-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
EFFECT OF THE PLACEMENT OF THE INERTIAL SENSOR ON THE HUMAN MOTION DETECTION
There are numerous possibilities of assessments of the human activity, offered by the ActimedARM -a wearable inertial sensor we developed. This device features a triaxial magnetometer, a trixial accelerometer, a micro-processing unit, a Zigbee module and a µSD card. Its embedded algorithms make it able to compute postures, transfers of the subject and also to characterize the walking episodes. We recently succeeded in computing the relative displacements of the sensors, from double integration of the acceleration signals, in order to qualify specific physical activities such as rising from chairs or stools. The experiments highlighted the impact of the location of the sensor on the body on the correlation between objective motion and signals processed from acceleration measurements, showing a better correlation coefficient of 11.41% when the sensor is located on the navel.
Keywords:
actimetry monitoring, embedded systems, inertial sensors
Authors: Julien Collet 1; Martin Cerny 2; Ludovic Delporte 3; Norbert Noury 1
Authors place of work: Institute of Nanotechnology of Lyon (INL-UMR5 70), INSA Lyon, Villeurbanne, France 1; VSB Technical University Ostrava, Ostrava, Czech Republic 2; Neurologic Science Center of Lyon (CNRL), Lyon, France 3
Published in the journal: Lékař a technika - Clinician and Technology No. 4, 2014, 44, 21-24
Category: Původní práce
Summary
There are numerous possibilities of assessments of the human activity, offered by the ActimedARM -a wearable inertial sensor we developed. This device features a triaxial magnetometer, a trixial accelerometer, a micro-processing unit, a Zigbee module and a µSD card. Its embedded algorithms make it able to compute postures, transfers of the subject and also to characterize the walking episodes. We recently succeeded in computing the relative displacements of the sensors, from double integration of the acceleration signals, in order to qualify specific physical activities such as rising from chairs or stools. The experiments highlighted the impact of the location of the sensor on the body on the correlation between objective motion and signals processed from acceleration measurements, showing a better correlation coefficient of 11.41% when the sensor is located on the navel.
Keywords:
actimetry monitoring, embedded systems, inertial sensorsIntroduction
Activities of Daily Living (ADL) are directed by vital needs as for example sleeping or feeding. These activities reflect both our physical and mental conditions. Furthermore, it is known that the ability of an individual to complete physical tasks can help diagnosing diseases such as chronic obstructive pulmonary diseases (COPD) [1] or mobility affecting pathologies [4].
Actimetry studies the succession of postures (sitting, standing, lying and walking) in which a subject was during a period of time to evaluate its characteristics. There are mainly two kinds of devices dedicated to this tracking: gold-standard systems using sets of cameras and inertial devices [2, 3]. The first category can produce objective and exhaustive assessment of the activity by implementing complex image-processing algorithms. But these systems are limited to laboratory premises and have important disadvantages such as requiring powerful calculation systems and the user to wear tags over the body. The other category –namely, the inertial sensors - have emerged due to the recent advances in the MEMS industry [2, 3]. The decreasing sizes of devices made possible the design of small wearable systems for the monitoring of the activity on a daily basis.
We designed and fabricated such an embedded system: The ActimedARM. The design was driven by consideration of energy consumption and acceptability. Recently, we performed experiments to investigate the feasibility of the assessment of the motion and the impact of the sensor location on an individual body. To achieve this, displacement was computed from ac-celeration measurements and compared to the data obtained from an objective source.
Materials and methods
Material
The ActimedARM was designed around a 32bit ARM powered processing unit. It also embeds a three-axis magnetometer, a three-axis accelerometer, a Zigbee (802.15.4) module and a µSD card. A 3.6 Volts Lithium-Ion battery [4], power the whole system.
