-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Evolution of Epigenetic Regulators and in Amniotes
CTCF is an essential, ubiquitously expressed DNA-binding protein responsible for insulator function, nuclear architecture, and transcriptional control within vertebrates. The gene CTCF was proposed to have duplicated in early mammals, giving rise to a paralogue called “brother of regulator of imprinted sites” (BORIS or CTCFL) with DNA binding capabilities similar to CTCF, but testis-specific expression in humans and mice. CTCF and BORIS have opposite regulatory effects on human cancer-testis genes, the anti-apoptotic BAG1 gene, the insulin-like growth factor 2/H19 imprint control region (IGF2/H19 ICR), and show mutually exclusive expression in humans and mice, suggesting that they are antagonistic epigenetic regulators. We discovered orthologues of BORIS in at least two reptilian species and found traces of its sequence in the chicken genome, implying that the duplication giving rise to BORIS occurred much earlier than previously thought. We analysed the expression of CTCF and BORIS in a range of amniotes by conventional and quantitative PCR. BORIS, as well as CTCF, was found widely expressed in monotremes (platypus) and reptiles (bearded dragon), suggesting redundancy or cooperation between these genes in a common amniote ancestor. However, we discovered that BORIS expression was gonad-specific in marsupials (tammar wallaby) and eutherians (cattle), implying that a functional change occurred in BORIS during the early evolution of therian mammals. Since therians show imprinting of IGF2 but other vertebrate taxa do not, we speculate that CTCF and BORIS evolved specialised functions along with the evolution of imprinting at this and other loci, coinciding with the restriction of BORIS expression to the germline and potential antagonism with CTCF.
Published in the journal: . PLoS Genet 4(8): e32767. doi:10.1371/journal.pgen.1000169
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000169Summary
CTCF is an essential, ubiquitously expressed DNA-binding protein responsible for insulator function, nuclear architecture, and transcriptional control within vertebrates. The gene CTCF was proposed to have duplicated in early mammals, giving rise to a paralogue called “brother of regulator of imprinted sites” (BORIS or CTCFL) with DNA binding capabilities similar to CTCF, but testis-specific expression in humans and mice. CTCF and BORIS have opposite regulatory effects on human cancer-testis genes, the anti-apoptotic BAG1 gene, the insulin-like growth factor 2/H19 imprint control region (IGF2/H19 ICR), and show mutually exclusive expression in humans and mice, suggesting that they are antagonistic epigenetic regulators. We discovered orthologues of BORIS in at least two reptilian species and found traces of its sequence in the chicken genome, implying that the duplication giving rise to BORIS occurred much earlier than previously thought. We analysed the expression of CTCF and BORIS in a range of amniotes by conventional and quantitative PCR. BORIS, as well as CTCF, was found widely expressed in monotremes (platypus) and reptiles (bearded dragon), suggesting redundancy or cooperation between these genes in a common amniote ancestor. However, we discovered that BORIS expression was gonad-specific in marsupials (tammar wallaby) and eutherians (cattle), implying that a functional change occurred in BORIS during the early evolution of therian mammals. Since therians show imprinting of IGF2 but other vertebrate taxa do not, we speculate that CTCF and BORIS evolved specialised functions along with the evolution of imprinting at this and other loci, coinciding with the restriction of BORIS expression to the germline and potential antagonism with CTCF.
Introduction
CCCTC-binding factor (CTCF) is a ubiquitously expressed protein that binds to more than 20,000 sites within the human genome [1]–[3]. The distribution of these binding sites, along with experimental data from several well-characterised loci (reviewed [4]) indicates that CTCF acts as an insulator protein genome-wide, defining boundaries for gene clusters or segregating alternative promoters. This can affect gene expression, for instance at the well-studied chicken ß–globin locus, where CTCF binding to the FII insulator leads to transcriptional silencing by blocking the effects of a nearby enhancer [5].
CTCF is also required for inter-chromosomal interactions such as pairing of the X chromosomes during initiation of X chromosome inactivation [6] and even co-localisation of non-homologous chromosomes [7]. It is now considered that CTCF contributes more broadly to the establishment of nuclear compartments where transcription is enhanced or repressed [8],[9], rather than functioning only to insulate neighbouring regions of the genome from each other. Given these diverse and significant roles, it is not surprising that CTCF is essential for life (reviewed [9]). Furthermore, point mutation and loss of heterozygosity of CTCF is associated with human cancer, identifying CTCF as an important candidate tumour-suppressor gene [10].
The CTCF protein, and the nucleotide sequence that encodes it, can conceptually be divided into three separate domains (Figure 1A). The central (ZF) domain contains ten Cys2His2 zinc-fingers (ZFs), and one Cys2HisCys ZF, combinations of which are used to bind various DNA sequences [11]. Flanking the ZF domain are the N - and C-terminal domains, which interact with other DNA-binding proteins, histones and histone modifying proteins, and the large subunit of polymerase II (reviewed [8]). In all three of its domains, CTCF shows extraordinary conservation throughout vertebrates [11]–[14], and even non-vertebrates [15], reflecting the considerable functional constraint CTCF must face due to its multiple essential roles and many interacting partners.
Fig. 1. Gene structure of CTCF and BORIS. 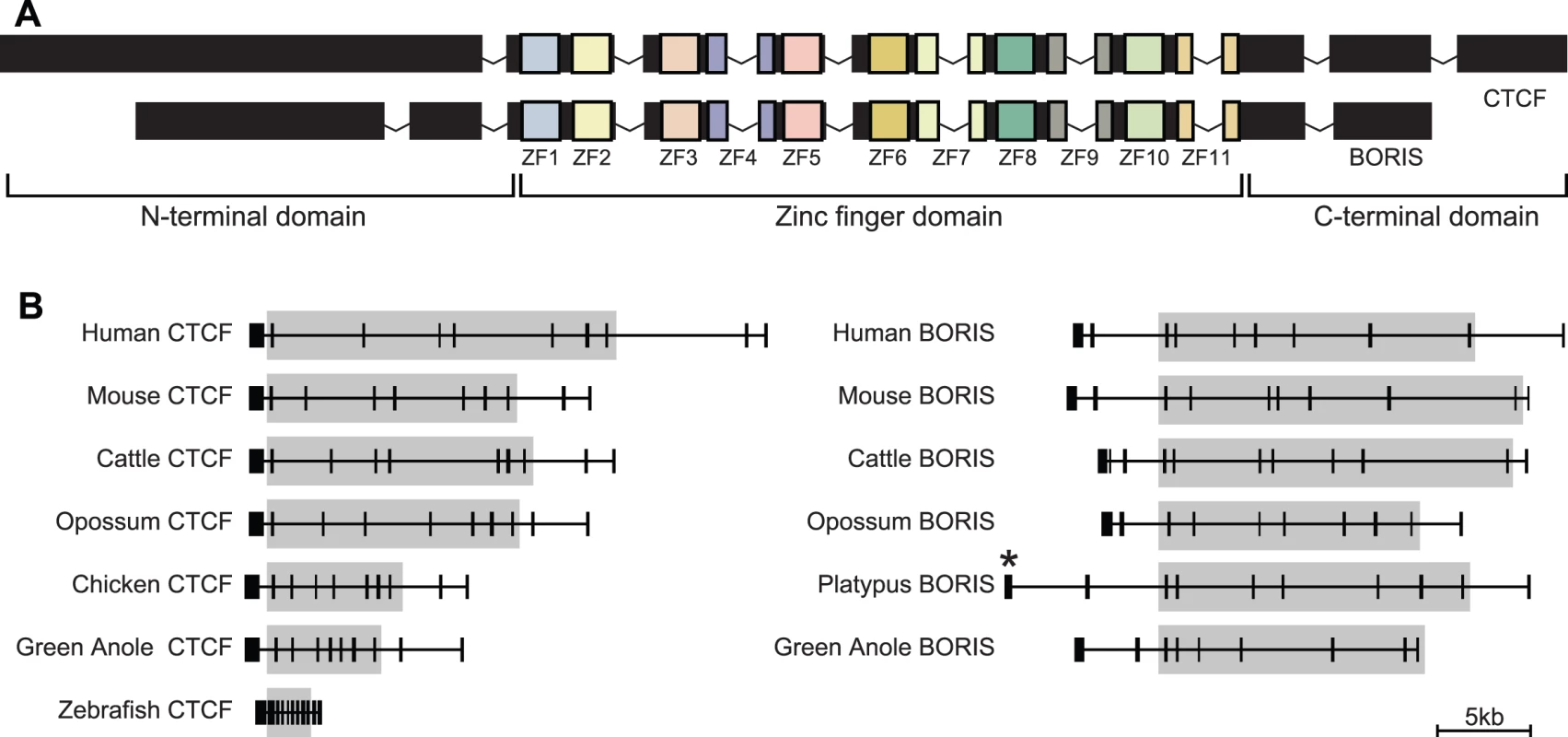
(A) CTCF and BORIS share a similar ZF domain, but different N- and C-terminal domains. (B) All vertebrate CTCF orthologues posses ten exons. Intron-exon boundaries are identical between all CTCF and BORIS orthologues within the ZF domain (grey). Note, genomic coverage of platypus BORIS is incomplete at the 5′ end (*). In humans and mice, a paralogue of CTCF has been identified known as CTCF-like (CTCFL), or as it was originally named (and how we will refer to it hereafter), Brother Of Regulator of Imprinted Sites (BORIS) [16]. Human and mouse BORIS posses a suite of ZFs with binding capability, sequence and underlying gene structure that is extremely similar to CTCF (Figure 1A). However, the N - and C-terminal domains of human and mouse BORIS show almost no similarity to CTCF, implying that although they can bind the same DNA, they are likely to act differently at these sites.
