-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEighteen months of teriparatide treatment leads to improvement of bone mineral density and trabecular bone score in patients with glucocorticoids induced osteoporosis: the results from prospective follow-up (registry OSTEO.sk)
18-mesačná liečba teriparatidom zlepšuje u pacientov s glukokortikoidmi indukovanou osteoporózou hodnoty hustoty kostného minerálu a trabekulárne kostné skóre: výsledky prospektívneho sledovania (register OSTEO.sk)
Úvod:
Liečba glukokortikoidmi má za následok rýchlu stratu denzity kostného minerálu (BMD) a glukokortikoidmi indukovaná osteoporóza (GIOP) je najčastejšou a závažnou formou sekundárnej osteoporózy. Liečba žien s postmenopauzálnou osteoporózou teriparatidom (TPTD) významne zvyšuje trabekulárny kostný objem a konektivitu, zlepšuje trabekulárnu morfológiu s posunom smerom k viac doštičkovitej štruktúre a zvyšuje hrúbku kortikálnej kosti.
Ciel štúdie:
Posúdení zmeny BMD a trabekulárneho kostného skóre (TBS) po 12 - a 18-mesačnej liečbe TPTD u pacientov s GIOP.
Pacienti a metódy:
Prospektívne multicentrické, nekontrolované sledovanie účinnosti a bezpečnosti liečby TPTD u pacientov s GIOP, uskutočňované v 5 špecializovaných centrách ustanovených pre liečbu TPTD. U všetkých pacientov zaradených do registru OSTEO.sk bolo pravidelne uskutočňované meranie BMD v bedrovej chrbtici a proximálnom femoru a sledovanie markerov kostného obratu. V podskupine účastníkov bolo merané TBS.
Výsledky:
Z celkového počtu 263 pacientov zaradených do registra bolo do záverečnej analýzy zahrnutých 129 pacientov (20 mužov vo veku 51,5 let/109 žien vo veku 59,7 let). Po liečbe TPTD bol zistený v porovnaní s východiskovými hodnotami nárast BMD v bedrovej chrbtici v 12. mesiaci (+ 8,3 %, p < 0,001) a v 18. mesiaci nárast BMD hlavica femoru (+ 3,85 %, p < 0,001), krčku femoru (+ 3,26 %, p < 0,001) a bedrovej chrbtice (+ 9,28 %, p < 0,001). V podskupine (n = 12) v priebehu prvých 12 mesiacov narástlo TBS o 2,4 % (p = 0,01). 18-mesačná liečba TPTD viedla k významnému zvýšení markerov kostného obratu.
Záver:
18-mesačná liečba TPTD viedla k významnému zvýšeniu BMD so súčasným zvýšením všetkých hodnotených markerov kostného obratu. Okrem toho bolo v podskupine účastníkov štúdie pozorované zvýšenie TBS po 12 mesiacoch liečby. Podľa týchto výsledkov bolo preukázané, že u pacientov s GIOP je TPTD vysoko účinný osteoanabolický liek.
Klíčová slova:
kostná minerálna denzita (BMD) – osteoporóza indukovaná glukokortikoidmi (GIOP) – trabekulárne kostné skóre (TBS) – teriparatide
Authors: Payer Juraj 1; Tomková Soňa 2; Killinger Zdenko 1; Brázdilová Kristina 1; Jackuliak Peter 1; Vaňuga Peter 3; Letkovská Alexandra 4; Masaryk Pavol 4; Kmečová Zlata 5; Kužma Martin 1
Authors place of work: Comenius University Medical Faculty, 5th Department of Internal Medicine, University Hospital Bratislava, Slovakia 1; Osteocentrum, Hospital Košice-Šaca, Košice, Slovakia 2; National Institute of Endocrinology and Diabetology, Lubochňa, Slovakia 3; National Institute of Rheumatic Diseases, Piešťany, Slovakia 4; Osteocentrum of F. D. Roosevelt Faculty Hospital, Banská Bystrica, Slovakia 5
Published in the journal: Clinical Osteology 2018; 23(4): 138-145
Category: Původní práce
Summary
Introduction:
Glucocorticoid therapy results in a rapid loss of bone mineral density (BMD) and glucocorticoid-induced osteoporosis (GIOP) is the most frequent and severe form of secondary osteoporosis. Teriparatide (TPTD) treatment of postmenopausal women with osteoporosis significantly increases cancellous bone volume and connectivity, improves trabecular morphology with a shift toward a more plate-like structure, and increases cortical bone thickness.
Aim of the study:
Assessment of change in BMD and trabecular bone score (TBS) after 12 - and 18-months treatment with TPTD in patients with GIOP.
Patients and methods:
Prospective multicentric, non-controlled follow-up of the effect and safety of TPTD treatment in patients with GIOP, performed in 5-referral centers specified at the treatment with TPTD, In all patients included in OSTEO.sk registry the measurements of BMD, at lumbar spine (LS) and proximal femur, and bone turnover markers were performed periodically. In the subset of participants, a TBS was measured.
Results:
From total number of 263 patients included in the registry, 129 patients (20 men aged 51,5 years/109 women aged 59,7 years) were included in the final analysis. An increase of lumbar spine (LS) BMD at month 12 (+8.3%, p < 0.001) and at month 18 an increase of total hip (+3.85%, p < 0.001), femoral neck (+3.26 %, p < 0.001) and LS BMD (+9.28%, p < 0.001) in comparison to baseline values after treatment with TPTD were observed. In the subset (n=12), TBS increase of 2,4% (p=0,01) during first 12 months. 18 months of TPTD therapy led to significant increase of bone turnover markers.
Conclusion:
18 month treatment with TPTD led to significant increase of BMD with concomitant increase in all assessed bone turnover markers. In addition, in subset of the study group and increase of TBS after 12 months was observed. According to these results, it has been proven that TPTD is highly effective osteoanabolic drug in patients with GIOP.
Keywords:
bone mineral density (BMD) – glucocorticoid-induced osteoporosis – TBS – teriparatide
Introduction
The prevalence of oral glucocorticoid (GC) use is 0.9% of the total adult population rising to 2.5% at age over 70 years [1]. GC therapy results in a rapid bone loss within the first weeks of treatment leading to osteoporotic fractures in 3 to 6 months from the beginning of GC treatment [2].