The core of the system, the STM32F103RE (STMicroelectronics), was chosen for its power in terms of calculations (Cortex-M3 core), its integrated peripherals (SPI, USART, ADC, RTC) and its low-consumption (28 mA maximum in operation, 25 µA in sleep mode). The µSD card offers a great compromise between storage capacity and physical dimensions. It is also natively handled by the processing unit (a SDIO port is available and a dedicated library is provided by the manufacturer).
The ADXL345 (Analog Device) is an autonomous system that communicates the values of accelerations on a SPI bus. It was chosen for its low-power characteristics (3.6 V, 65 µA@25 Hz) and is in sleep mode most of the time [4].
The HMC1043 (Honeywell) is a three dimensional magnetometer and implements a Wheatstone bridge type circuit and an amplification stage to feed the analog-to-digital converter. The system also features a reset circuit to compensate the drift in time of the magneto-resistors [4] and is usually read each second by the processing unit.
Mechanical considerations and hardware performances
The design stage was driven by considerations of acceptability. In terms of physical dimensions, the ActimedARM has the following dimensions: 28x30x12 mm without the power battery. The privileged location for the sensor was on the bust, for a better acceptability and discretion. Also, the bust was found to be more representative of the whole body motion and therefore more suitable for a global asses-sment of the activity. During the latest experiments, other locations were investigated such as on the navel or on the pelvis.
The second design goal was to create a system able to monitor the activity during long periods of time. To do so, its consumption must be kept low. In radio-operation mode (the radio module is on), the autonomy is 8 days (resp. 24 days) on a 1200 mAh battery (resp. 3600 mAh). In data-logger mode (the radio module is off), the autonomy reaches 10 days (resp. 32 days) [4].
Embedded Algorithms
In normal conditions, the software iterates every second (1 Hz). In this iteration, 25 samples of acceleration and one sample of magnetic field are stored and processed. The processing starts with low-pass filtering of the acceleration signals then the detection of the posture, the transfers and the walking episodes. The quaternion representing the attitude of the subject is then computed and, depending on the configuration file, the data is sent through the ZigBee module to the host computer and/or written on dedicated files on the on board µSD card [4].
Fig. 1: The ActimedARM (center), a μSD card (right) and a battery (left). 
The detection of the posture is elaborated from the vertical component of the acceleration modulus being maximum (resp. minimum) when the subject is standing (resp. lying),
The classification of the transfers is realized by analyzing the changes in postures over time for stand to lying and lying to stand transfer. In the more complex case of sit-to-stand and stand-to-sit, an algorithm searches for a phase-shift which occurs during transfers between vertical and horizontal signals.
Fig. 2: Location of the 2 sensors on the subject. 
When walking, a pseudo-periodic signal due to foot impacts can be detected on vertical accelerations. We can therefore detect walking episodes by tracking peaks in the frequency range 1.1 Hz – 5.4 Hz [4].
Experiments
Two series of experiments were performed, involving 4 male subjects (36 +11.5 yrs, 87.7 +32.57 kg, 1.73 +0.10 m) wearing two ActimedARM: one on the navel and the other on the pelvis.
The experiments consisted in a series of sit-to-stand transitions from a chair and from a stool. The orientation data, computed from the sensors, were then compared to position plot from the video system composed of 7 cameras (type: Eagle, sampling frequency: 200 Hz, resolution: 1.3M pixels, manufacturer: Motion Analysis Inc.). For the motion analysis, thirteen tags were placed on the sensors and on the body.
The inertial sensors were sampled at a frequency of 25 Hz for accelerations and 1 Hz for magnetic field components. One quaternion was computed every second during the experimentations. The sensors modules were in data-logging mode (Zigbee communication module off).
The motion plot was obtained by integrating twice the vertical acceleration recorded by the ActimedARM and opposed to the motion plot from the gold standard-video system, under the Matlab Environment. The algorithm is composed of a first step designed to reduce the integration error by removing the DC component of the signal (subtraction of the mean value), then, two numerical integration steps are processed with a high-pass F.I.R filter (0.12 Hz) used in between to reduce the amount of error on the processed signal.