One example of how CTCF and BORIS may function differently comes from their effects on the regulation of genomic imprinting, which is responsible for parent-of-origin specific, mono-allelic gene expression in about 100 mammalian genes [17]. The most extensively studied imprinted gene, insulin-like growth factor 2 (IGF2), is expressed exclusively from the paternally-derived chromosome in eutherian (‘placental’) mammals [18]–[21] and marsupial mammals [22],[23]. Located downstream of IGF2 is the untranslated RNA H19, which is expressed solely from the maternally derived chromosome [24],[25]. Biallelic expression of IGF2 was discovered in the egg-laying monotreme mammals [26], birds [22],[27] and fish [28], implying that imprinting of this region evolved at the same time as viviparity, 180-210MYA [29].
In mice, imprinted expression of Igf2/H19 depends on the imprint control region (ICR), an insulator element located between these two genes. The ICR is methylated during spermatogenesis, specifically marking the paternally-derived chromosome [30]. CTCF binds to the ICR, but only on the unmethylated, maternally-derived chromosome. When bound to the maternally-derived ICR, CTCF performs many functions including protecting the ICR from methylation [31]–[33], blocking Igf2 access to a downstream enhancer (resulting in Igf2 silencing in cis [34],[35]), and simultaneously activating H19 expression [33]. CTCF is thought to orchestrate these events through the formation of maternal-specific chromosomal loops [36] and the establishment of local chromatin modifications [37]. Thus, CTCF acts somatically to ‘interpret’ the differential methylation mark of the ICR acquired during gametogenesis, resulting in imprinted expression of Igf2/H19.
In contrast, BORIS appears to be essential for the establishment of differential methylation at the IGF2/H19 ICR [38]. In mouse testes, BORIS is bound to the Igf2/H19 ICR during the time when the ICR becomes methylated. Methylation is accomplished by members of the de novo methyltransferase 3 family, of which DNMT3L is essential to this process [39],[40] and DNMT3A/3B are partially redundant [41]. Transgenes containing the mouse ICR were methylated in Xenopus oocytes only when co-injected with BORIS, DNMT3L, one of DNMT3A/3B and a histone modifier called protein arginine methyltransferase 7 (PRMT7) [38]. Thus, BORIS and CTCF both bind to the ICR through their common ZF domain, yet appear to act differently at this site. BORIS establishes differential methylation of the ICR and later CTCF interprets this mark, resulting in imprinted expression of Igf2/H19.
Significantly, in humans and mice CTCF and BORIS show mutually exclusive expression; BORIS is transcribed only in certain parts of the developing and adult testes, whereas CTCF is expressed in all other regions tested [16],[38]. The only reported instances of BORIS expression outside of the testes is in various types of cancers [42]–[47]. This mutually exclusive expression pattern could be explained in part by the recent discovery that CTCF actually binds to the promoter of BORIS and negatively regulates its expression [44].
BORIS is associated with a large group of potentially oncogenic “cancer-testis” (CT) genes, which also show testis-specific, or gonad-specific, expression in healthy individuals, but are highly expressed in cancers [48]. CTCF binds to the promoter of many CT-genes in healthy somatic tissue where these genes are silenced [42],[43],[49],[50]. However, this repression is disrupted by conditional expression of BORIS, which replaces CTCF binding at the promoter and subsequently causes local demethylation and gene activation [42],[43],[49]. Similarly, CTCF-binding has a repressive effect on the promoter of the anti-apoptotic gene BAG1, whereas BORIS performs oppositely, altering histone methylation and upregulating BAG1 expression [51]. The discovery that CTCF and BORIS have opposite effects on transcription of BAG1, some CT-genes, and on the epigenetic status of the IGF2/H19 ICR, has lead to the (albeit controversial [47]) hypothesis that CTCF and BORIS are antagonistic regulators of the common loci to which they bind, and that inappropriate interactions between them is cancer promoting [16],[52].
Comparisons between the genomes of mammals and other vertebrates are powerful tools in understanding how human genes and their products are regulated, what their function is and how and why they evolved [53],[54]. Indeed, much of CTCF function has been characterised in chicken, including its capacity as an insulator protein [5] and recent studies have revealed the extreme conservation of CTCF sequence and function in amphibians [13], fish [14] and even invertertebrates such as Drosophila [15].
Despite this, CTCF has not been characterised in non-eutherian mammals or reptiles and whether BORIS exists outside humans and mice is not even known. From reported failures to find BORIS sequence in chicken and fish [14],[16] it has been proposed that BORIS arose recently from duplication of CTCF in an early mammal [16]. However, here we report that BORIS orthologues are present in all major mammalian groups and at least two reptilian species, proving that BORIS evolution occurred much earlier than has been recognised. We examined the expression pattern of CTCF and BORIS in the three major mammalian clades and a reptile, discovering that although CTCF is ubiquitously expressed in all species, BORIS became progressively specialised to testis throughout amniote evolution. We consider these new data with respect to current theories regarding CTCF and BORIS as antagonistic epigenetic regulators and their roles in governing genomic imprinting at the IGF2/H19 locus.
Results
We isolated, sequenced and characterised CTCF and BORIS homologues in eutherians, marsupials, monotremes and reptiles, and studied their expression profiles in one species from each of these vertebrate groups.
Cloning and Characterisation of CTCF and BORIS Orthologues in Vertebrates
Homologues of CTCF and BORIS were amplified from a range of amniotes by reverse-transcriptase PCR (RT-PCR) and rapid amplification of cDNA ends, using primers designed from sequenced genomic data or evolutionarily conserved regions (Table S1). Full-length or near full-length protein coding cDNA sequences were retrieved in this way from our model eutherian, marsupial, monotreme and reptilian species; domestic cattle (Bos taurus), tammar wallaby (Macropus eugenii), duck-billed platypus (Ornithorhynchus anatinus) and central bearded dragon (Pogona vitticeps) respectively (accession numbers EU527852-EU527858). Similarity searches, using these sequences and other annotated CTCF and BORIS sequences as queries, were conducted in a variety of databases hosted at NCBI (http://www.ncbi.nlm.nih.gov) and Ensembl (http://www.ensembl.org). This approach identified a further 37 homologues of these genes in vertebrates (Table S2).
From the largest region of common overlap between these homologues, a neighbour joining tree was constructed, revealing two distinct clusters of sequence (Figure 2). One of these clusters contained previously annotated copies of CTCF from human (NM_006565), mouse (NM_007794), rat (NM_031824), cattle (NM_001075748), chicken (NM_205332) and zebrafish (NM_001001844). The other cluster contained annotated BORIS sequence from human (NM_080618) and mouse (NM_001081387). The branch separating these two clusters was supported by a 100% bootstrap value. This unambiguously defined which sequences were CTCF orthologues and which were BORIS orthologues.
