; hint of a hooked tip can be sometimes be seen. D ("egg"): sperm head has an oval shape with no mark of a hooked tip. The head is less elongated than in category B; E ("ball"): sperm head has a round shape with no hint of a hooked tip, is smaller than in all other categories, and is strongly stained indicative of high DNA condensation; F ("drumstick"): sperm head is elongated, has no traces of a hooked tip, is longer and thinner than in category A&B but shorter than in G. G ("club"): sperm head is clearly elongated with no hint of a hooked tip, and very poorly condensed. Scale = 5 μm. Altogether, our data support that presence of Zfy2 enables round spermatids to initiate and undergo head morphogenesis and complete tail development. The Zfy2 in XE,Z2Y*XSry makes this transition much more effectively than Zfy2/1 in XESxrbY*X but does not reach the level observed in males with Y gene contribution provided by Sxra. In all genotypes this restructuring does not proceed normally and yields sperm with severely amorphous heads.
Addition of Zfy2 to XEOSry and XEY*XSry males increases the success of round spermatids injection (ROSI) and allows for successful ICSI yielding live offspring
In our recent study we have shown that ROSI success with XEOSry and XEY*XSry males was below 10% (9% and 6%, respectively) while with XESxrbO and XEY*XSxrb males ROSI efficiency increased by ~2–2.5 fold (20% and 16%, respectively), suggesting that a gene/s encoded within Sxrb provides some benefit for assisted reproduction success [5]. Considering the Zfy2 role in meiotic progression [11] and spermatid elongation (Figs 2 and 3, S3 & S4 Figs) we decided to test whether Zfy2 is beneficial for germ cell function. When ROSI was performed with round spermatids from XE,Z2OSry and XE,Z2Y*XSry males (Fig 1B, S1 Table) live offspring rate increased, reaching 27% and 43%, respectively (Table 2).
Tab. 2. The results of round spermatid injection (ROSI) and intracytoplasmic sperm injection (ICSI) with germ cells from males with limited Y gene complement. 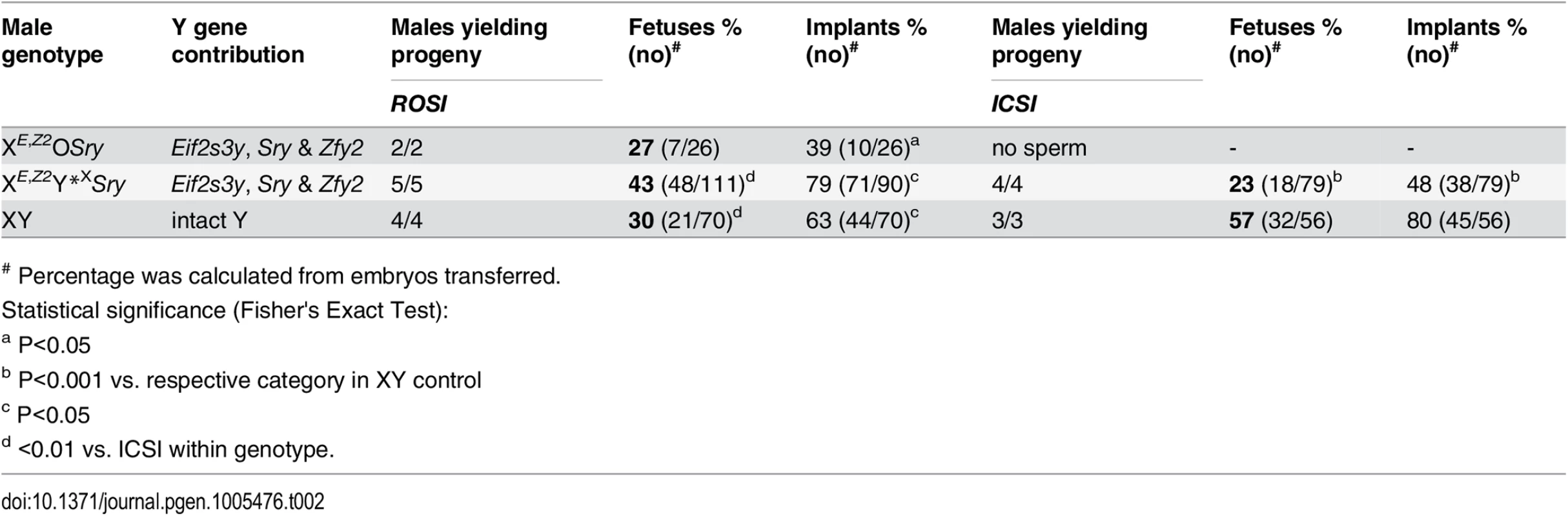
# Percentage was calculated from embryos transferred. Sperm from XESxrbO and XESxrbY*X males were not functional in assisted fertilization, and for XESxrbY*X males it could be attributed to Y gene deficiency. To test whether Zfy2 influences ICSI outcome, we performed injections with sperm from an XE,Z2Y*XSxrb male, which had both Sxrb (encoding the Zfy2/1 fusion gene) and the Zfy2 transgene (Fig 1B). We had only one male of this genotype available as sperm donor for ICSI, and only 7 embryos were transferred but those yielded 2 live offspring (29%, 2/7). Encouraged by this result we moved on to test sperm from XE,Z2Y*XSry males. Out of 5 males examined, 4 had testicular sperm and yielded live ICSI offspring (Table 2). The efficiency of ICSI with sperm from XE,Z2Y*XSry males was lower than with sperm wild-type XY controls (23% vs. 57%, P<0.001). Because each XE,Z2Y*XSry and XY male provided both round spermatids (ROSI) and sperm (ICSI) for injections, we were able to perform a direct comparison of these two types of germ cells for their ability to participate in successful assisted fertilization. In XY males, as expected, the efficiency of ROSI was lower than ICSI (Table 2, 30% vs. 57%, P<0.01). Interestingly, this pattern was reversed in XE,Z2Y*XSry males, in which ROSI success was significantly better (Table 2, 43% vs. 23%, P<0.01).