Histomorphometric analysis of biopsies from GC-treated individuals have demonstrated a reduction in bone formation at the cellular and tissue level, resulting in reduced bone volume and trabecular thickness. Higher doses and the long-term use of GC, however, also may be associated with an increase in bone resorption, leading to greater bone loss and disruption of cancellous bone architecture [3–7]. GC therapy is associated with reduced intestinal and renal calcium absorption and increased urinary calcium excretion, therefore increasing calcium intake seems to be a logical approach to slow decrease in bone mass and microarchitecture [8,9].
Teriparatide (TPTD) increases periosteal and endocortical bone formation – total bone area, cortical area and bone strength. TPTD treatment of postmenopausal women with osteoporosis significantly increases cancellous bone volume and connectivity, improves trabecular morphology with a shift toward a more plate-like structure, and increases cortical bone thickness, leading to reducing in vertebral and non-vertebral fracture risk [2,10–17].
In last years, a trabecular bone score (TBS), indirect non-invasive bone parameter to describe degradation of trabecular microstructure is widely used [18] And high TBS value indicates better whereas lower TBS indicates worse trabecular bone structure and meaning greater risk of osteoporosis fractures [19–22]. There are several studies proving positive effect of TPTD on TBS in comparison or following to bisphosphonate treatment in patients with GC-induced osteoporosis (GIOP) [23–25].
This study was aimed to assess changes in bone mineral density (BMD) and TBS after 18-month period of treatment with TPTD in patients with GIOP concentrated into referral TPTD treatment centers in Slovakia.
Patients and methods
Patients
From January 2010 till January 2014, a prospective follow-up on effect and safety of TPTD treatment in GIOP patients in 5 referral centers specified at the treatment with TPTD, was performed.
The patients inclusion criteria were as follows:
- bone mineral density T-score < -2.9 or one or more osteoporotic fractures
- the use > 5 mg corticoids for more than 3 months
The regional medical ethical committees in each centre gave approval for the study
Treatment
All patients were treated with 20 μg of recombinant humane parathyroid hormone [1–34] – teriparatide (Forsteo®, Eli Lilly and Company, Netherlands BV), administered daily, subcutaneously. All patients enrolled in the study received daily supplements of 500 to 1 000 mg of Calcium and 400 to 800 IU of Vitamin D.
Outcome measures
The primary outcomes were defined as change in bone mineral density (BMD) at femoral neck (FN), total hip (TH) and lumbar spine (LS) and bone turnover markers after 12 and 18 months of TPTD treatment. Secondary outcomes included prevalence of clinical fractures, changes in TBS, tolerability and safety of the treatment. It was the prospective, open label, non-randomized, 18-months study in five centers in Slovakia (January 2010 – January 2014).
Methods
In all patients, LS BMD, FN and TH region was measured at the baseline, month 12 and 18 using dual-energy X-ray absorptiometry (Hologic Discovery). In all five centers Hologic densitometer (Discovery) with identical software and normative database was used.
All patients were assessed for serum levels of procolagen type 1, aminoterminal propeptide (P1NP), β-crosslaps (CTx) and osteocalcin (OC) at month 0, 6, 12 and 18. the same method (ECLIA) and Ca in routine manner by local laboratories.
TBS derived from lumbar spine DXA was assessed by the iNsight® v. 1.0 (Medimaps, France) in the subset of patients.
Statistics
Standard descriptive parametric and nonparametric statistics were used for the description of the data. Continuous variables were described using mean and standard deviation or median supplemented by 5th and 95th percentile; categorical variables were described using absolute and relative frequencies of categories (percentage). Statistical significance of time related changes in pair-wise comparisons was analyzed using pair-wise t-test or Wilcoxon paired test for detailed comparisons of two time points. The results were considered statistically significant at the level of alpha < 0.05 in all applied analyses. Analyses were performed using IBM SPSS 22.0.0 (IBM Corporation, 2013).
Results
From total of 263 GIOP patients on TPTD treatment a number of 129 patients (20 men/109 women), who properly terminated TPTD treatment after 18 months, were included in the study. Figure 1 and Figure 2 shows doses of GC used by patient and number of all fractures at the beginning of the follow-up. Baseline characteristics are in Table 1.