Then, the computation of the correlation coefficient between signals from ActimedARM and video systems was done from the second sit-to-stand until the last one.
Results
The average correlation between the gold standard video system and the ActimedARM located on the pelvis (respectively on the navel) is 72.37% (resp. 83.78%) as can be seen on Table 1.
Tab. 1. Correlation coefficients between displacement data computed from ActimedARM raw acceleration values and data from the video system. 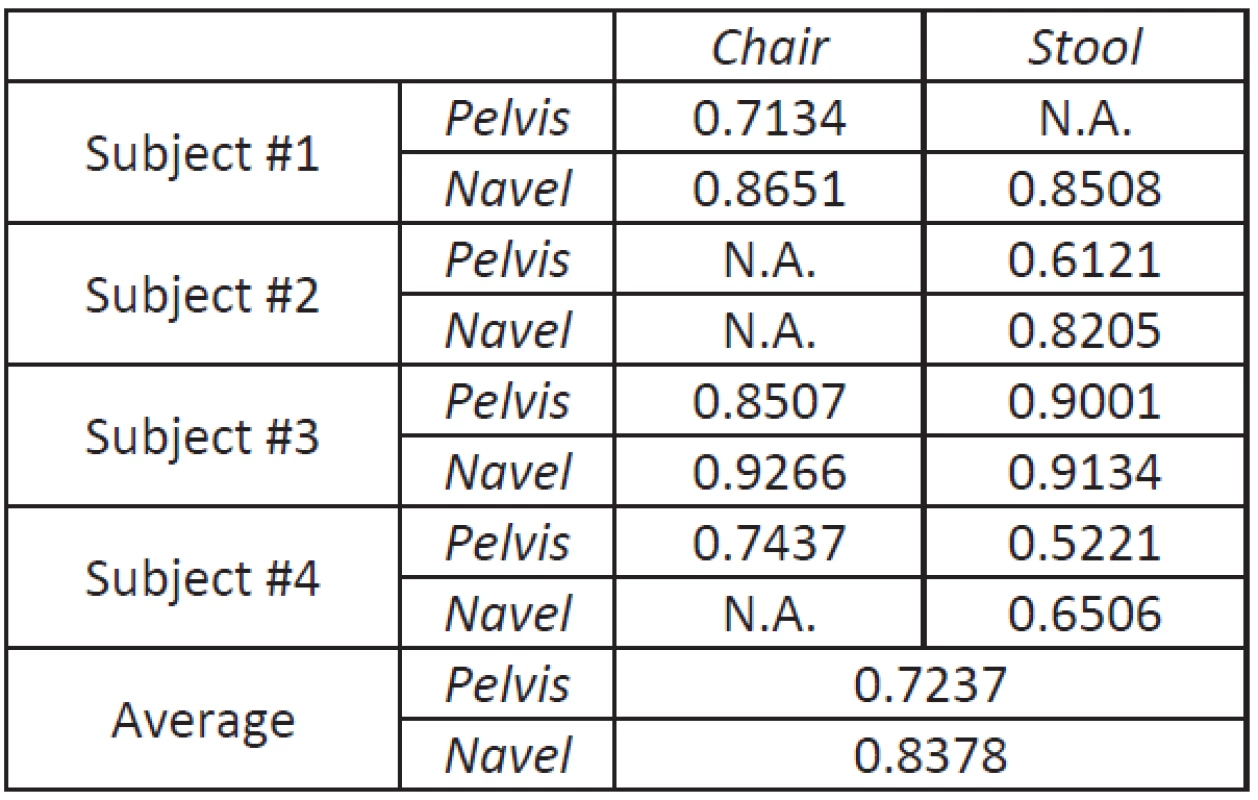
The error between signals from our sensors and the objective motion plots from the video system is mainly due to the cumulative errors brought by the integration process when computing displacements data from raw acceleration signals.