Fig. 2. Neighbour-joining tree showing relationships between members of the CTCF and BORIS gene family. 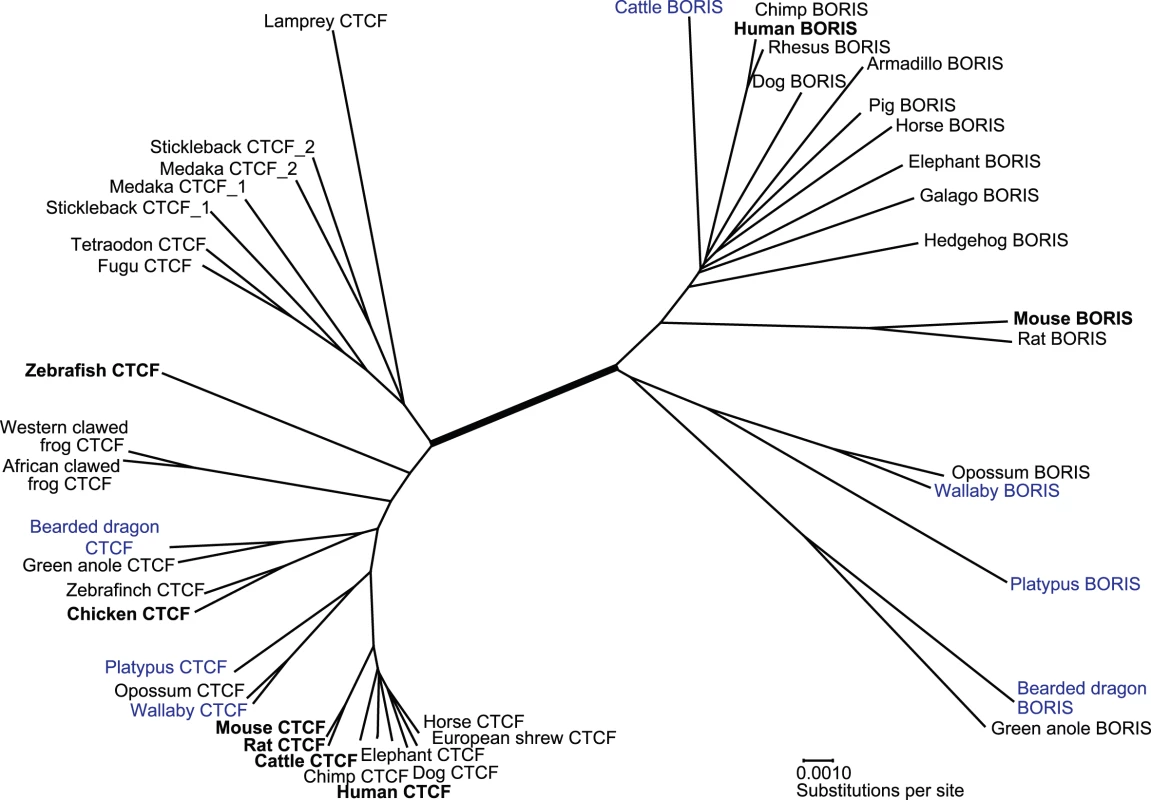
Sequence we determined experimentally (blue) and discovered by in silico similarity searches (black) form two distinct clusters with previously annotated CTCF and BORIS orthologues (bold). These clusters are separated from each other by a branch with 100% bootstrap value (thick line). Accession numbers and scientific names for these sequences and species are shown in Table S2. In line with previous studies, we detected CTCF orthologues in all major vertebrate groups [12]–[14]. Included in this cluster were closely related duplicate CTCF sequences (designated CTCF1 and CTCF2) from stickleback and medaka. The BORIS cluster included, as well as orthologues from many eutherian species, clear orthologues in two marsupials (Gray short-tailed opossum, Monodelphis domestica, and wallaby), a monotreme (platypus) and two reptiles (bearded dragon and green anole, Anolis carolinensis). No orthologues of BORIS could be detected using nucleotide BLAST, or translated BLAST searches in genomes of any avian (chicken, Gallus gallus; and zebra finch, Taeniopygia guttata), amphibian (Western-clawed frog, Xenopus tropicalis), teleost fish (puffer fish, Takifugu rubripes and Tetraodon nigroviridis; zebrafish, Danio rerio; stickleback, Gasterosteus aculeatus; and medaka Oryzias latipes) or primitive vertebrate (sea lamprey, Petromyzon marinus).
Although BLAST searches failed to identify any sequence orthologous to BORIS in bird, amphibian and fish genomes, it remained possible that BORIS is present in these genomes but is too diverged to detect using standard alignment methods. This seemed particularly likely for chicken, as birds are a sister taxon to the reptiles, in which we discovered BORIS orthologues. Applying a strategy previously used in the search for divergent genes [55], we sought orthologues of markers on either side of human BORIS. We located such sequences in multiple species, and searched the dividing spaces for BORIS-like sequence.
We found that genes flanking BORIS in humans were part of a single large block of genes (TMEPAI-BMP7) clustered together in the same orientation in all tetrapods (data not shown). Genes from this block were either not clustered together, or were not present in sea lamprey and teleost fish genomes.
We aligned the regions containing genes immediately adjacent to BORIS (PCK1 and RBM38) between human, mouse, dog, opossum, platypus, chicken, green anole and frog (Figure 3). As before, we could detect no BORIS orthologues in frog, but found some similarity between the first zinc finger of BORIS and a 108-bp region of the chicken PCK1-RBM38 intergenic sequence. When this sequence was used a query for reciprocal BLAST against the entire human genome, the best alignments were to the first zinc finger of CTCF and BORIS, indicating that these sequences were homologous. We could uncover no evidence for this sequence being part of an active gene other than finding that it overlaps with an Ensembl ab-initio gene prediction (GENSCAN00000030237). We therefore conclude that the sequence is a degraded relic of BORIS.
Fig. 3. Human genomic sequence encompassing PCK1-BORIS-RBM38 (top) compared to the orthologous regions in other amniotes. 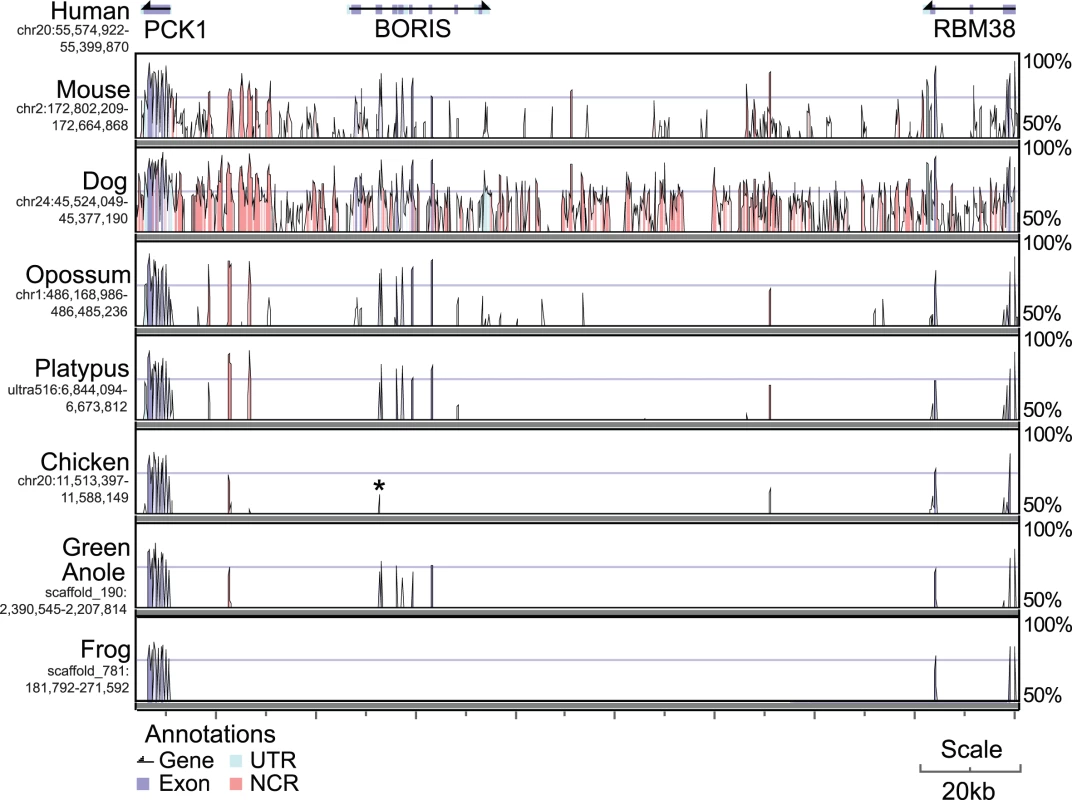
High similarity over a 100bp window is seen for most exonic sequence (blue) and some untranslated regions (UTR, light blue) or non coding regions (NCR, pink). Despite no similarity to any other region of human BORIS to the chicken PCK1-RBM38 region, the peak labelled with a star is homologous to the first ZF of BORIS. Sequence Analysis and Gene Structure of CTCF and BORIS in Vertebrates
We aligned predicted CTCF and BORIS proteins and found that, like previously annotated versions of these proteins, all possessed eleven ZFs, ten of which belong to the Cys2His2 class, and one which belongs to the Cys2HisCys class (Figure S1). As previously reported for human, mouse, chicken, zebrafish and frog [12]–[14],[16], we found that all vertebrate CTCF orthologues are extremely highly conserved throughout their entire length. From pairwise alignments over the entire length of its sequence, we found 92% average identity between human CTCF and other selected vertebrate CTCF sequences (Table 1). In comparison, similarity of BORIS orthologues, to each other and to CTCF, was largely restricted to the region encoding the ZFs. When human BORIS was compared to other BORIS sequences the average identity was 80.4% within the ZF domain, but less than 35% similar in the other two regions. Moreover, comparisons of human BORIS with CTCF sequences produced an average identity of 74.1% within the ZF domain, but less than 15% conservation within the other regions.