XE,Z2Y*XSry males generate several types of gametes, which consequently lead to several possible progeny genotypes. Genotyping of all progeny obtained after ROSI and ICSI revealed that anticipated offspring types were produced and their frequency met the expectancy, with 4 predominating genotypes accounting for 98.5% of all genotypes and distributed within 16%-31% range, and 1 rare genotype, which originated from atypical segregation of sex chromosomes (Fig 4 and S2 Table).
Fig. 4. Progeny genotype frequencies. 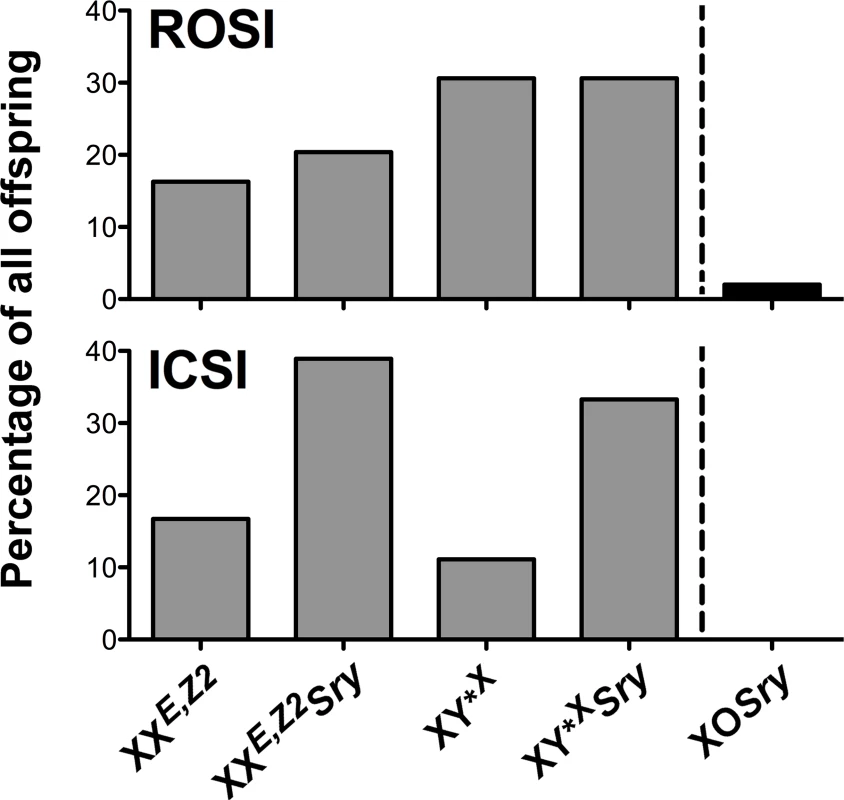
Frequency of the offspring genotypes obtained after assisted reproduction with XE,Z2Y*XSry males. Four predominant genotypes (grey bars) and one rare genotype derived from untypical sex chromosome segregation (black bar on the right side of the dashed line) were observed. These genotypes are expected from XE,Z2Y*XSry males. Number of genotyped progeny was 49 for ROSI and 18 for ICSI. See also S2 Table. Zfy expression analysis supports the role of Zfy2, and not Zfy1, in sperm function
To correlate spermiogenic phenotype with Zfy expression we performed Zfy transcript quantification on whole testes from XEY*XSry (no Zfy), XESxrbY*X (Zfy2/1 fusion gene), XE,Z2Y*XSry (Zfy2 transgene), with XY*XSxra (endogenous Zfy2 and Zfy1 and no NPYq) and XY (intact Y chromosome) serving as controls. Because Zfy2 and Zfy1 are very similar (97% and 94% for transcript and amino acid identity, respectively) we failed to design real-time PCR primers that were specific to Zfy2. We therefore quantified the expression of Zfy1, and Zfy1 and Zfy2 combined (global) (Fig 5 and S5 Fig).
