-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Late Functional and Morphological Findings after Methylalcohol Poisoning
Authors: J. Lešták 1; P. Diblík 2; S. Zacharov 3; M. Fůs 1; M. Kynčl 1; Jaroslav Tintěra 1; J.. Heissigerová 2
Authors place of work: Oční klinika JL Fakulty biomedicínského inženýrství ČVUT v Praze 1; Oční klinika Lékařské fakulty Univerzity Karlovy a Všeobecné Fakultní nemocnice v Praze 2; Toxikologické informační středisko, Klinika pracovního lékařství, 1. LF UK a VFN v Praze 3
Published in the journal: Čes. a slov. Oftal., 76, 2020, No. 6, p. 278-285
Category: Kazuistika
doi: https://doi.org/10.31348/2020/39Summary
Aim: The aim of the study was to determine the morphological and functional findings in a patient after methanol poisoning.
Examination methods: The patient (male, 38 years old) was suffered methanol poisoning in eight years ago (2012). The following tests and examinations were performed: neurological visual field XR test (Medmont M700), retinal nerve fibre layer (RNFL), ganglion cell complex (GCC) and peripapillary vessel density (all using Avanti RTvue, Optovue), pattern electroretinography (PERG) and pattern visual evoked potential (PVEP) examination according to ISCEV methodology (Roland Consult Instrument) and brain MRI examination (Philips Achieva Dstream 3 T).
Results: The biggest changes were found in RNFL and VD. PERG also showed damage to retinal ganglion cell axons. In left eye we determined decrease in oscillations (in comparison with contralateral eye) at N35-P50 and P50-N95. VEPs in both eyes were significantly reduced, almost inconspicuous in the left eye. Extension of latency time of P100 was not identified. Functional MRI showed a bilateral decrease in voxel activity with a greater decrease in the left eye. There were postmalatical changes in the dorsal parts of the putamen on MRI. The width of the optic nerve and chiasm was physiological.
Conclusion: Asymmetric damaging of RNFL and cortical centres of the brain were determined. We registered large pathological changes in VD, which are probably responsible for the deepening of optic nerve excavation and further loss of nerve fibers of retinal ganglion cells, which have not yet been described in the literature. Following these results is possible to define direct damage of nerve structures and blood vessels by toxins of methanol metabolism in the acute stage and upcoming reparation processes in following periods.
Keywords:
methanol poisoning – visual field – OCT – vessel density – electrophysiological examinations – magnetic resonance
INTRODUCTION
Methylalcohol poisoning is a life-threatening condition, which in those who survive causes toxic neuropathy of the optic nerve, with potential long-term consequences for sight. Naturally, in a country where the consumption of alcoholic beverages is not prohibited, this is not a rare occurrence, and as a result we find studies on this theme also in our periodical. The first was conducted by Votočková, who describes stiff pupils in medium mydriasis, while the anterior segment was otherwise within the norm, in a 44 year old patient 24 hours after the consumption of methylalcohol. On the fundus there were worn edges of the papillae and barely perceptible blurred landscapes around the posterior pole. Vision was fingers to 3 m. As regards the other organs, the study in question describes minor haemorrhages also in the meninges and the brain, which was oedematous. It also cites the study by Pick and Bielschowsky, who in 1912 presented the first descriptions of changes in the layer of the retinal ganglion cells. As against these changes, lesions of the optic nerve are only imperceptible, the tracts and further pathway are not damaged [1].
A second study was conducted by Cigánek et al., who described visual functions in 18 soldiers who had survived methylalcohol poisoning. Nine of them suffered changes in the visual fields, even despite values of 1.0 visual acuity. Fluorescence angiography was performed on these patients. In the arteriovenous phase, infiltration of the contrast substance was evident from the edges of the optic nerve papilla and in the surrounding area, which persisted into the venous phase. The blood vessels were slightly raised at the crossing of the edge of the papilla. Electroretinographic findings on a flash stimulus demonstrated a decrease of wave a in 100 % of cases and wave b in 33 %. In visually evoked potentials (VEP) to a reverse stimulus, change of latency was in only 22 %. In the pathogenesis of changes, the authors consider potential circulatory malfunction [2].
A further study was also conducted by Czech authors, but its results were published in a foreign periodical. It describes 42 patients observed from 5 to 50 months after methylalcohol poisoning. Changes in the retinal nerve fibre layer (RNFL) were observed in 33 % of cases, and their progression was recorded in 24 %. Changes of latency of P1 oscillation of VEP was recorded in 18/42 right eyes and 21/42 left eyes. Abnormal amplitude of N1-P1 was observed in 10/42. A significant association was present between chronic neurodegeneration of the retina, progressive loss of visual functions and necrotic lesions in the brain. An improvement of conductivity of the optic nerve was observed in more than 80 % of patients, but the amplitude of the evoked potential had a tendency to a further decrease over the course of 4 years of observation [3].