Tab. 1. Average pairwise similarity (%) between regions of human CTCF/BORIS and other vertebrate orthologues. 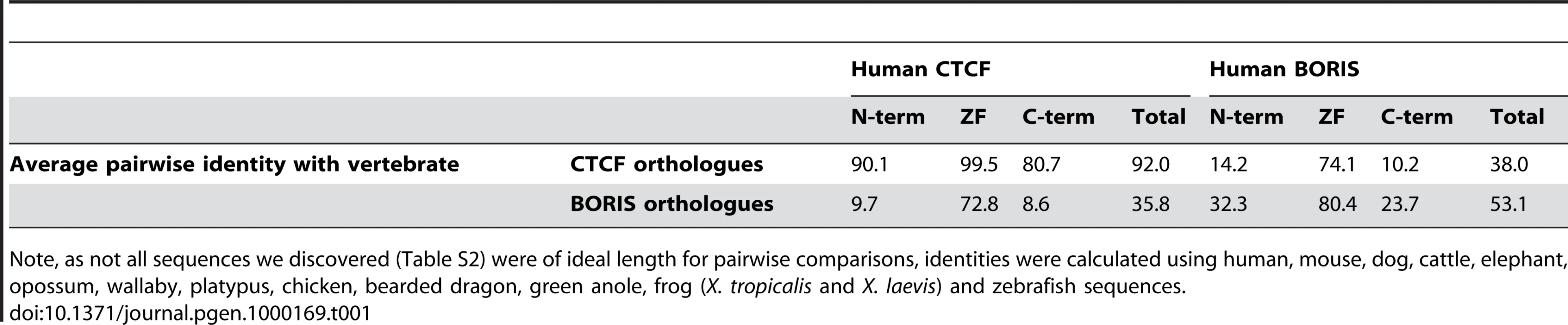
Note, as not all sequences we discovered (Table S2) were of ideal length for pairwise comparisons, identities were calculated using human, mouse, dog, cattle, elephant, opossum, wallaby, platypus, chicken, bearded dragon, green anole, frog (X. tropicalis and X. laevis) and zebrafish sequences. CTCF genomic sequence from human, mouse, zebrafish and frog were all reported to have ten protein-encoding exons when they were first characterised [13],[14],[16]. In contrast, chicken CTCF was reported by Klenova et al. [12] to only have seven protein coding exons, four of which contained all eleven zinc fingers. We analysed the gene structure of CTCF in all species from which there was full genomic sequence and found that all sequences, including chicken CTCF, contained ten exons in total, with seven ZF exons (Figure 1B). BORIS orthologues were also found to have a very similar structure, especially within the ZF domain where intron-exon boundaries were identical.
Gene Expression Analysis
One of the most remarkable characteristics of CTCF and BORIS is that in humans and mice they show apparently mutually exclusive expression. BORIS is transcribed only in specific parts of the testis, while CTCF is expressed in all tissues except those expressing BORIS [16],[38]. This expression pattern underpins the hypothesis that BORIS is the key regulator establishing the male germline imprint of IGF2, that it acts antagonistically to CTCF and defines its inclusion within the cancer-testis group of genes.
To determine if this expression pattern is conserved more widely in vertebrates, we examined the transcription of CTCF and BORIS in cattle, wallaby, platypus and bearded dragon. Initially, we performed 35 cycles of RT-PCR on a series of tissues using CTCF/BORIS primers anchored within at least one of the ZFs and a surrounding non-zinc finger region (Table S1). CTCF transcripts were detected in this way for all tissues and animals tested (Figure 4A). BORIS transcripts were detected only in the gonads of cattle and wallaby; strongly in testes, and weakly in ovarian samples. In contrast, BORIS was amplified from a much wider set of somatic and reproductive tissues in platypus (brain, heart, liver, kidney and testis); and bearded dragon (brain, lung, liver, kidney, spleen, testis and ovary). To minimise the possibility we were observing tissue specific splice variants, we repeated our RT-PCR experiments using primers from different regions of BORIS (Table S1), and found similar results (data not shown).
Fig. 4. Expression analysis of CTCF and BORIS. 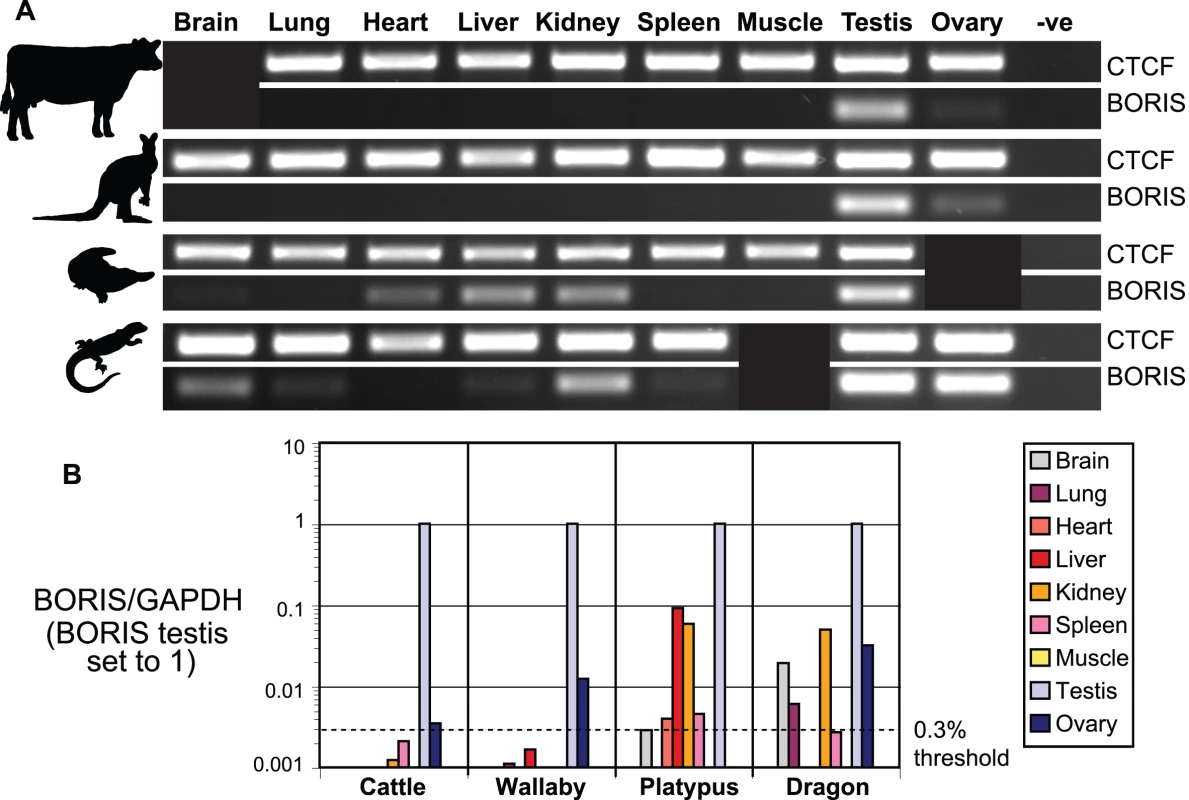
(A) Conventional RT-PCR of CTCF and BORIS after 35 cycles. Note, cattle brain, platypus ovary and bearded dragon muscle could not be tested due to tissue unavailability or poor RNA quality. (B) BORIS transcript levels relative to the positive control gene GAPDH as quantified by real-time PCR. To assist comparisons between species, we set BORIS expression in the testis to 1 and adjusted all other values within the same species proportionally. As found in humans, expression of BORIS in somatic tissues of cattle and wallaby did not exceed 0.3% of that found in testis, although ovarian expression is just on or above this threshold. In contrast, levels of BORIS expression in platypus (liver and kidney) and bearded dragon (brain, kidney and ovary) are well above this threshold and in some cases match CTCF expression (Figure S2). Despite these discoveries, due to the nature of conventional ‘end-point observed’ RT-PCR our initial experiments were semi-quantitative at best. Thus, we were unsure if the expression we were observing was at a level which was biologically relevant. A recent publication using the quantitative real-time PCR technique found that although BORIS expression is considered to be restricted to the testis and some tumours, BORIS transcripts could be detected in other tissues up to 0.3% of the level of BORIS in the testis [47]. The authors concluded from this that expression of BORIS less than 0.3% of the level in testis was not biologically relevant.