Tab. 1. Baseline characteristics of the study sample 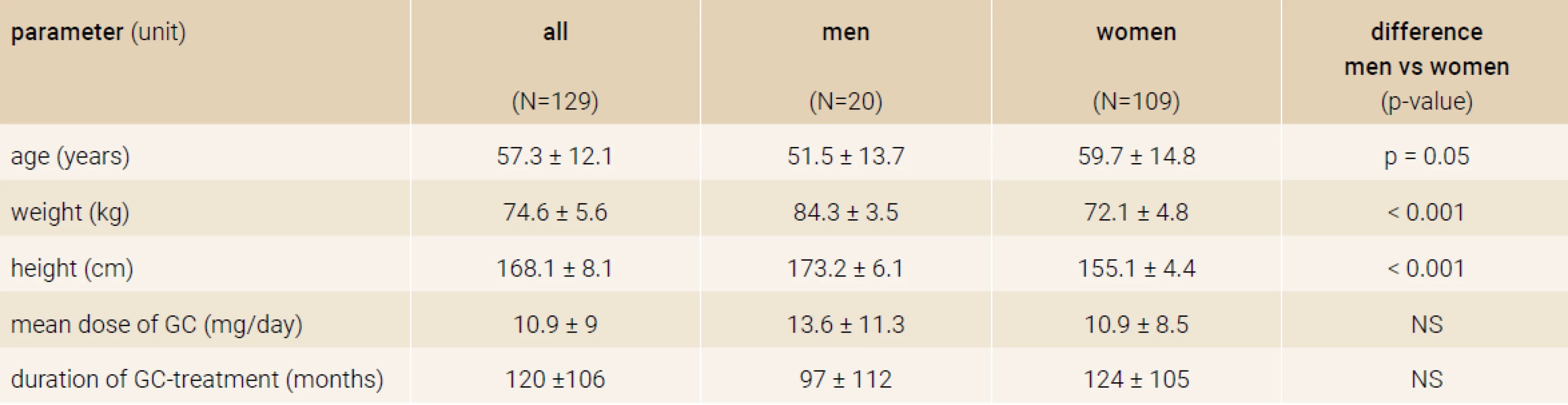
*values are expressed as mean ±SD (Standard Deviation) Fig. 1. Doses of glucocorticoids in the study group at the beginning of the follow-up 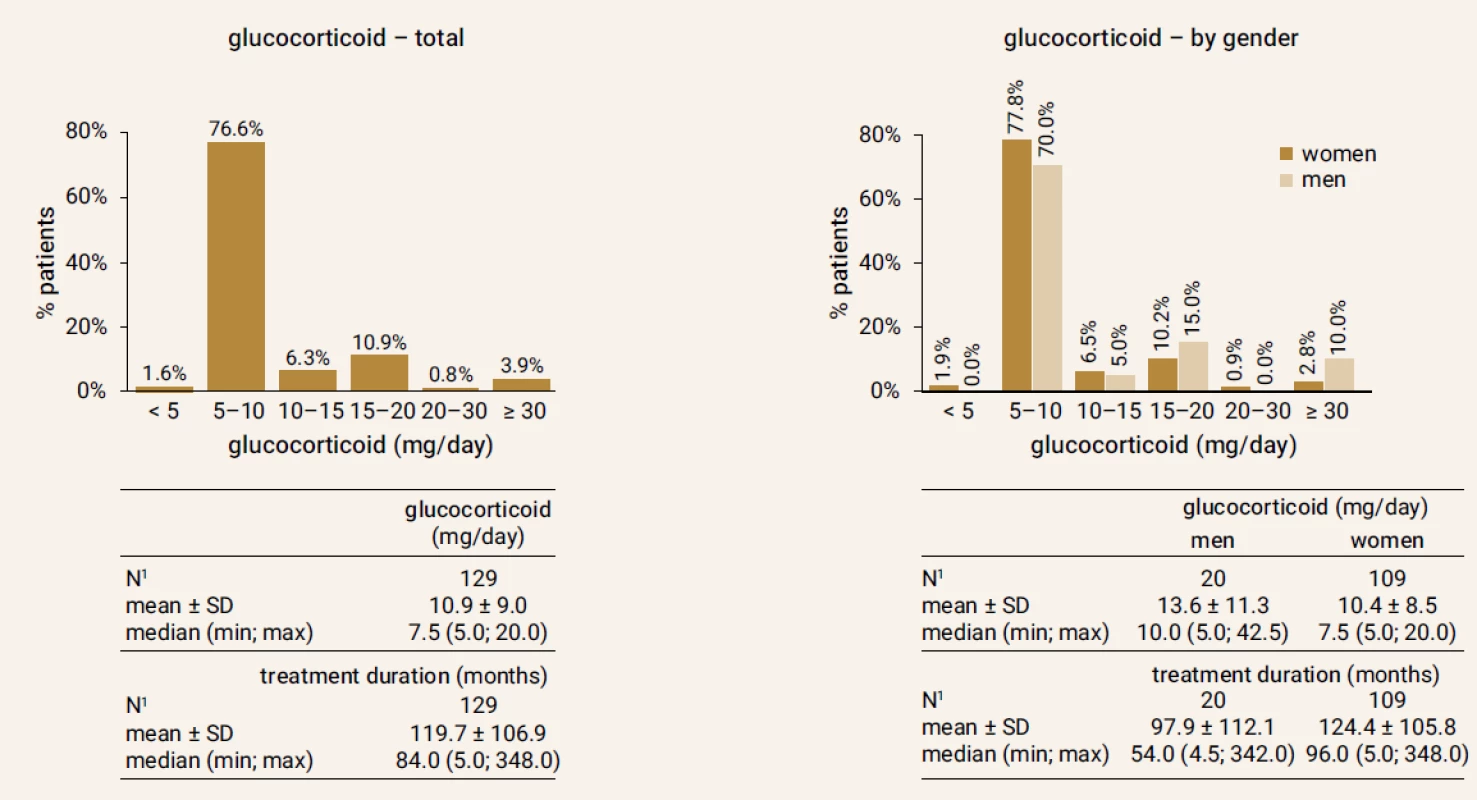
1Patients with complete information SD – Standard Deviation Fig. 2. Precentage of patients with fractures (vertebral and non-vertebral) before start of the treatment in study population 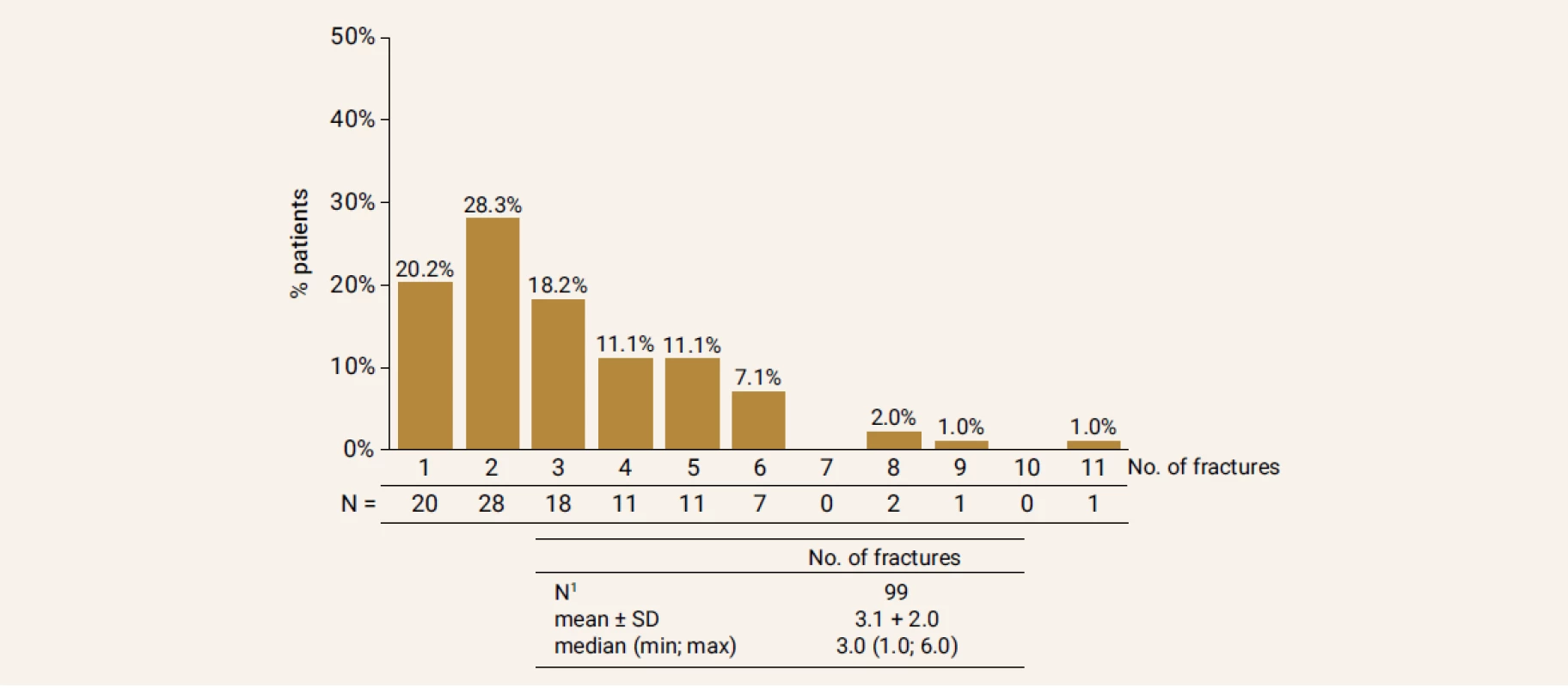
1Patients with complete information SD – Standard Deviation Change in bone mineral density