Fig. 5. Zfy expression. 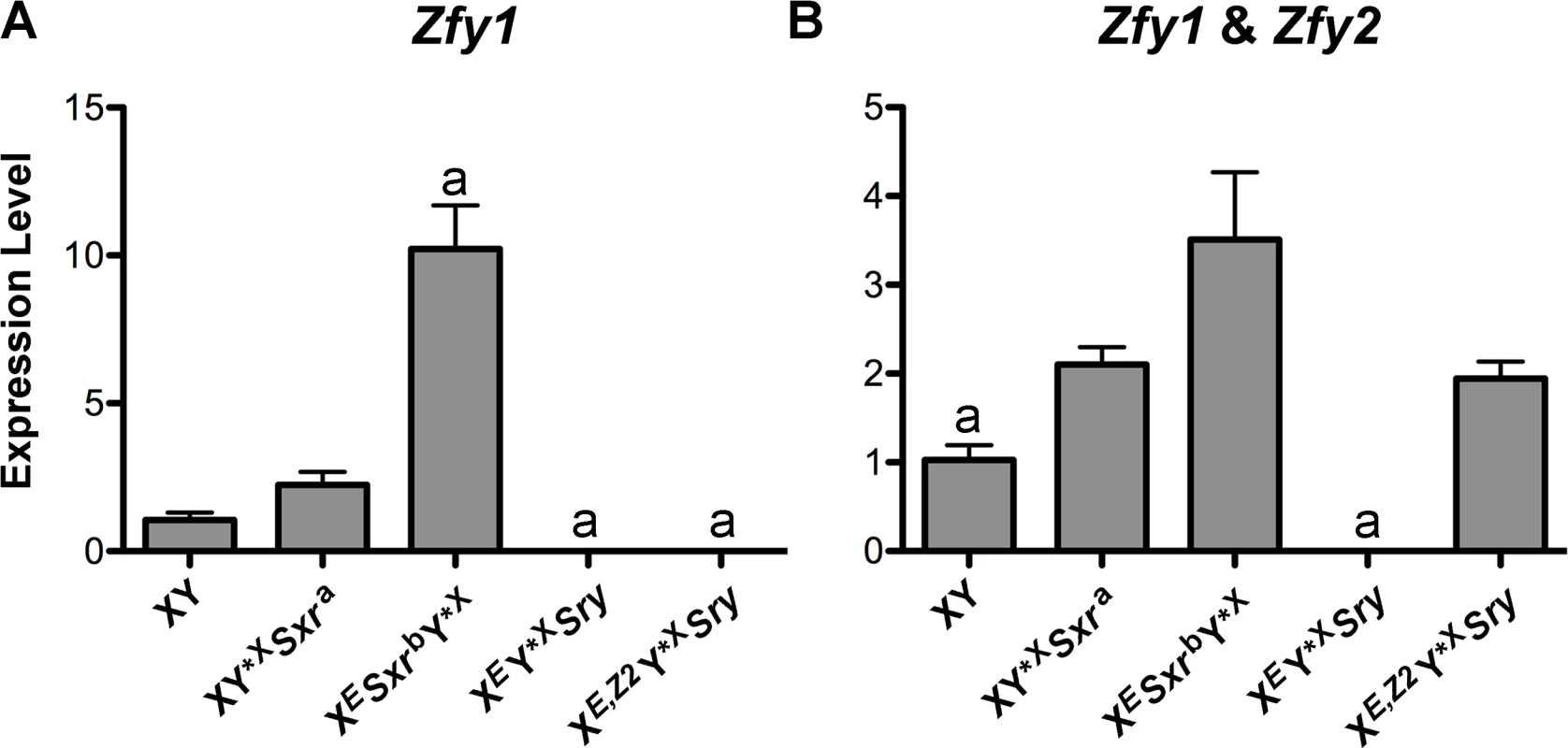
(A) Zfy1 transcript levels in genotypes of interest (n = 3 per genotype) obtained by real-time PCR. The loading controls were two ubiquitously expressed genes (actin and Sdha) and two spermatid-specific genes (Act and Acrv), and normalization was achieved by geometric averaging of these genes. (B) Zfy1 and Zfy2 (global Zfy) transcript levels were examined as in A. Values are mean ± SEM. Statistical significance: a different than all others (except zero to zero values comparison); * P < 0.05. For explanation of genotypes see Fig 1, S1 Table, and text. Primer sequences are shown in S3 Table. The data normalized to individual reference genes are shown in S5 Fig. As expected, no Zfy1 transcripts were detected in XEY*XSry and XE,Z2Y*XSry males. XY*XSxra males had ~2-fold higher Zfy1 levels than XY males but the difference did not reach significance (P = 0.08). This minor increase is likely due to the lack of NPYq genes known to result in the upregulation of sex chromosome genes, including Zfy [20]. In XESxrbY*X males Zfy1 levels were ~10-fold and ~5-fold higher than in XY and XY*XSxra, respectively, representing a combined effect of the NPYq absence and activity of a strong Cypt-derived spermatid-specific promoter driving expression of the Zfy2/1 fusion gene. The global Zfy expression was again higher in XESxrbY*X and XY*XSxra than in XY but the difference was lower in magnitude. Zfy global levels in XE,Z2Y*XSry males were similar to those of XY*XSxra, and higher than in XY. When compared to XESxrbY*X, Zfy global levels in XE,Z2Y*XSry males were ~2-fold lower but the difference did not reach significance (P = 0.1). In XE,Z2Y*XSry males the global levels were reflective exclusively of Zfy2, while in XESxrbY*X males primarily of Zfy1 since the Zfy2/1 fusion gene encodes Zfy1 coding region under the control of Zfy2 promoter [17].
When the spermiogenic phenotype is viewed in the context of Zfy expression our data support that it is Zfy2, and not Zfy1 (even if present in abundance), that enables the formation of sperm functional in ICSI.
Discussion
Y chromosome encoded zinc finger protein genes, Zfy, have once been in the center of attention as potential candidates for the testis-determining factors [21–23]. When the fame went to another Y gene, Sry [1–3], Zfy genes were quickly forgotten and it has taken more than two decades for these genes to re-emerge with newly ascribed spermiogenic roles. Zfy1 and Zfy2 were shown to play spermatogenic quality functions during the pachytene stage of meiosis and during MI by triggering the apoptotic elimination of spermatocytes [24,25] and to facilitate the second meiotic division [11]. It has also been shown that a gene/s from Sxra, partially retained in Sxrb, is necessary for the initiation of sperm morphogenesis [7] and increases the efficiency of round spermatid injection [5]; Zfy genes were proposed as the most likely candidates. Here we tested this assumption by investigating the effects of transgenic Zfy2 addition into Y chromosome deficient males, which have a postmeiotic arrest at the round spermatid stage. We demonstrated that Zfy2 is responsible for formation of sperm functional in assisted fertilization.