We performed real-time PCR amplifications of CTCF and BORIS on all our available tissues in triplicate and comparatively quantified their respective levels using the Corbett Research Rotorgene system, with SYBR Green as the fluorescent DNA-binding dye. Differences in template concentration within a species were taken into consideration by normalising our results to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In agreement with our initial RT-PCR experiments, amplification of CTCF occurred in all tissues and species reproducibly, but with up to 50-fold variation between tissues (Figure S2) not unlike that seen previously in developing zebrafish and frog [13],[14].
Like previous experiments, we found that BORIS amplifications by real-time PCR were predominantly from the testis, with consistently high expression between 10% and 100% of the level of GAPDH (Figure S2). As expected, BORIS amplification was also detected on multiple occasions outside of the testis, particularly within platypus and bearded dragon, and at levels well within the expected limitations of our assay (see methods). When we applied the 0.3% cut-off defined by Kholmanskikh et al., [47] to our results, we found that as in humans, levels of BORIS in somatic tissues were below this threshold in cattle and wallaby (<0.2% of testis expression), while ovarian BORIS levels were just on (cattle) or above (wallaby) this threshold (Figure 4B).
Expression of BORIS outside of the testis in platypus and bearded dragon was much higher. BORIS transcripts in the liver and kidney of platypus was within 6–10% of that found in platypus testis (Figure 4B), and was at a level comparable to CTCF expression found in these tissues (Figure S2). Likewise, levels of BORIS transcripts in bearded dragon brain, kidney and ovary were 2–5% of the level of BORIS in testis. BORIS transcripts were detected in other five other somatic tissues of platypus (brain, heart and spleen) and bearded dragon (lung and spleen) at levels just on or above the 0.3% threshold.
Discussion
We examined the sequence and expression of key epigenetic regulators CTCF and BORIS in vertebrates through cloning, sequencing, bioinformatic analysis and quantitative gene expression experiments. Our results are at variance with the hypothesis that BORIS arose recently by duplication of CTCF in mammals, and was quickly specialised for a role in germ cell imprinting that was complementary, or even antagonistic, to the role of CTCF.
BORIS First Arose in Early Amniotes
Previous studies established that CTCF is a highly conserved and ubiquitous gene in humans and mice, as well as other vertebrates including birds, fish and amphibians [12]–[14]. Our studies on cattle, wallaby, platypus and dragon lizard confirm the expectation that CTCF is highly conserved in all vertebrate groups, and is expressed to varying degrees in all tissues of eutherian, marsupial and monotreme mammals, as well as reptiles (Figures 4A and S2).
In contrast to the well-studied CTCF gene, much less is known about the evolutionary history and function of BORIS. BORIS sequence was previously determined only in humans and mice [16], and no orthologue was detected in chicken. This gave rise to the speculation that BORIS duplicated from CTCF only recently in the mammal lineage. In addition, chicken CTCF was reported to have a gene structure significantly different from that of mammal CTCF and BORIS. Chicken was therefore considered to represent the ancestral gene structure, and an alteration of CTCF gene structure was proposed to have occurred in the mammalian ancestor, followed by a duplication to give rise to BORIS.
We found that chicken CTCF was not, after all, different in structure from mammal CTCF as was previously reported [12],[16] (Figure 1B). The chicken genome project had not been undertaken when chicken CTCF was initially sequenced, so sequence coverage from this early study may not have been sufficient to build a reliable assembly of the region. Alternatively, the CTCF clone that was sequenced may have been a cDNA and genomic DNA chimaera.
Unexpectedly, we found orthologues of BORIS in at least two reptilian species (bearded dragon and green anole). This means that the duplication of CTCF which gave rise to BORIS must have occurred prior to the divergence of sauropsids (birds and reptiles) and mammals 210–310 million years ago (Figure 5). In agreement with Loukinov et al. [16] we could find no full orthologue of BORIS within the chicken genome. However, by analysing the intergenic region between markers flanking the expected site of BORIS in chicken, we did discover a small 108-bp segment of DNA homologous to the first zinc finger of BORIS (Figure 3). Although this region of DNA may be part of another functional gene, we consider that it is unlikely to be functionally related to other BORIS orthologues, given that no other regions showed conservation, even the usually well-conserved zinc fingers. We conclude that either BORIS succumbed to pseudogenisation in birds some time after they diverged from reptiles, or underwent a rapid functional change leaving behind only small traces of its evolutionary past.
Fig. 5. Proposed model of CTCF and BORIS evolution in amniotes. 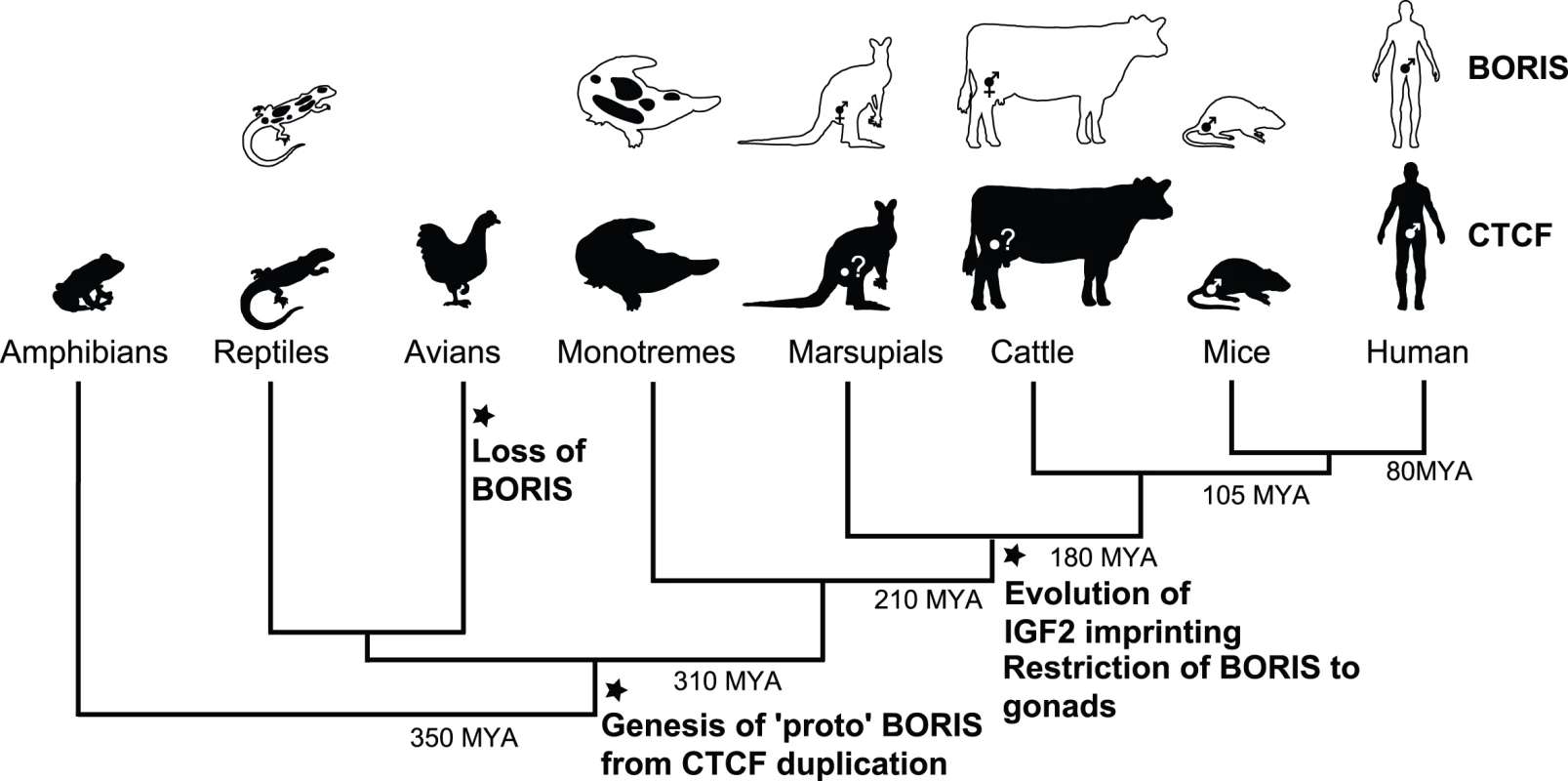
The expression of CTCF and BORIS is indicated (black = expressed, white = not expressed) within various taxa. The ancestral expression pattern of BORIS is wide, including multiple somatic tissues (reptiles and monotremes), but becomes progressively restricted in therian mammals with gonad specific expression in marsupials and cattle (Figure 4) and testis-specific expression in humans and mice [16],[38],[47]. Significant events in the evolution of CTCF and BORIS are marked with respect to the phylogenetic tree. In an extension of previous studies [16], we found extremely high conservation between vertebrate CTCF orthologues, but observed that BORIS homologues were similar to each other, and to CTCF, only within the ZF domains (Table 1). These observations support the prediction of Loukinov et al. that any major differences between CTCF and BORIS function are probably attributable to the N - and C-terminal domains, given these are the most divergent. In fact, the N - and C-terminal domains of CTCF and BORIS contained only small pockets of sequence that were obviously alignable (Figure S1). Interestingly, two of these conserved regions overlapped the start and end of these proteins, implying that the duplication that gave rise to BORIS must have involved the entire CTCF sequence.