After TPTD treatment, an increase of lumbar spine (LS) BMD at month 12 (+8.3%, p < 0.001) and total hip (+3.85%, p < 0.001), femoral neck (+3.26 %, p < 0.001) and LS BMD (+9.28%, p < 0.001) at month 18 was observed (Figure 3).
Fig. 3. Changes in BMD over time periods. Treatment with TPTD led to an increase in BMD of total hip, femoral neck and LS assessed by means of BMD at month 12 and 18 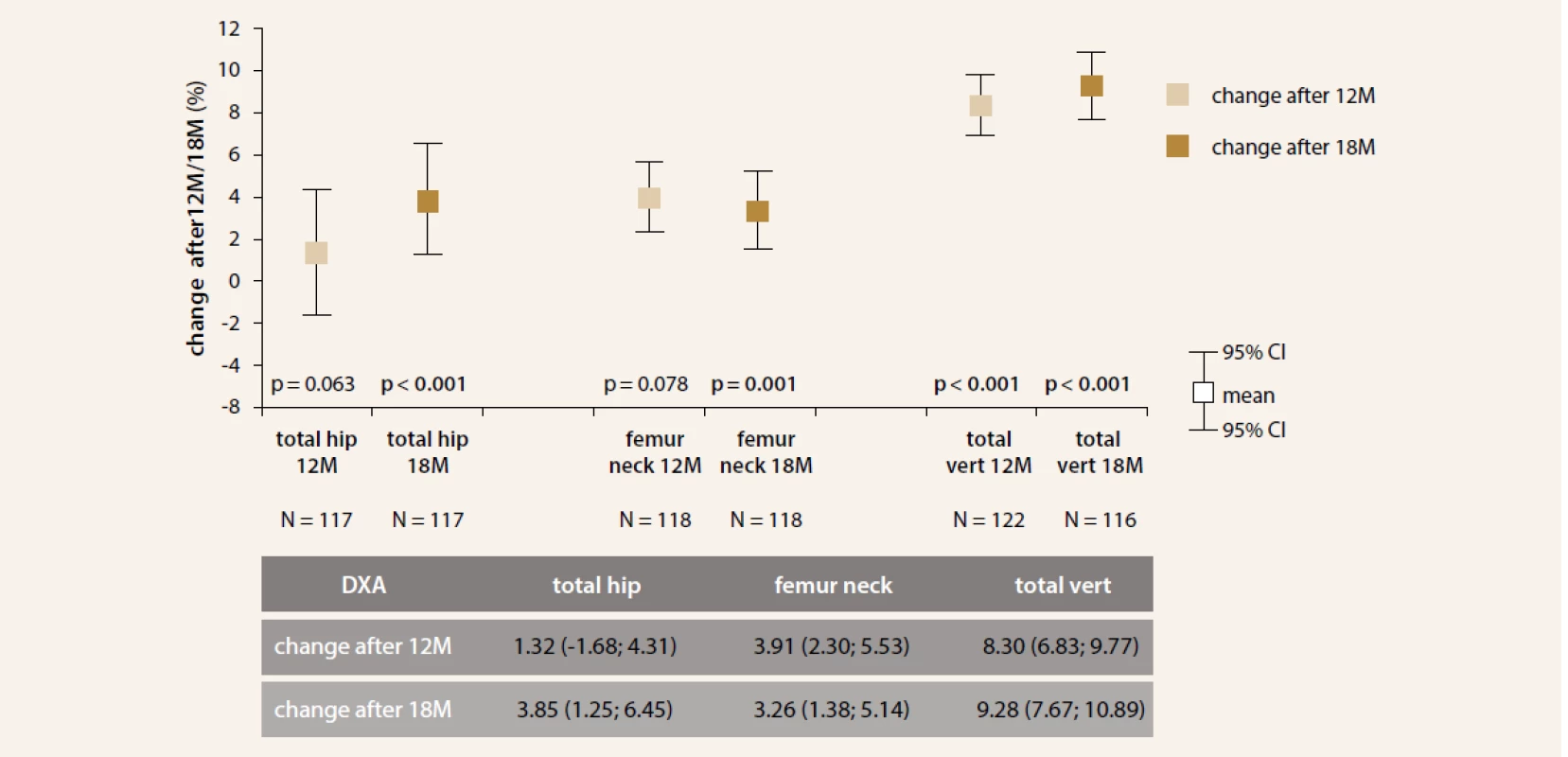
Change in bone turnover markers
During study period, P1NP increase of 233%; 304.5% and 176.3% at month 6; 12 and 18 (all p < 0.001), respectively, see Figure 4 for further details.