In our previous study we reported that only two Y chromosome genes, the testis determinant factor Sry and the spermatogonial proliferation factor Eif2s3y, are sufficient to make a male mouse whose germ cells are functional in assisted fertilization (ROSI) and yield live progeny [5]. When Y chromosome contribution was expanded by substituting Sry for Sxrb, ROSI efficiency improved, and we speculated that this was due to the presence of the Zfy2/1 fusion gene, which facilitated the second meiotic division in the testis in the presence of Y*X, or in the oocytes after fertilization when the meiotic pairing partner was missing [5]. Spermatid elongation and occasional formation of mature testicular sperm were previously observed in males with Sxrb [5,7] but their function in fertilization has not been tested. Here we have shown that these sperm are not successful in assisted fertilization (ICSI). In XESxrbO males the great majority of elongating spermatids are diploid [7] and so are the testicular sperm as shown in this study. The fact that live offspring were obtained with ROSI, and not with ICSI, could therefore be due to the highly condensed nature of the sperm chromatin. In diploid round spermatids from XESxrbO males the chromatin is still histone-bound and the homologous chromosomes are presumably still paired as in meiosis II. Secondary spermatocytes, with the same chromosomal state prematurely condense upon injection into oocytes and complete meiosis II along with the maternal chromatin, expelling a polar body-like structure with the haploid complement of paternal DNA [26]. We have proposed that a similar process occurs with the round spermatids from XESxrbO males, resulting in normal, diploid zygotes [5]. The diploid spermatozoa, however, cannot have the normal histone component because in order to complete spermiogenesis most of the histones would have had to be replaced by protamines. This sperm chromatin must be completely reorganized, which normally takes one to two hours after ICSI [27]. By this time, the maternal chromatin has already completed meiosis II, and the zygote can no longer support the completion of meiosis II for the paternal DNA. Congruent with this explanation, ICSI should be successful with sperm from XESxrbY*X males, which yielded predominantly diploid zygotes. However, only one offspring was obtained, suggesting that sperm ability to support embryonic and fetal development was highly impaired. We have previously shown that XY*XSxra males can be reproduced by ICSI [19]. Thus, one or more Y genes that are present and active in Sxra, and not in Sxrb, are likely to be responsible for rendering sperm functional. We now show that this gene is Zfy2, and that sperm dysfunction in XESxrbY*X males can be overcome with the transgenic addition of Zfy2.
Why is it that Zfy2 renders sperm functional in assisted fertilization while the Zfy2/1 fusion gene does not? The Zfy2/1 fusion gene, present within Sxrb, encodes a protein that is almost identical to that encoded by Zfy1 but the transcription is driven by a Cypt-derived Zfy2 specific promoter [17]. Both Zfy2 and Zfy2/1 are therefore strongly expressed postmeiotically because both have the Cypt-derived promoter, which drives strong expression in spermatids [14]. However, in the case of the Zfy2/1 fusion gene, the transcript produced is almost identical to that of Zfy1. Alternative splicing results in the majority of Zfy1 transcripts lacking exon 6, which encodes the ZFY protein transactivating domain (TA), while most of the Zfy2 transcripts retain exon 6 [17]. The protein encoded by Zfy1 lacking exon 6 is expected to bind but not transactivate target genes and consequently can serve as a competitive inhibitor of full length ZFY proteins. Moreover, the TA domain in ZFY1 protein, when present, is ~5.5-fold less active than that of ZFY2 protein [11]. The Zfy2/1 fusion gene produces transcripts that are spliced like those of Zfy1 so that a substantial proportion of them lack the exon 6 encoding the TA domain transcription factor function [17] and when TA domain is present, it is equivalent to that encoded by ZFY1 and therefore less potent. These transcript and protein specific differences explain why Zfy2/1 in XESxrbY*X males is not sufficient for promoting sperm function, and why addition of Zfy2 to this genotype rescues this defect.
The Zfy2 levels in XE,Z2Y*XSry males were ~1.9 fold higher than in XY. It therefore cannot be disregarded that the spermiogenic phenotype results from Zfy2 overexpression. The Zfy2 transgene was provided as a single copy Zfy2 BAC inserted by cassette mediated exchange (CME) into the Hprt locus on the X chromosome [24,25]. This transgene, because of its localization on the X chromosome, behaves in the same way as the endogenous gene, i.e. undergoes meiotic sex chromosome silencing (MSCI) [24], and its level of expression should be equivalent to that of the endogenous gene. The overexpression observed in XE,Z2Y*XSry males is likely due to the lack of Y chromosome long arm genes, known to result in global upregulation of several sex chromosome genes, including Zfy2 [20]. The fact that we see Zfy overexpression in XY*XSxra males, which have endogenous Zfy1 and Zfy2 but lack the Y chromosome long arm, supports this case. To bring the Zfy2 expression to the physiological level XE,Z2Y*XSry males, we would need to provide the genes from the Y long arm but those would likely influence spermiogenic phenotype. Thus, with the current tools, we cannot test whether Zfy2 expression at physiological levels would also induce spermatid elongation and promote sperm function. The resolution can come from the analysis of Zfy2 knockout mice, interpreted in the context of the expression data presented here.