Fig. 3: Subject #3, Displacement of the patient during sit-to-stand and stand-to-sit on a chair. Pelvis located ActimedARM processed signals (r.), video (b.). 
Fig. 4: Subject #3, Displacement of the patient during sit-to-stand and stand-to-sit on a stool. Pelvis located ActimedARM processed signals (r.), video (b.). 
Conclusion
When looking to results (Table 1), it seems pretty obvious that the navel is a privileged location when assessing the motion during sit-to-stand transitions, with an average correlation coefficient better by 11.41% when located on the navel.
Further experiments still needs to be done to check if this location is optimal for the evaluation of other kinds of physical activities or for the overall assessment of the motion both in terms of accuracy and in terms of acceptability.
Improvements can be made on the motion-processing algorithm to reduce errors due to the integration of noise and numerical errors.
Eventually, a new version of the system is under design to obtain a more accurate and low-power inertial sensor using the latest, more frugal components.
Acknowledgement
This paper has been elaborated in the framework of the project “Support research and development in the Moravian-Silesian Region 2013 DT 1 - International research teams“ (RRC/05/2013). Financed from the budget of the Moravian-Silesian Region.
The authors thank team IMPACT (CRNL, Lyon) for accessing their MouvHandi motion platform. Further-more, a grateful thank is addressed at Etienne Grenier and Osée Rajaiah for their voluntary participation to experimentations.
References
Prof. Norbert Noury
Institute of Nanotechnology of Lyon
INSA Lyon,
Villeurbanne, F-69621
France
E-mail: norbert.noury@insa-lyon.fr
Phone: (+33)4 72 43 74 54
Zdroje
[1] B. Aguilaniu, H. Roth, J. Gonzalez, M. Jondot, J. Maitre, F. Denis, T. Similowski. A simple semi-placed three minutes chair rise test (3CRT) for routine exercise tolerance testing in COPD. Int Journal of COPD, 2014 (under publication).
[2] G. A. L. Meijer, K. R. Westerterp, F. M. H. Verhoeven, H. B. M. Koper, F. Ten Hoor. Methods to assess physical activity with special reference to motion sensors and accelerometers, IEEE-TBME. 1991;38 : 221-229.
[3] Bouten C et al. A triaxial accelerometer and portable data processing unit for the assessment of daily physical activity. IEEE-TBME. 1997; 44(3):136-47.
[4] N. Noury, B. Perriot, J. Collet, E. Grenier, M. Cerny B. Massot, E. McAdams. ActimedARM – Design of a Wearable System to Monitor Daily Actimetry, 35th Annual International Conference of the IEEE EMBS, Osaka, 3-7 July, 2013.
Štítky
Biomedicína
Článek vyšel v časopiseLékař a technika

2014 Číslo 4-
Všechny články tohoto čísla
- Solution of the Cross-talk problem in cell impedance analysis of cardiac myocytes
- Differences in sleep patterns among healthy sleepers and patients after stroke
- EFFECT OF THE PLACEMENT OF THE INERTIAL SENSOR ON THE HUMAN MOTION DETECTION
- Impact of different heart rates and arterial elastic moduli on pulse wave velocity in arterial system model
- Pulmonary fluid accumulation and its influence on the Impedance Cardiogram: CompariSON Between a Clinical Trial AND FEM Simulations
- REAL-TIME visualization of multichannel ECG signals using the parallel CPU threads
- Lékař a technika
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- REAL-TIME visualization of multichannel ECG signals using the parallel CPU threads
- Differences in sleep patterns among healthy sleepers and patients after stroke
- Pulmonary fluid accumulation and its influence on the Impedance Cardiogram: CompariSON Between a Clinical Trial AND FEM Simulations
- EFFECT OF THE PLACEMENT OF THE INERTIAL SENSOR ON THE HUMAN MOTION DETECTION
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