Fig. 4. Changes in bone turnover markers after TPTD treatment. Treatment led to significant increase in all three evaluated bone turnover markers 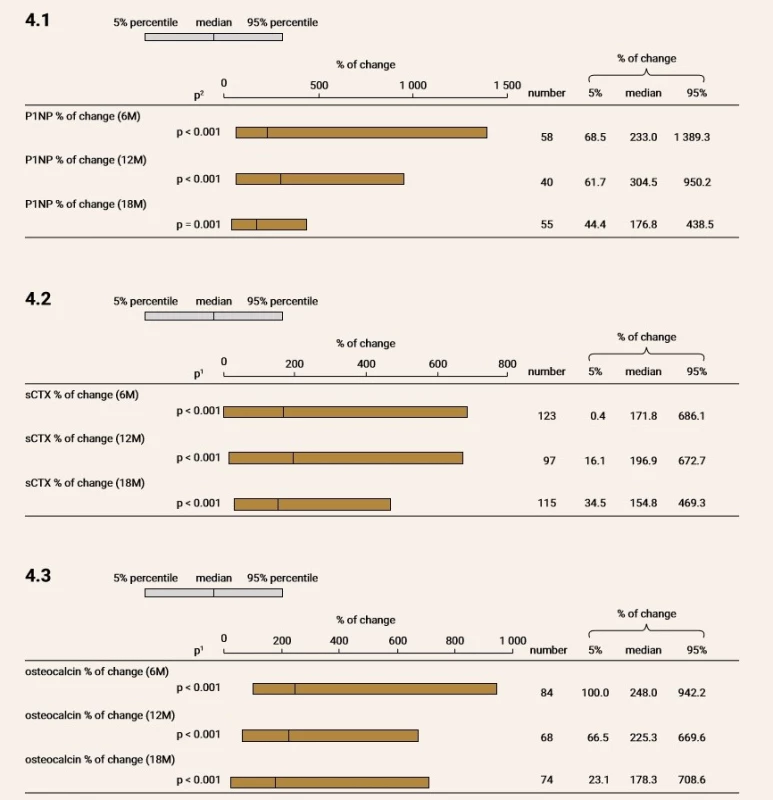
M – month P1NP – procollagen type 1 amino-terminal propeptide sCTX – serum C-terminal telopeptide CTx increased of 171.8% (p < 0.001); 196.9% (p < 0.001) and 154.8% (p < 0.001) at month 6, 12 and 18, respectively. Similarly, OC increased of 248% (p < 0.001); 225.3% (p < 0.001) and 178.3% (p < 0.001) at month 6, 12 and 18, respectively.
Serum calcium levels above reference range (2.7 mmol/l) were present in 3,2%; 8.6%; 5.8% and 3.4% of patients at the baseline, month 6, 12 and 18, respectively (Figure 5).
Fig. 5. Calcium levels at the baseline and after 6,12 and 18 months of treatment 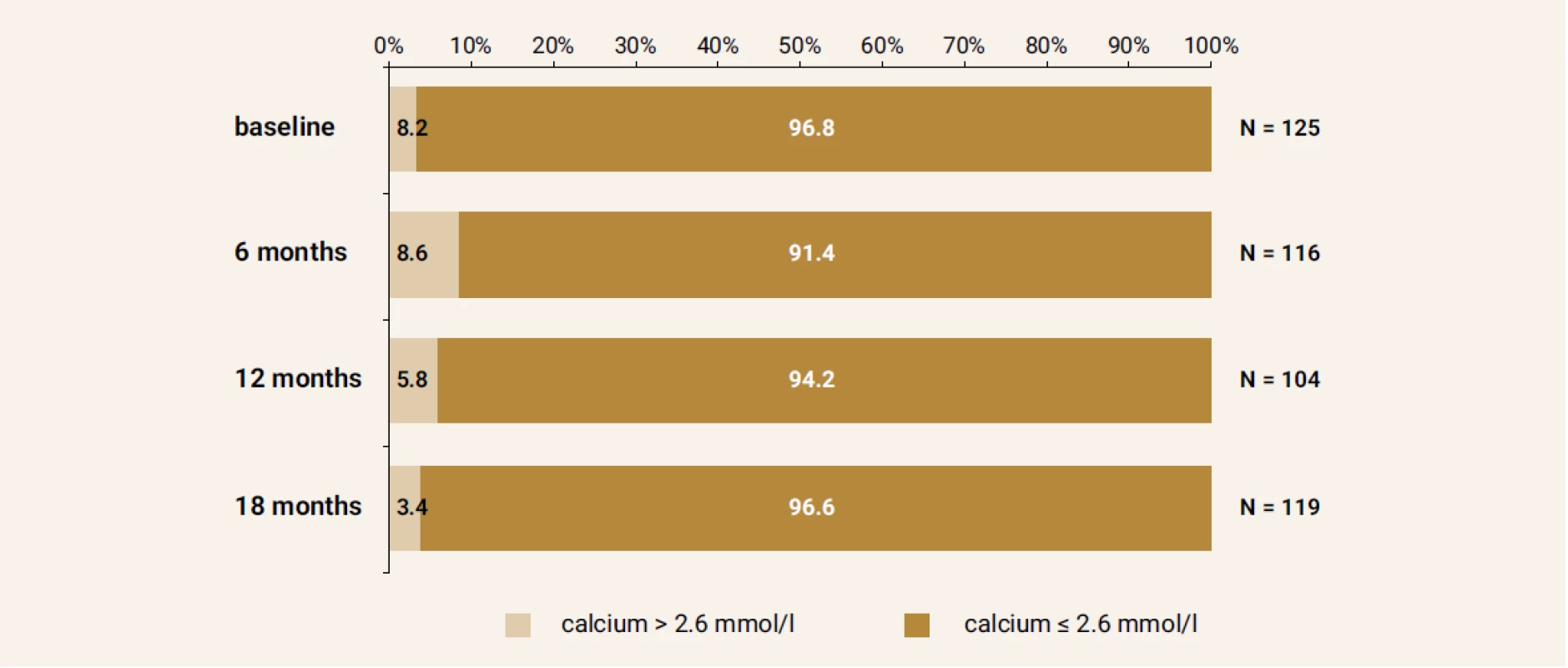
Effect on fractures
After 18 months of treatment a decrease in number of new onset fractures (2 high trauma, 1 low trauma wrist fracture) was observed compared to the baseline (6 high trauma, 0 low trauma fractures), Table 2.