The fact that we obtained ICSI offspring from XE,Z2Y*XSry males represents a significant advancement in establishing a minimum Y complement compatible with successful assisted fertilization. Although we have shown earlier that only two Y genes are sufficient to generate progeny [5], this was achieved with round spermatid injection (ROSI), a method which is considered experimental in human ART due to concerns regarding the safety of injecting immature germ cells and technical difficulties [28]. Intracytoplasmic sperm injection (ICSI), however, is a common procedure in human ART, rendering our mouse data more directly translational.
With the reemergence of Zfy genes from the backstage and their recently acknowledged roles during spermatogenesis [5,7,11,17,24,25] (and this study), it will now be important to characterize the mechanism and identify the target genes that these transcription factors regulate. In humans, there is a single ZFY gene on the Y chromosome, which is ubiquitously expressed. No mutations of ZFY have been described and there is therefore no information concerning its possible contribution to human germ cell development or male fertility. The newly acquired mouse data regarding the role of Zfy gene in spermatogenesis may therefore trigger re-evaluation of ZFY function in humans.
Materials and Methods
Ethics statement
The mice were maintained in accordance with the guidelines of the Laboratory Animal Services at the University of Hawaii and guidelines presented in National Research Council’s (NCR) “Guide for Care and Use of Laboratory Animals” published by Institute for Laboratory Animal Research (ILAR) of the National Academy of Science, Bethesda, MD, 2011. Anesthesia, necessary for performing embryo transfers and semi-castration, was achieved by intraperitoneal injection of Avertin. Euthanasia was achieved by cervival dislocation. MAW has an active protocol for animal handling and treatment procedures (protocol number 06–010), reviewed and approved by local Animal Care and Use Committees annually.
Chemicals and media
Pregnant mares’ serum gonadotrophin (eCG) and human chorionic gonadotrophin (hCG) were purchased from Calbiochem (San Diego, CA). All other chemicals were obtained from Sigma Chemical Co. (St Louis, MO) unless otherwise stated. Sperm and oocyte collection and subsequent manipulation, including microinjections were done in HEPES-buffered CZB medium (HEPES-CZB) [29]. Culture of injected oocytes and embryos was done in CZB medium [30].
Animals
The mice of interest in this study were mice with limited Y gene complement (Fig 1, S1 Table):
XEif2s3yOSry (abbreviated as XEOSry) are males carrying an autosomally-encoded transgene of testis determinant Sry [31] and the X chromosome-located transgene encoding spermatogonial proliferation factor Eif2s3y [32]. These mice have only one sex chromosome (hence the designation XO).
XEif2s3yY*XSry (abbreviated as XEY*XSry) males have the same Y gene complement as XEOSry but carry a minute X chromosome derivative (Y*X) with a complete pseudoautosomal region (PAR) but lacking most of the other X genes [33].
XEif2s3ySxrbO (abbreviated as XESxrbO) males have the X chromosome carrying an Eif2s3y transgene [32] together with Tp(Y)1CtSxr-b, a Sxra derivative with a 1.3 Mb deletion that has removed the majority of the Yp gene complement and created a Zfy2/1 fusion gene [34,35].
XEif2s3ySxrbY*X (abbreviated as XESxrbY*X) have the same Y gene complement as XESxrbO but carry also Y*X.
XY*XSxra have a single X chromosome and Tp(Y)1CtSxr-a [36] attached distal to the Y*X PAR.
Mice with Zfy2 transgene. The Zfy2 transgene (abbreviated as Z2) was added to the genotypes described in 1, 2 & 4. It was provided as a single copy Zfy2 BAC inserted by cassette mediated exchange (CME) into the Hprt locus on the X chromosome [24,25].
The XEOSry and XEY*XSry males were produced ‘in house’ by breeding XPafO or XPafY*X females [37] carrying the X-linked coat marker Patchy-fur [38] and XEif2s3yYTdym1Sry males that have the X chromosome carrying an Eif2s3y transgene [32] and a Y chromosome with an 11-kb deletion removing the testis determinant Sry (dl1Rlb) [1,39], complemented by an autosomally-located Sry transgene [Tg(Sry)2Ei] [31]. The XESxrbO and XESxrbY*X males were produced ‘in house’ by breeding XPafO or XPafY*X females described above and XEif2s3yYSxrb males that have the X chromosome carrying an Eif2s3y transgene [32] and a Y-chromosome that has Tp(Y)1CtSxr-b sex reversal factor [40,41] attached distal to its PAR region. The XY*XSxra males were produced by ICSI with sperm from males of the same genotype and oocytes from wild-type females. Males transgenic for Zfy2 (XE,Z2OSry, XE,Z2Y*XSry and XE,Z2SxrbY*X) were produced as described above but with the father carrying XE,Z2 rather than XE.
The crosses utilized in production of mice with limited Y gene complement yield a variety of progeny genotypes. The males of interest were identified among the progeny by genotyping for X and Y chromosome markers, scoring fur appearance, and evaluation of testes size. All mice were on MF1 genetic background, except for XY*XSxra which was C57BL/6. XYRIII MF1 were used as wild-type controls; YRIII chromosome is the strain of Y chromosome from which Sxra and Sxrb derive.
For assisted reproduction, six-to-twelve week-old B6D2F1 (C57BL/6 x DBA/2) females (NCI, Raleigh, NC) were used as oocyte donors and CD-1 (Charles River, Wilmington, MA) or Swiss Webster (NCI, Raleigh, NC) mice were used as vasectomized males and surrogate/foster females for embryo transfer.