The ZF domain of CTCF orthologues we examined showed an almost perfect (99.5% average) identity with human CTCF. In comparison, the average conservation between the ZF domain of human BORIS and other BORIS orthologues was much lower (80.4%). This suggests that BORIS experienced a decrease in functional constraint relative to CTCF, initially because it was a duplicate, and presently because it only binds a subset of the sites bound by CTCF. Alternatively, BORIS may bind some sequences not recognised by CTCF [38]. In support of this, we found that although all of the amino acids thought to perform protein-DNA interactions [56] were 100% conserved for vertebrate CTCF, many were not conserved in some, or all BORIS orthologues (Figure S1). It would be interesting to investigate this further by mapping BORIS binding sites in the genome relative to the published CTCF binding sites [2],[3].
Expression of Ancestral BORIS in Somatic Tissue
Our RT-PCR experiments showed two main patterns of BORIS expression (Figure 4). In the marsupial and the eutherian (wallaby and cattle respectively) we found predominantly testis-specific expression with some ovarian expression, whereas in the reptile (bearded dragon) and the monotreme (platypus) we detected expression of BORIS in multiple somatic tissues as well as the gonads. When these experiments were repeated using quantitative real-time PCR, we discovered that after 45 cycles of PCR, some BORIS transcripts could be detected outside of the germline in cattle and wallaby. However, we found that these levels of BORIS were extremely low, approaching the limits of detection and falling under a previously defined threshold for meaningful expression of BORIS [47]. Thus, we expect that BORIS function in cattle and wallaby is absent from somatic tissues, just as is predicted in humans and mice. More experiments will be required to determine if the ovarian expression of BORIS in cattle and particularly wallaby is functionally significant.
The highest levels of BORIS expression outside of the testis were found in platypus and bearded dragon. The most striking examples of these came from the liver and kidney of platypus and the brain, kidney and ovary of bearded dragon, which were at levels 2-10% of BORIS expression in the testis (Figure 4B). In two cases (platypus liver and kidney) this level of expression was close to the level of CTCF expression within the same tissues (Figure S2). These results strongly suggest that BORIS in platypus and bearded dragon functions outside of the testes, including in the ovary and multiple somatic tissues. This finding is of significance because it indicates that BORIS had wide expression in an ancestral amniote, similar to that of CTCF, the gene from which it arose by duplication.
The question then arises, why was the CTCF duplicate (or “proto-BORIS”) initially retained and why did it succumb to evolutionary change? The high degree of CTCF conservation throughout vertebrates implies that it is a gene under extreme functional constraint. Accordingly, perhaps it is not surprising that a CTCF duplicate in a new genomic environment would be retained and undergo sub-functionalisation, alleviating some mutational load upon CTCF. Subfunctionalisation may also explain why duplicate copies of CTCF have been retained in the genomes of medaka and stickleback (Figure 2), following whole-genome duplication of early teleost fish [57].
Although we observed CTCF and BORIS expression alongside each other in some tissues of monotremes and reptiles, these genes are apparently not co-expressed in humans and mice and may even be antagonistic. CTCF and BORIS bind competitively to common sites and display opposing effects on the epigenetic status of the Igf2/H19 ICR and transcription of BAG1 and the CT-genes [16],[42],[43],[49],[52]. Thus, at some stage during the evolution of therian mammals, CTCF and BORIS evolved mutually exclusive expression and potential antagonism. As our studies were performed on whole tissues, we could not resolve whether CTCF and BORIS show mutually exclusive expression amongst the many discrete cell-types in testis and ovary in wallaby and cattle, so we cannot pinpoint when mutually exclusive expression arose in therian mammals after their divergence from the monotremes.
BORIS Specialisation Correlates with the Evolution of Imprinting
To date, the only non-pathological function proposed for BORIS is the establishment of paternal-specific methylation at the Igf2/H19 ICR in mice [16],[38]. If found to be true for mice, it seems likely that this function is conserved in humans, since they also possess a paternally methylated CTCF-dependent insulator (the ICR) [34],[35] and testis-specific BORIS expression which is exclusive of CTCF [16]. Moreover, differential methylation of CTCF/BORIS binding sites upstream of a maternally-expressed H19 orthologue has been discovered in sheep and wallaby [58]-[60], suggesting that the mouse model of Igf2/H19 imprinted regulation and BORIS function may be conserved throughout all therians. Yet, BORIS is not expected to have this function in reptiles and monotremes, or the amniotic ancestor from which BORIS first arose, as IGF2 imprinting evolved after the divergence of monotremes from therian mammals. Our finding that BORIS expression is gonad-specific in wallaby and cattle, both of which possess imprinting of IGF2 [21],[23], implies that restriction of BORIS expression to the germline correlates with the evolution of genomic imprinting at IGF2/H19 and other loci (reviewed [61]).
In support of this, the evolution of another essential regulator of the Igf2/H19 ICR is also strongly correlated with the evolution of imprinting. Orthologues of the de novo methyltransferase family member DNMT3L are present in eutherians and marsupials (which posses imprinting), but apparently not in chicken, fish [62] or platypus (T.H., unpublished data) which are thought to lack imprinting.
Model of CTCF and BORIS Evolution in Amniotes
We propose that a duplication of CTCF occurred in a common ancestor of all amniotes, probably some time after their divergence from amphibians 350-310MYA (Figure 5). We predict that originally this ‘proto-BORIS’ functioned alongside CTCF, perhaps subfunctionalising to take on tissue-specific roles from the highly conserved and functionally constrained CTCF protein. When genomic imprinting arose in early therian mammals 210-180MYA, BORIS was recruited to perform imprint establishment in germ cells, and CTCF imprint interpretation at IGF2/H19 and potentially other imprinted genes. We speculate that this specialisation marked the start of antagonism between BORIS and CTCF, through the development of opposing epigenetic effects at the common loci to which they bound. The result of this was restriction of BORIS expression to the gonads of early therian mammals, and later restriction to the testes in the ancestor of humans and mice.
The divergent nature and proposed clash of function between CTCF and BORIS has often been described as ‘sibling-rivalry’ [16],[52]. Our results show that this rivalry did not always exist, and ironically may have evolved in response to the evolution of genomic imprinting, which is in turn thought to have evolved from other conflicts in the family [63].
Materials and Methods
Tissue
Adult cattle tissue was sourced from commercial abattoirs processing farmed animals from New South Wales, Australia. Tissue from adult wallaby and platypus were sourced from a captive breeding colony of wallabies and a platypus tissue collection, both held at the Research School of Biological Sciences, Australian National University, Canberra, Australia. Juvenile central bearded dragon tissues samples were sourced from a captive breeding colony held at the University of Canberra, Australia. All tissue (excluding testes samples) was from females, except for platypus tissue which was male. The captivity and sacrifice of all animals was approved by the Australian National University (wallaby and platypus) and University of Canberra (bearded dragon) Animal Experimentation Ethics Committees (AEECP R.CG.08.03, R.CG.02.00 and CEAE 04/04 respectively). Sourcing of cattle tissue was exempt from AEEC approval, as these animals were not sacrificed primarily for research purposes (Simon Bain, ANU AEEC).