Tab. 2. The number of new onset fractures in the study group in each time period 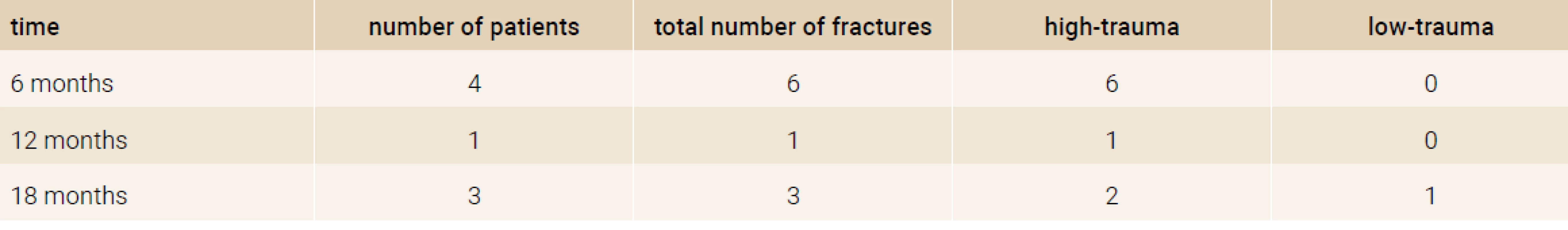
Treatment with TPTD was well tolerated and no serious side effects were observed.
Effect on trabecular bone score
TBS was evaluated in the subset of 12 patients (7 women, 5 males). An increase 2,4% (p = 0,01) of TBS was observed at month 12 of TPTD treatment (Figure 6).
Fig. 6. Change in TBS values after treatment with TPTD. Significant increase was observed at month 12 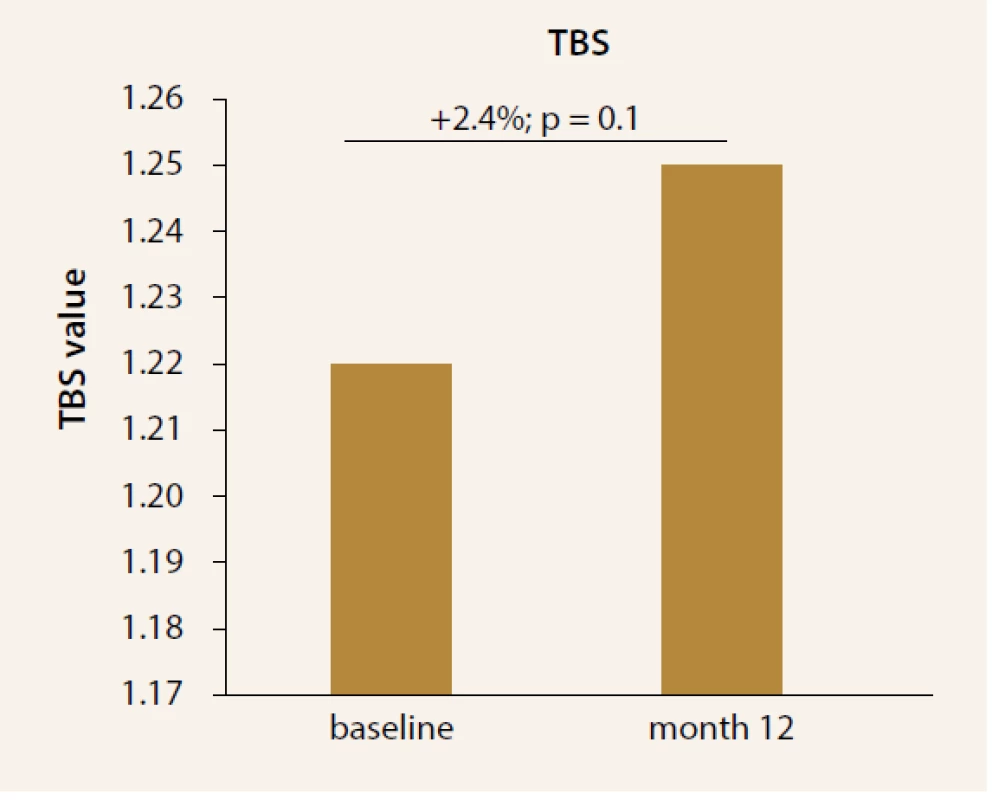
Discussion
The beneficial effects of daily injection of TPTD have been shown in several randomized, placebo-controlled trials with glucocorticoid-induced osteoporosis [2,15–17].