The mice were fed ad libitum with a standard diet and maintained in a temperature and light-controlled room (22°C, 14h light/10h dark), in accordance with the guidelines of the Laboratory Animal Services at the University of Hawaii and guidelines presented in National Research Council’s (NCR) “Guide for Care and Use of Laboratory Animals” published by Institute for Laboratory Animal Research (ILAR) of the National Academy of Science, Bethesda, MD, 2011. The protocol for animal handling and treatment procedures was reviewed and approved by local Animal Care and Use Committees. Anesthesia, necessary for performing embryo transfers and semi-castration, was achieved by intraperitoneal injection of Avertin. Euthanasia was achieved by cervival dislocation. MAW has an active protocol for animal handling and treatment procedures (protocol number 06–010), reviewed and approved by local Animal Care and Use Committees annually.
Histology analysis
For histology analysis, part of the testes were fixed in Bouin overnight and then stored in 70% ethanol prior to embedding in paraffin wax, sectioning at 5 μm, and staining with hematoxylin-eosin (H&E) and Periodic acid Schiff and hematoxylin (PAS-H). The stages of seminiferous tubules were identified based on the composition of cells near the basal membrane according to the method described by Ahmed [42], and as described by us before [5]. This was necessary because of meiotic and post-meiotic arrests present in males with limited Y gene complement, which prevented staging based on the changes of acrosome and nuclear morphology of spermatids.
Sperm morphology analysis
Sperm morphology was examined on surface spreads of spermatogenic cells prepared from frozen testis tissue as described earlier [25]. The images of sperm were captured at 1000x magnification.
Round spermatid injection (ROSI) and intracytoplasmic sperm injection (ICSI)
Injections with testicular cells were performed as described before [5,19]. Testes were collected twice from each male following initial semi-castration, and used for preparation of testicular cell suspensions for injections. The metaphase II (MII) oocytes for ROSI were collected from superovulated (5 iu eCG and 5 iu hCG given 48 hrs apart) female mice and incubated at 37°C, 5% CO2 until injection. Testicular sperm suspension was diluted with HEPES-CZB containing 1% (w/v) polyvinyl pyrrolidone (PVP) on the injection dish. Spermatids were injected individually into the oocytes. The total duration of ROSI was no longer than 1 hour. The oocytes were activated shortly after injection by incubation in Ca2+-free CZB medium supplemented with 2.5 mM SrCl2 at 37°C, 5% CO2 for 4 hrs, after which time they were transferred into standard CZB medium for subsequent culture. At ~6–8 hrs after injection the oocytes were assessed for polar body extrusion and pronuclei development. Normally fertilized oocytes exhibiting two pronuclei (PN) and extruded second polar body (PBII) were allowed to cleave and were subjected to embryo transfer. Surrogate mothers were subjected to caesarian section on day 18 of pregnancy to allow for scoring of the numbers of fetuses and implantation sites.
Zygote chromosome analysis
Chromosome preparation and analysis were performed as previously described [8]. In wild-type males the spermatid used for injection is considered chromosomally normal when resulting zygote contains 40 normal metaphase chromosomes, 20 maternal and 20 paternal. Males with limited Y gene complement either lack the Y chromosome or carry a minute Y*X chromosome variant. Thus, for these genotypes lack of one chromosome in the paternal chromosome complement and the presence of one small variant were considered normal.
Progeny genotyping
Offspring produced with ICSI and ROSI were genotyped by PCR to identify presence of Eif2s3y, Zfy2, and Sry transgenes. Presence of Y*X was recognized by copy number assessment. Genomic DNA was isolated from mouse tails using phenol chloroform extraction and ethanol precipitation. DNA was used to amplify single copies of X-linked Prdx4 (absent in Y*X) and Amelx (present in Y*X), and Atr (on chromosome 9) for normalization using Power SYBR Green PCR Master Mix on a Quant Studio 12K Flex machine (Applied Biosystems). The following conditions were used: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. Two PCR reactions were used to detect the presence of Y*X and the number of X-chromosomes. An 82-bp Prdx4 fragment were amplified using primers Prdx4-F and Prdx4-R and a 162-bp Amelx fragment with primers Amelx-F and Amelx-R. All samples were tested in quadruplicate per assay using XO samples as a reference control. Copy number estimation for each gene was calculated with the ΔΔCt method. Briefly, ΔCt values were calculated as difference between tested gene and Atr. ΔΔCt values were calculated by subtracting ΔCt of tested genes from the reference samples. The copy numbers were calculated by raising 2 to the power of ΔΔCt (2ΔΔCt). The genotypes were inferred from the copies of each target gene: XO, 1 Prdx4 + 1 Amelx; XY*X, 1 Prdx4 + 2 Amelx; XX, 2 Prdx4 + 2 Amelx; XXY*X, 2 Prdx4 + 3 Amelx. Primer sequences are shown in S3 Table.
Real-time RT-PCR
For real-time reverse transcriptase polymerase chain reaction (RT-PCR), total testis RNA was extracted using Trizol and DNaseI treatment (Ambion, Austin, TX,USA), and purified using an RNeasy kit (Qiagen, Valencia, CA, USA). Reverse transcription of polyadenylated RNA was performed with Superscript Reverse Transcriptase III, according to the manufacturer’s guidelines (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed using SYBR Green PCR Master mix on an ABI QuantStudio 12K Flex machine (Applied Biosystems, Carlsbad, CA, USA). PCR reactions were incubated at 95°C for 10 min followed by 40 PCR cycles (10 s at 95°C and 60 s at 60°C). For analysis of Zfy expression, two types of PCR reactions were performed: (1) ‘Zfy1’ amplifying only Zfy1 transcripts and (2) ‘Zfy Global‴ amplifying both Zfy1 and Zfy2. Three mice per genotype were analyzed, all reactions were carried out in quadruplicates per assay, and four different loading controls, two ubiquitously expressed genes (actin and Sdha) and two spermatid-specific genes (Act and Acrv) were used. DCt value for each individual sample was calculated by subtracting either the average Ct or geometric mean of loading control(s) from the average Ct of a tested gene. DDCt value was calculated by subtracting the DCt of each tested male from the average DCt of wild-type XY males, which served as references. The data were expressed as a fold value of expression level.