Nucleic Acid Extraction, Amplification, and Sequencing
Genomic DNA extraction was performed on liver tissue samples following the standard protocol for mammalian tissue [64]. Total RNA was extracted using the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich) according to the manufacturer's instructions. Eluted RNA was treated by DNAse digestion, using the DNA-free Dnase kit (Ambion) as recommended by the manufacturer. All samples were checked for quality and purity on a 1.2% denaturing formaldehyde agarose gel [64]. RNA was tested for genomic DNA contamination by PCR prior to first strand synthesis of cDNA. Approximately 800 ng of purified RNA was used to create cDNA using the SuperScript III Reverse Transcriptase system (Invitrogen) according to manufacturer's instructions. All first strand synthesis reactions were undertaken using random hexamer primers except for Rapid Amplification of cDNA Ends (RACE) experiments, where the GeneRacer Oligo dT primer (Invitrogen) was used. Conventional PCR amplifications were performed in a 50 µL reaction, including either 1 µL of undiluted cDNA or 200 ng of genomic DNA as a template, 0.2 µM of each primer (Table S1) and the following reagents from Invitrogen; 1X PCR Buffer, 0.8 mM dNTP mixture (0.2 mM each), 1.5 mM MgCl2 and 0.2 µL of Platinum Taq DNA Polymerase. Cycling conditions used were as follows; 94°C, 2 min; 34×(94°C, 30 sec; 61°C, 30 sec; 72°C, 1 min); 72°C, 10 min. When amplifications over 1000 bp were performed, extension times were increased by 1 min/kb. Nested PCR amplifications for 3′ RACE were also undertaken using this protocol, except with reduced cycle numbers, modified primer concentration and increased annealing temperatures as stipulated in the GeneRacer kit (Invitrogen) protocol.
For initial gene expression studies 5 µL of CTCF and BORIS amplified products were combined together with 6 µL of loading buffer (30% glycerol, with light Bromophenol blue staining) and subjected to electrophoresis for 40min at 7.6 V/cm on a 1% agarose gel with TAE buffer and SYBR Safe DNA gel stain (Invitrogen). Gels photographs were illuminated with blue light and exposed using the Gel Logic 100 Imaging System (Kodak). Other than cropping, no alterations to these images were performed.
Full length CTCF and BORIS cDNAs were amplified from liver and testes samples respectively and cloned using the TOPO TA Cloning Kit. Recombinant plasmid DNA was purified using the Wizard Plus SV Miniprep System and then combined with relevant primers (Table S1) for sequencing at the Australian Genome Research Facility.
Real-Time PCR
Real-time PCR was performed in 20 µL reactions using the QuantiTect SYBR Green PCR Kit (Qiagen) according to manufacturer's instructions. Amplifications were performed and detected with a Rotorgene 3000 cycler (Corbett Research) using the following cycling conditions; 95°C, 15 min; 45×(94°C, 30 sec; 58°C, 30 sec; 72°C, 20 sec); 72°C, 10 min. All experimental amplifications were performed in triplicate and averaged over two or three concordant results which varied by Ct values of less than 0.7. Levels of CTCF and BORIS relative to GAPDH in each tissue and species were calculated using the comparative quantitation software supplied by Rotorgene. All products were checked for specificity by melt-curve analysis and electrophoresis.
Primers used in this analysis were designed for each species from similar intron-spanning regions of CTCF, BORIS and GAPDH (Table S1). These primers were selected for high amplification efficiency (>1.65) and low primer-dimer. A 10-fold serial dilution of testis cDNA was undertaken to determine the amplification range and performance of BORIS primers at low template concentrations, because BORIS (unlike CTCF and GAPDH) is known to have low or undetectable expression in many tissues [16],[47]. We found that BORIS transcripts could be detected reliably down to the 10−3 dilution. Primers for the positive control gene GAPDH (Table S1) were designed from sequence deposited on NCBI for cattle (NM_001034034.1), platypus (EH003224) and wallaby (EF654515 and trace archive data). For bearded dragon, GAPDH primers were designed from sequence we determined ourselves by PCR amplification and sequencing (EU784660).
Bioinformatic Analysis
Homology searches were performed using BLASTn and tBLASTn [65] against the non-redundant, expressed sequence tag and trace archive databases at the NCBI website (http://www.ncbi.nlm.nih.gov) or release 46 of the Ensembl website (http://www.ensembl.org). For species in which gene prediction was not available, or was unrealistic, we performed our own gene predictions using Genomescan [66] and local alignment. A multiple alignment of the resulting set of predicted and experimentally determined cDNA sequence (Table S2) was produced using ClustalW2 with default parameters (http://www.ebi.ac.uk/Tools/clustalw2). Phylogenetic analysis was performed on aligned cDNA sequences by the neighbour-joining method with uncorrected distance measure, using the phylogenetic program PAUP* version 4.0 b 10 [67]. 1,000 replications were performed for bootstrap analysis. Protein coding predictions of these cDNA sequences were also made and aligned using ClustalW2. This alignment was then used to calculate pairwise identity between selected orthologues using MacVector v9.5.2.
The conserved block of genes orthologous to the region surrounding human BORIS was identified in amniote species by BLAST with the criteria of unique reciprocal best-hits back to the query sequence in the human genome. Genomic sequence from these orthologous blocks was extracted from Ensembl and aligned using the LAGAN algorithm [68] available on the mVISTA website with default parameters (http://genome.lbl.gov/vista/mvista/submit.shtml).
Supporting Information
Zdroje
1. LobanenkovVV
NicolasRH
AdlerVV
PatersonH
KlenovaEM
1990 A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene 5 1743 1753
2. KimTH
AbdullaevZK
SmithAD
ChingKA
LoukinovDI
2007 Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 128 1231 1245
3. BarskiA
CuddapahS
CuiK
RohTY
SchonesDE
2007 High-resolution profiling of histone methylations in the human genome. Cell 129 823 837
4. ValenzuelaL
KamakakaRT
2006 Chromatin insulators. Annu Rev Genet 40 107 138
5. BellAC
WestAG
FelsenfeldG
1999 The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98 387 396
6. XuN
DonohoeME
SilvaSS
LeeJT
2007 Evidence that homologous X-chromosome pairing requires transcription and Ctcf protein. Nat Genet 39 1390 1396
7. LingJQ
LiT
HuJF
VuTH
ChenHL
2006 CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312 269 272
8. WallaceJA
FelsenfeldG
2007 We gather together: insulators and genome organization. Curr Opin Genet Dev 17 400 407
9. FilippovaGN
2008 Genetics and epigenetics of the multifunctional protein CTCF. Curr Top Dev Biol 80 337 360
10. FilippovaGN
QiCF
UlmerJE
MooreJM
WardMD
2002 Tumor-associated zinc finger mutations in the CTCF transcription factor selectively alter tts DNA-binding specificity. Cancer Res 62 48 52
11. FilippovaGN
FagerlieS
KlenovaEM
MyersC
DehnerY
1996 An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol Cell Biol 16 2802 2813
12. KlenovaEM
FagerlieS
FilippovaGN
KretznerL
GoodwinGH
1998 Characterization of the chicken CTCF genomic locus, and initial study of the cell cycle-regulated promoter of the gene. J Biol Chem 273 26571 26579
13. BurkeLJ
HollemannT
PielerT
RenkawitzR
2002 Molecular cloning and expression of the chromatin insulator protein CTCF in Xenopus laevis. Mech Dev 113 95 98
14. PugachevaEM
KwonYW
HukriedeNA
PackS
FlanaganPT
2006 Cloning and characterization of zebrafish CTCF: Developmental expression patterns, regulation of the promoter region, and evolutionary aspects of gene organization. Gene 375 26 36
15. MoonH
FilippovaG
LoukinovD
PugachevaE
ChenQ
2005 CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator. EMBO Rep 6 165 170
16. LoukinovDI
PugachevaE
VatolinS
PackSD
MoonH
2002 BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A 99 6806 6811