In this study, an effect of osteoanabolic treatment with TPTD on BMD, bone turnover markers and TBS was evaluated. Daily TPTD administration for the period of 18 month had positive effect on BMD and bone formation as documented by increase of bone turnover markers. In addition, in the small subset, 12 month treatment resulted in increase of TBS. In past years, there were several studies concerning the effect of TPTD on BMD and the risk of osteoporotic fractures. In the randomized, double-blind clinical trial of 36 months effect of TPTD in comparison to daily alendronate administration in GIOP patients showed significant skeletal benefits (documented by increase in BMD) in patients with GIOP, compared to alendronate [26–28]. Although, our analysis was limited because of absence of control group, the effect of TPTD on BMD was obvious, preferably after 18 months of treatment. The effect of TPTD was supported by increase of bone turnover markers followed with slight decrease at month 18. In other trials comparing a bisphosphonate with TPTD in severe postmenopausal osteoporosis, TPTD therapy was associated with increased areal and volumetric BMD and bone strength compared to alendronate [28,29]. This response may reflect the characteristic ability of glucocorticoids to inhibit osteoblast and osteocyte function profoundly by several mechanisms, including the stimulation of apoptosis [30]. Interestingly, Reid et al. in two of the previous studies with TPTD documented a reduction in non-vertebral fractures in postmenopausal women with osteoporosis [31,32]. Another study documented in a group of postmenopausal women with prevalent vertebral fracture a significant decrease in risk of vertebral and non-vertebral fractures after 18-month administration. The treatment was associated with significant improvement in quality of life [33].
Our study, however not aimed to evaluate incidence of fractures, supports the finding with a decreasing number of newly developed high trauma fractures (despite the 1 new low trauma fracture). The standard of care for patients at risk for glucocorticoid-associated bone loss and osteoporosis includes a choice of antiresorptive agents. However, for patients with established osteoporosis who are at high risk for fracture, more effective therapy may be warranted [30,34–39]. In 18-month trial in men with glucocorticoid-induced osteoporosis, TPTD showed also larger improvements in spinal BMD and bone strength and microstructure than risendronate [40]. This is also supported by the effect of TPTD on TBS in our study.
Conclusion
According to the previous studies TPTD as the anabolic agent is appropriate therapeutic strategy for patients at high risk for fracture, such as patients on GC treatment. In this study, treatment with TPTD resulted in BMD increase followed by significant changes in bone turnover markers. The effect of TPTD on bone structure was additionally documented by its positive effect on TBS, Hovewer, larger studies to evaluate TBS are needed. According to these findings, TPTD is highly effective osteoanabolic drug in patients with GIOP.
Acknowledgments
The authors confirm that all the research meets the ethical guidelines, including adherence to the legal requirements of Slovakia. We assert that there are no conflicts of interest (both personal and institutional) regarding specific financial interests that are relevant to the work conducted or reported in this manuscript.
doc. MUDr. Martin Kužma, PhD.
Received | Doručené do redakcie | Doručeno do redakce 26. 10. 2018
Accepted | Prijaté po recenzii | Přijato po recenzi 28. 11. 2018
Zdroje
- Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10): 777–787. Dostupné z DOI: <http://dx.doi.org/10.1007/s001980200108>.
- Payer J, Brazdilova K, Jackuliak P. Management of glucocorticoid-induced osteoporosis: prevalence, and emerging treatment options. Drug Healthc Patient Saf 2010; 2 : 49–59.
- Dale Carbonare L, Arlot ME, Chavassieux PM et al. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J Bone Miner Res 2001; 16(1): 97–103. Dostupné z DOI:<http://dx.doi.org/10.1359/jbmr.2001.16.1.97>.
- Van Staa TP, Leufkens HGM, Abenhaim L et al. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology 2000; 39(12): 1383–1389.
- Van Staa T, Leufkens HGM, Abenhaim L et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000; 15(6): 993–1000. Dostupné z DOI:<http://dx.doi.org/10.1359/jbmr.2000.15.6.993>.
- De Gregório LH, Lacativa PG, Melazzi AC et al. Glucocorticoid-induced osteoporosis. Arq Bras Endocrinol Metabol 2006; 50(4): 793–801.
- Van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2006; 79(3): 129–137. Dostupné z DOI:<http://dx.doi.org/10.1007/s00223–006–0019–1>.
- Migliaccio S, Brama M, Malavolta N. Management of glucocorticoids-induced osteoporosis: role of teriparatide. Ther Clin Risk Manag 2009; 5(2): 305–310.
- Lekamwasam S, Adachi JD, Agnusdei D et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 2012; 23(9): 2257–2276. Dostupné z DOI: <http://dx.doi.org/10.1007/s00198–012–1958–1>.
- Jiang Y, Zhao JJ, Mitlak BH et al. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 2003; 18(11): 1932–1941. Dostupné z DOI: <http://dx.doi.org/10.1359/jbmr.2003.18.11.1932>.
- Neer RM, Arnaud CD, Zanchetta JR et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344(19): 1434–1441. Dostupné z DOI: <http://dx.doi.org/10.1056/NEJM200105103441904>.
- Rizzoli R, Adachi JD, Cooper C et al. Management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2012; 91(4): 225–243. <http://dx.doi.org/10.1007/s00223–012–9630–5>.
- Uihlein AV, Leder BZ. Anabolic therapies for osteoporosis. Endocrinol Metab Clin North Am 2012; 41(3): 507–525. Dostupné z DOI: <http://dx.doi.org/10.1016/j.ecl.2012.05.002>.
- Vanuga P, Tomkova S, Masaryk P et al. Slovak register of patient with severe osteoporosis on osteoanabolic treatment. Osteoporos Int 2012; 23(Suppl 2): S239-S240.
- Bultink IE, Baden M, Lems WF. Glucocorticoid-induced osteoporosis: an update on current pharmacotherapy and future directions. Expert Open Pharmacother 2013;14(2):185–97. Dostupné z DOI: <http://dx.doi.org/10.1517/14656566.2013.761975>.
- Amiche MA, Albaum JM, Tadrous M et al. Efficacy of osteoporosis pharmacotherapies in preventing fracture among oral glucocorticoid users: a network meta-analysis. Osteoporos Int 2016; 27(6): 1989–1998. Dostupné z DOI: <http://dx.doi.org/10.1007/s00198–015–3476–4>.
- Blick SK, Dhillon S, Keam SJ. Teriparatide: a review of its use in osteoporosis. Drugs 2008; 68(18): 2709–2737. Dostupné z DOI: <http://dx.doi.org/10.2165/0003495–200868180–00012>.
- Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 2008; 42(4): 775–787. Dostupné z DOI: <http://dx.doi.org/10.1016/j.bone.2007.11.018>.
- Hans D, Goertzen AL, Krieg MA et al. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 2011; 26(11): 2762–2769. Dostupné z DOI: <http://dx.doi.org/10.1002/jbmr.499>.