Statistical analyses
Fisher's Exact Test was used to assess the differences between the genotypes for ROSI and ICSI and zygotic chromosome analysis data. Student t-test was used for gene expression and histology analyses.
Supporting Information
Zdroje
1. Gubbay J, Collignon J, Koopman P, Capel B, Economou A, et al. (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346 : 245–250. 2374589
2. Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, et al. (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346 : 240–244. 1695712
3. Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351 : 117–121. 2030730
4. Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, et al. (2014) Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 508 : 494–499. doi: 10.1038/nature13206 24759411
5. Yamauchi Y, Riel JM, Stoytcheva Z, Ward MA (2014) Two Y genes can replace the entire Y chromosome for assisted reproduction in the mouse. Science 343 : 69–72. doi: 10.1126/science.1242544 24263135
6. Soh YQ, Alfoldi J, Pyntikova T, Brown LG, Graves T, et al. (2014) Sequencing the mouse y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell 159 : 800–813. doi: 10.1016/j.cell.2014.09.052 25417157
7. Vernet N, Mahadevaiah SK, Ellis PJ, de Rooij DG, Burgoyne PS (2012) Spermatid development in XO male mice with varying Y chromosome short-arm gene content: evidence for a Y gene controlling the initiation of sperm morphogenesis. Reproduction 144 : 433–445. doi: 10.1530/REP-12-0158 22869781
8. Yamauchi Y, Riel JM, Stoytcheva Z, Burgoyne PS, Ward MA (2010) Deficiency in mouse Y chromosome long arm gene complement is associated with sperm DNA damage. Genome Biol 11: R66. doi: 10.1186/gb-2010-11-6-r66 20573212
9. Burgoyne PS, Mitchell MJ (2007) The roles of mouse Y chromosome genes in spermatogenesis. In: LYFCaC W.Y., editor. The Y Chromosome and Male Germ Cell Biology in Health and Diseases. New Jersey: World Scientific Publishing Co. Inc. pp. 1–25.
10. Ferguson L, Ellis PJ, Affara NA (2009) Two novel mouse genes mapped to chromosome Yp are expressed specifically in spermatids. Mamm Genome 20 : 193–206. doi: 10.1007/s00335-009-9175-8 19308643
11. Vernet N, Mahadevaiah SK, Yamauchi Y, Decarpentrie F, Mitchell MJ, et al. (2014) Mouse Y-linked Zfy1 and Zfy2 are expressed during the male-specific interphase between meiosis I and meiosis II and promote the 2nd meiotic division. PLoS Genet 10: e1004444. doi: 10.1371/journal.pgen.1004444 24967676
12. Capel B, Swain A, Nicolis S, Hacker A, Walter M, et al. (1993) Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73 : 1019–1030. 7684656
13. Jeske YW, Bowles J, Greenfield A, Koopman P (1995) Expression of a linear Sry transcript in the mouse genital ridge. Nat Genet 10 : 480–482. 7670499
14. Hansen MA, Nielsen JE, Tanaka M, Almstrup K, Skakkebaek NE, et al. (2006) Identification and expression profiling of 10 novel spermatid expressed CYPT genes. Mol Reprod Dev 73 : 568–579. 16477651
15. Nagamine CM, Chan K, Hake LE, Lau YF (1990) The two candidate testis-determining Y genes (Zfy-1 and Zfy-2) are differentially expressed in fetal and adult mouse tissues. Genes Dev 4 : 63–74. 1968414
16. Nagamine CM, Chan KM, Kozak CA, Lau YF (1989) Chromosome mapping and expression of a putative testis-determining gene in mouse. Science 243 : 80–83. 2563174
17. Decarpentrie F, Vernet N, Mahadevaiah SK, Longepied G, Streichemberger E, et al. (2012) Human and mouse ZFY genes produce a conserved testis-specific transcript encoding a zinc finger protein with a short acidic domain and modified transactivation potential. Hum Mol Genet 21 : 2631–2645. doi: 10.1093/hmg/dds088 22407129
18. Kitamura K, Iguchi N, Kaneko Y, Tanaka H, Nishimune Y (2004) Characterization of a novel postacrosomal perinuclear theca-specific protein, CYPT1. Biol Reprod 71 : 1927–1935. 15286030
19. Yamauchi Y, Riel JM, Wong SJ, Ojarikre OA, Burgoyne PS, et al. (2009) Live offspring from mice lacking the Y chromosome long arm gene complement. Biol Reprod 81 : 353–361. doi: 10.1095/biolreprod.109.076307 19420387
20. Cocquet J, Ellis PJ, Yamauchi Y, Mahadevaiah SK, Affara NA, et al. (2009) The multicopy gene Sly represses the sex chromosomes in the male mouse germline after meiosis. PLoS Biol 7: e1000244. doi: 10.1371/journal.pbio.1000244 19918361
21. Mardon G, Page DC (1989) The sex-determining region of the mouse Y chromosome encodes a protein with a highly acidic domain and 13 zinc fingers. Cell 56 : 765–770. 2493989
22. Page DC, Mosher R, Simpson EM, Fisher EM, Mardon G, et al. (1987) The sex-determining region of the human Y chromosome encodes a finger protein. Cell 51 : 1091–1104. 3690661
23. Sinclair AH, Foster JW, Spencer JA, Page DC, Palmer M, et al. (1988) Sequences homologous to ZFY, a candidate human sex-determining gene, are autosomal in marsupials. Nature 336 : 780–783. 3144651
24. Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, et al. (2010) Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 20 : 2117–2123. doi: 10.1016/j.cub.2010.11.010 21093264
25. Vernet N, Mahadevaiah SK, Ojarikre OA, Longepied G, Prosser HM, et al. (2011) The Y-encoded gene zfy2 acts to remove cells with unpaired chromosomes at the first meiotic metaphase in male mice. Curr Biol 21 : 787–793. doi: 10.1016/j.cub.2011.03.057 21530259
26. Kimura Y, Yanagimachi R (1995) Development of normal mice from oocytes injected with secondary spermatocyte nuclei. Biology of Reproduction 53 : 855–862. 8547481
27. Ajduk A, Yamauchi Y, Ward MA (2006) Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol Reprod 75 : 442–451. 16775225
28. Practice Committee of American Society for Reproductive M, Practice Committee of Society for Assisted Reproductive T (2008) Round spermatid nucleus injection (ROSNI). Fertil Steril 90: S199–201. doi: 10.1016/j.fertnstert.2008.08.033 19007630
29. Kimura Y, Yanagimachi R (1995) Intracytoplasmic sperm injection in the mouse. Biol Reprod 52 : 709–720. 7779992
30. Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I (1989) An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 86 : 679–688. 2760894
31. Mahadevaiah SK, Odorisio T, Elliott DJ, Rattigan A, Szot M, et al. (1998) Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum Mol Genet 7 : 715–727. 9499427
32. Mazeyrat S, Saut N, Grigoriev V, Mahadevaiah SK, Ojarikre OA, et al. (2001) A Y-encoded subunit of the translation initiation factor Eif2 is essential for mouse spermatogenesis. Nat Genet 29 : 49–53. 11528390
33. Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A (1998) The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet 80 : 37–40. 9678332
34. Burgoyne PS, Levy ER, McLaren A (1986) Spermatogenic failure in male mice lacking H-Y antigen. Nature 320 : 170–172. 3951555
35. Sutcliffe MJ, Burgoyne PS (1989) Analysis of the testes of H-Y negative XOSxrb mice suggests that the spermatogenesis gene (Spy) acts during the differentiation of the A spermatogonia. Development 107 : 373–380. 2632229
36. Cattanach BM, Pollard CE, Hawker SG (1971) Sex-reversed mice: XX and XO males. Cytogenetics 10 : 318–337. 5156366
37. Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, et al. (1991) The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet 57 : 221–230. 1743079
38. Lane PW, Davisson MT (1990) Patchy fur (Paf), a semidominant X-linked gene associated with a high level of X-Y nondisjunction in male mice. J Hered 81 : 43–50. 2332613
39. Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P, et al. (1992) Inverted repeat structure of the Sry locus in mice. Proc Natl Acad Sci U S A 89 : 7953–7957. 1518820
40. Mazeyrat S, Saut N, Sargent CA, Grimmond S, Longepied G, et al. (1998) The mouse Y chromosome interval necessary for spermatogonial proliferation is gene dense with syntenic homology to the human AZFa region. Hum Mol Genet 7 : 1713–1724. 9736773
41. Simpson EM, Page DC (1991) An interstitial deletion in mouse Y chromosomal DNA created a transcribed Zfy fusion gene. Genomics 11 : 601–608. 1774064
42. Ahmed EA, de Rooij DG (2009) Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol 558 : 263–277. doi: 10.1007/978-1-60761-103-5_16 19685330
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 12- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
-
Všechny články tohoto čísla
- Data Sharing Policy: In Pursuit of Functional Utility
- Catching a (Double-Strand) Break: The Rad51 and Dmc1 Strand Exchange Proteins Can Co-occupy Both Ends of a Meiotic DNA Double-Strand Break
- Why Are tRNAs Overproduced in the Absence of Maf1, a Negative Regulator of RNAP III, Not Fully Functional?
- "Women Who Don't Give a Crap"
- A Point Mutation in Suppressor of Cytokine Signalling 2 () Increases the Susceptibility to Inflammation of the Mammary Gland while Associated with Higher Body Weight and Size and Higher Milk Production in a Sheep Model
- Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s
- A Simple Model-Based Approach to Inferring and Visualizing Cancer Mutation Signatures
- An Empirical Bayes Mixture Model for Effect Size Distributions in Genome-Wide Association Studies
- Mouse Y-Encoded Transcription Factor Is Essential for Sperm Formation and Function in Assisted Fertilization
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- "Women Who Don't Give a Crap"
- A Point Mutation in Suppressor of Cytokine Signalling 2 () Increases the Susceptibility to Inflammation of the Mammary Gland while Associated with Higher Body Weight and Size and Higher Milk Production in a Sheep Model
- Data Sharing Policy: In Pursuit of Functional Utility
- Catching a (Double-Strand) Break: The Rad51 and Dmc1 Strand Exchange Proteins Can Co-occupy Both Ends of a Meiotic DNA Double-Strand Break
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