17. MorisonIM
RamsayJP
SpencerHG
2005 A census of mammalian imprinting. Trends Genet 21 457 465
18. DeChiaraTM
RobertsonEJ
EfstratiadisA
1991 Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64 849 859
19. OgawaO
EcclesMR
SzetoJ
McNoeLA
YunK
1993 Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms' tumour. Nature 362 749 751
20. RainierS
JohnsonLA
DobryCJ
PingAJ
GrundyPE
1993 Relaxation of imprinted genes in human cancer. Nature 362 747 749
21. DindotSV
FarinPW
FarinCE
RomanoJ
WalkerS
2004 Epigenetic and genomic imprinting analysis in nuclear transfer derived Bos gaurus/Bos taurus hybrid fetuses. Biol Reprod 71 470 478
22. O'NeillMJ
IngramRS
VranaPB
TilghmanSM
2000 Allelic expression of IGF2 in marsupials and birds. Dev Genes Evol 210 18 20
23. SuzukiS
RenfreeMB
PaskAJ
ShawG
KobayashiS
2005 Genomic imprinting of IGF2, p57(KIP2) and PEG1/MEST in a marsupial, the tammar wallaby. Mech Dev 122 213 222
24. BartolomeiMS
ZemelS
TilghmanSM
1991 Parental imprinting of the mouse H19 gene. Nature 351 153 155
25. RachmilewitzJ
GoshenR
ArielI
SchneiderT
de GrootN
1992 Parental imprinting of the human H19 gene. FEBS Lett 309 25 28
26. KillianJK
NolanCM
StewartN
MundayBL
AndersenNA
2001 Monotreme IGF2 expression and ancestral origin of genomic imprinting. J Exp Zool 291 205 212
27. NolanCM
KillianJK
PetitteJN
JirtleRL
2001 Imprint status of M6P/IGF2R and IGF2 in chickens. Dev Genes Evol 211 179 183
28. LawtonBR
SevignyL
ObergfellC
ReznickD
O'NeillRJ
2005 Allelic expression of IGF2 in live-bearing, matrotrophic fishes. Dev Genes Evol 215 207 212
29. WoodburneMO
RichTH
SpringerMS
2003 The evolution of tribospheny and the antiquity of mammalian clades. Mol Phylogenet Evol 28 360 385
30. TremblayKD
DuranKL
BartolomeiMS
1997 A 5′ 2-kilobase-pair region of the imprinted mouse H19 gene exhibits exclusive paternal methylation throughout development. Mol Cell Biol 17 4322 4329
31. SchoenherrCJ
LevorseJM
TilghmanSM
2003 CTCF maintains differential methylation at the Igf2/H19 locus. Nat Genet 33 66 69
32. PantV
MarianoP
KanduriC
MattssonA
LobanenkovV
2003 The nucleotides responsible for the direct physical contact between the chromatin insulator protein CTCF and the H19 imprinting control region manifest parent of origin-specific long-distance insulation and methylation-free domains. Genes Dev 17 586 590
33. EngelN
ThorvaldsenJL
BartolomeiMS
2006 CTCF binding sites promote transcription initiation and prevent DNA methylation on the maternal allele at the imprinted H19/Igf2 locus. Hum Mol Genet 15 2945 2954
34. HarkAT
SchoenherrCJ
KatzDJ
IngramRS
LevorseJM
2000 CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405 486 489
35. BellAC
FelsenfeldG
2000 Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405 482 485
36. KurukutiS
TiwariVK
TavoosidanaG
PugachevaE
MurrellA
2006 CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci U S A 103 10684 10689
37. HanL
LeeDH
SzaboPE
2008 CTCF is the master organizer of domain-wide allele-specific chromatin at the H19/Igf2 imprinted region. Mol Cell Biol 28 1124 1135
38. JelinicP
StehleJC
ShawP
2006 The testis-specific factor CTCFL cooperates with the protein methyltransferase PRMT7 in H19 imprinting control region methylation. PLoS Biol 4 e355
39. KanedaM
OkanoM
HataK
SadoT
TsujimotoN
2004 Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429 900 903
40. Bourc'hisD
BestorTH
2004 Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431 96 99
41. KatoY
KanedaM
HataK
KumakiK
HisanoM
2007 Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet 16 2272 2280
42. VatolinS
AbdullaevZ
PackSD
FlanaganPT
CusterM
2005 Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res 65 7751 7762
43. HongJA
KangY
AbdullaevZ
FlanaganPT
PackSD
2005 Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res 65 7763 7774
44. RenaudS
PugachevaEM
DelgadoMD
BraunschweigR
AbdullaevZ
2007 Expression of the CTCF-paralogous cancer-testis gene, brother of the regulator of imprinted sites (BORIS), is regulated by three alternative promoters modulated by CpG methylation and by CTCF and p53 transcription factors. Nucleic Acids Res 35 7372 7388
45. RisingerJI
ChandramouliGV
MaxwellGL
CusterM
PackS
2007 Global expression analysis of cancer/testis genes in uterine cancers reveals a high incidence of BORIS expression. Clin Cancer Res 13 1713 1719
46. Woloszynska-ReadA
JamesSR
LinkPA
YuJ
OdunsiK
2007 DNA methylation-dependent regulation of BORIS/CTCFL expression in ovarian cancer. Cancer Immun 7 21
47. KholmanskikhO
LoriotA
BrasseurF
De PlaenE
De SmetC
2008 Expression of BORIS in melanoma: lack of association with MAGE-A1 activation. Int J Cancer 122 777 784
48. SimpsonAJ
CaballeroOL
JungbluthA
ChenYT
OldLJ
2005 Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5 615 625
49. KangY
HongJA
ChenGA
NguyenDM
SchrumpDS
2007 Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene 26 4394 4403
50. KouprinaN
NoskovVN
PavlicekA
CollinsNK
Schoppee BortzPD
2007 Evolutionary diversification of SPANX-N sperm protein gene structure and expression. PLoS ONE 2 e359
51. SunL
HuangL
NguyenP
BishtKS
Bar-SelaG
2008 DNA methyltransferase 1 and 3B activate BAG-1 expression via recruitment of CTCFL/BORIS and modulation of promoter histone methylation. Cancer Res 68 2726 2735
52. KlenovaEM
MorseHC3rd
OhlssonR
LobanenkovVV
2002 The novel BORIS+CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol 12 399 414
53. O'BrienSJ
Menotti-RaymondM
MurphyWJ
NashWG
WienbergJ
1999 The Promise of Comparative Genomics in Mammals. Science 286 458 481
54. VolffJ-N
2006 Vertebrate genomes. Basel; New York Karger vii, 216
55. HoreTA
KoinaE
WakefieldMJ
Marshall GravesJA
2007 The region homologous to the X-chromosome inactivation centre has been disrupted in marsupial and monotreme mammals. Chromosome Res 15 147 161
56. PaboCO
PeisachE
GrantRA
2001 Design and selection of novel Cys2His2 zinc finger proteins. Annu Rev Biochem 70 313 340
57. TaylorJS
BraaschI
FrickeyT
MeyerA
Van de PeerY
2003 Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res 13 382 390
58. YoungLE
SchniekeAE
McCreathKJ
WieckowskiS
KonfortovaG
2003 Conservation of IGF2-H19 and IGF2R imprinting in sheep: effects of somatic cell nuclear transfer. Mech Dev 120 1433 1442
59. ThurstonA
TaylorJ
GardnerJ
SinclairKD
YoungLE
2008 Monoallelic expression of nine imprinted genes in the sheep embryo occurs after the blastocyst stage. Reproduction 135 29 40
60. SmitsG
MungallAJ
Griffiths-JonesS
SmithP
BeuryD
2008 Conservation of the H19 noncoding RNA and H19-IGF2 imprinting mechanism in therians. Nat Genet. In press doi:10.1038/ng.168
61. HoreTA
RapkinsRW
GravesJA
2007 Construction and evolution of imprinted loci in mammals. Trends Genet 23 440 448
62. YokomineT
HataK
TsudzukiM
SasakiH
2006 Evolution of the vertebrate DNMT3 gene family: a possible link between existence of DNMT3L and genomic imprinting. Cytogenet Genome Res 113 75 80
63. MooreT
HaigD
1991 Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet 7 45 49
64. SambrookJ
ManiatisT
FritschEF
1989 Molecular cloning : a laboratory manual. Cold Spring Harbor, N.Y Cold Spring Harbor Laboratory
65. AltschulSF
GishW
MillerW
MyersEW
LipmanDJ
1990 Basic local alignment search tool. J Mol Biol 215 403 410
66. YehRF
LimLP
BurgeCB
2001 Computational inference of homologous gene structures in the human genome. Genome Res 11 803 816
67. SwoffordDL
2003 PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). 4 ed. Sunderland, Massachusetts Sinauer Associates
68. BrudnoM
DoCB
CooperGM
KimMF
DavydovE
2003 LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res 13 721 731
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2008 Číslo 8- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
Nejčtenější v tomto čísle- Rise of the Machines
- A Tribute to Evgenii V. Ananiev, 1947–2008
- Analysis of Transposon Interruptions Suggests Selection for L1 Elements on the X Chromosome
- The Evolution of Epigenetic Regulators and in Amniotes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