- Briot K, Paternotte S, Kolta S et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone 2013; 57(1): 232–236. Dostupné z DOI: <http://dx.doi.org/10.1016/j.bone.2013.07.040>.
- Boutroy S, Hans D, Sornay-Rendu E et al. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 2013; 24(1): 77–85. Dostupné z DOI: <http://dx.doi.org/10.1007/s00198–012–2188–2>.
- Iki M, Tamaki J, Kadowaki E et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res 2014; 29(2): 399–407. Dostupné z DOI: <http://dx.doi.org/10.1002/jbmr.2048>.
- Senn C, Gunther B, Popp AW et al. Comparative effects of teriparatide and ibandronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: a 2-year open-label study. Osteoporos Int 2014; 25(7): 1945–1951. Dostupné z DOI: <http://dx.doi.org/10.1007/s00198–014–2703–8>.
- Saag KG, Agnusdei D, Hans D et al. Trabecular Bone Score in Patients With. Chronic Glucocorticoid Therapy-Induced Osteoporosis Treated With Alendronate or Teriparatide. Arthritis Rheumatol 2016; 68(9): 2122–2128. Dostupné z DOI: <http://dx.doi.org/10.1002/art.39726>.
- Miyaoka D, Imanishi Y, Ohara M et al. Effects of Teriparatide and Sequential Minodronate on Lumbar Spine. Bone Mineral Density and Microarchitecture in Osteoporosis. Calcif Tissue Int 2017; 101(4): 396–403. Dostupné z DOI: <http://dx.doi.org/10.1007/s00223–017–0295-y>.
- Saag KG, Zanchetta JR, Devogelaer JP et al. Effects of teriparatide versus alendronate for treating of glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 2009; 60(11): 3346–3355. Dostupné z DOI: <http://dx.doi.org/10.1002/art.24879.
- Saag KG, Shane E, Boonen S et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 2007; 357(20): 2028–2039. Dostupné z DOI: <http://dx.doi.org/10.1056/NEJMoa071408>.
- McClung MR, San Martin J, Miller PD et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 2005; 165(15): 1762–1768. Dostupné z DOI: <http://dx.doi.org/10.1001/archinte.165.15.1762>.
- Keaveny TM, Donley DW, Hoffmann PF et al. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res 2007; 22(1): 149–157. Dostupné z DOI: <http://dx.doi.org/10.1359/jbmr.061011>.
- O´Brien CA, Jia D, Plotkin LI et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strenght. Endocrinology 2004; 145(4): 1835–1841. <http://dx.doi.org/10.1210/en.2003–0990>.
- Reid DM, Hughes RA, Laan RF et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. J Bone Miner Res 2000; 15(6): 1006–1013. Dostupné z DOI: <http://dx.doi.org/10.1359/jbmr.2000.15.6.1006>.
- Reid DM, Adami S, Devogelaer JP et al. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int 2001; 69(4): 242–247.
- Neer RM, Claude DA, Jose RZ et al. Effect of Parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344(19): 1434–1441. Dostupné z DOI: <http://dx.doi.org/10.1056/NEJM200105103441904>.
- Adachi JD, Saag KG, Delmas PD et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized double-blind, placebo-controlled extension trial. Arthritis Rheum 2001; 44(1): 202–211. Dostupné z DOI: <http://dx.doi.org/10.1002/1529–0131(200101)44 : 1<202::AID-ANR27>3.0.CO;2-W>.
- Sambrook PN, Kotowicz M, Nash P et al. Prevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. J Bone Miner Res 2003; 18(5): 919–924. Dostupné z DOI: <http://dx.doi.org/10.1359/jbmr.2003.18.5.919>.
- Wallach S, Cohen S, Reid DM et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 2000; 67(4): 277–285.
- Neer RM, Arnaud CD, Zanchetta JR et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344(9): 1434–1441. Dostupné z DOI: <http://dx.doi.org/10.1056/NEJM200105103441904>.
- Murphy DR, Smolen LJ, Klein TM et al. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord 2012; 13 : 213. Dostupné z DOI: <http://dx.doi.org/10.1186/1471–2474–13–213>
- Bultink IEM, Baden M, Lems WF. Glucocorticoid-induced osteoporosis: An update on current pharmacotherapy and future directions. Expert Opin Pharmacother 2013; 14(2): 185–197. Dostupné z DOI: <http://dx.doi.org/10.1517/14656566.2013.761975>.
- Gluer CC, Marin F, Ringe JD et al. Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Rheumatol 2012; 39(3): 600–609.
Štítky
Biochemie Dětská gynekologie Dětská radiologie Dětská revmatologie Endokrinologie Gynekologie a porodnictví Interní lékařství Ortopedie Praktické lékařství pro dospělé Radiodiagnostika Rehabilitační a fyzikální medicína Revmatologie Traumatologie Osteologie
Článek vyšel v časopiseClinical Osteology
Nejčtenější tento týden
2018 Číslo 4- Alergie na antibiotika u žen s infekcemi močových cest − poznatky z průřezové studie z USA
- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
-
Všechny články tohoto čísla
- Eighteen months of teriparatide treatment leads to improvement of bone mineral density and trabecular bone score in patients with glucocorticoids induced osteoporosis: the results from prospective follow-up (registry OSTEO.sk)
- Osteoporosis and fractures in multiple sclerosis: pathogenesis, risk factors, treatment options, and prevention
- Incidence of hip fractures in Slovakia in 2000–2016
- The role of vitamin D in the treatment of secondary osteoporosis in cancer patients
- Clinical perspective of RANK/RANKL/OPG system components in rheumatoid arthritis
- An interdisciplinary approach to a patient with acute lymphoblastic leukemia
- Clinical Osteology
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clinical perspective of RANK/RANKL/OPG system components in rheumatoid arthritis
- An interdisciplinary approach to a patient with acute lymphoblastic leukemia
- Incidence of hip fractures in Slovakia in 2000–2016
- Osteoporosis and fractures in multiple sclerosis: pathogenesis, risk factors, treatment options, and prevention
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