CASE REPORT AND EXAMINATION METHODS
In May 2020 a 38 year old man was examined, who had been treated in September 2012 for acute methylalcoholy poisoning following the consumption of approximately 250 ml of adulterated rum. The consumption of the beverage took place over a period of 2-3 hours, the alcohol was consumed without food, in addition to it the patient drank only cola. After 16 hours the first symptoms of poisoning appeared (fluctuating malfunction of vision, the patient stated that “everything was white, I could barely distinguish colours”). The first contact with a doctor took place 36 hours after the consumption of the alcohol. The patient was examined at internal medicine and neurology departments; in addition to malfunction of vision he stated pain throughout his whole body, abdominal cramps, headache and breathlessness. At the examination he was agitated and restless, as a result diazepam was administered. CT of the brain was performed, with a negative finding. The results of the toxicological examination of blood serum demonstrated severe methylalcohol poisoning: the concentration of methylalcohol in serum was 2779 mg/l (toxic concentration 200 mg/l), concentration of formic acid 898 mg/l, ethanol in serum undetectable. The patient was transferred to the intensive care department, during transit he received 80 ml of 40 % ethanol solution applied via a nasogastric probe. Due to deepening malfunction of consciousness, orotracheal intubation was performed, and artificial lung ventilation was commenced, the patient was administered midazolam 10 mg and succinylcholine 100 mg intravenously. Upon admission to the department the patient was in a coma (Glasgow Coma Scale 3 points), haemodynamically stable (MAP over 80 mmHg without noradrenaline). The baseline laboratory values corresponded to a condition of severe metabolic acidosis (pH of arterial blood 6.71, bicarbonate 4.3 mM/l, base deficit 38 mM/l, anion gap 55 mM/l, serum lactate 11.4 mM/l), stress hyperglycaemia (18.4 mM/l) and acute renal failure (creatinine in serum – 175 uM/l). The patient was administered bicarbonate for correction of acidemia (total 950 ml of 8.4 % NaHCO3 solution), leucovorin for substitution of folate, and the antidote fomepizole (ADH inhibitor) was applied in a dose of 1 g every 4 hours (total 5 g). One hour after admission to the department, the patient was attached to intermittent haemodialysis with maximum flow speed (flow speed of dialysate 30 l/h, flow speed of blood 250 ml/min), which persisted for 10 hours. After dialysis, the follow-up level of formic acid was negative, concentration of methylalcohol 233 mg/l, after normalisation of the internal environment further therapy continued with ethanol intravenously. During the course of hospitalisation at the intensive care department there was a development of aspiration pneumonia on the right side, as a result of which amoxycillin and metronidazole were applied intravenously. A bacteria culture test determined haemophilus influenzae on a massive scale, and medication was changed to ampicillin and sulbactam. A further complication during the course of treatment was the onset of delirium in reaction to the combination of neuroleptics. The total period of hospitalisation in the intensive care department was 6 days, after which the patient was transferred to the standard internal medicine department. During hospitalisation in the internal medicine department the patient stated a malfunction of vision of the character of a dark stain upon frontal gaze, black spots in the visual field, blurred vision in both eyes, more pronounced in the left eye, and stinging in both eyes. The conclusion of the ophthalmological consultation was as follows: subjective malfunctions of vision, more pronounced in left eye, distance vision of right eye within the norm, in left eye reduced by 1 row, near vision without correction within the norm bilaterally. The perimeter could not be evaluated due to the low validity of the examination. The finding on the retina was within a physiological framework bilaterally. After 7 days of hospitalisation in the internal medicine department the patient was discharged for home treatment.
In his anamnesis the patient stated past abuse of marijuana and methamphetamine, smoking and alcohol abuse (approximately 1 litre of rum per week). Of secondary illnesses the patient had arterial hypertension being treated by beta-blockers, mixed hyperlipidaemia (cholesterol concentration 6.1 mM/l, lipids 4.8 mM/l), hyperuricaemia (concentration of uric acid 474 uM/l), chronic hepatopathy evidently of alcoholic etiology (GGT 2.94 µkat/l). The patient continued to be observed and examined at regular intervals during the years 2013-2018.
Results of a subsequent examination 10 months after poisoning: V: 1.0 naturally, Jaeger no. 1 naturally. Parallel, motility free, isocoria, optic media clear, temporally pale and bordered on papilla of fundus. Retina without pathological finding. Global RNFL had decreased since the first examination bilaterally by 3 µm. After stimulation of the left eye an examination of PVEP recorded slight extension of P100 latency to 118 ms. In the right eye the finding was within the norm.
On magnetic resonance of the brain the patient had symmetrical deposits in the putamen of increased signal in T2W, reduced in T1W without changes after application of KL, without signs of haemorrhage.
Visual acuity was 1.0 naturally 4 years after poisoning. On the fundus in the right eye the optic nerve papilla was temporally pale, in the left eye pale. The RNFL was unchanged from the previous examination. Progression was also not demonstrated by a perimetric examination. Visually evoked potentials had normalised. MR examination on a 3T instrument detected that necrosis in the region of the putamen was partially haemorrhagic.
The results 6 years after poisoning were as follows: V 1.0 naturally. On the ocular fundus the papillae were bilaterally pale with c/d = 0.5. We did not determine progression in the perimeter or RNFL. PVEP showed a normal finding in the right eye, borderline finding in the left eye.
A control MR examination of the brain confirmed symmetrical lesions of the putamen bilaterally, which corresponded to necrosis, with slight signs of haemorrhage. In comparison with the previous examination there was no evident progression of the extent of the finding.
During the course of the entire observation period the patient had normal serum concentration of vitamins B12 (234-264 ng/l), B1 (48-73 µg/l) and TSH (2.8-4.7 mlU/l).
In addition to an ocular examination, we also conducted an examination of colour sensitivity with the aid of Isihara pseudoisochromatic plates (Kanehara and co., Japan). We examined the visual field with a fast threshold program within the range of 0-30 degrees on the instrument Medmont M700 (Medmont International Pty Ltd., Australia). We examined the fibre layer (RNFL), Ganglion cell complex (GCC) and peripapillary vessel density (VD) on the instrument Avanti RT-Vue XR (Optovue, USA). Examination of pattern electroretinogram (PERG) and pattern visually evoked potentials (PVEP) was performed according to the ISCEV method on a Roland Consult instrument (Germany). The difference from the recommended ISCEV method was the distance of the eye from the stimulation surface (instead of the recommended 50 cm, in our case the distance was 30 cm).
Structural and functional MR was performed on the system Philips Achieva DStream 3 Tesla Version sw. 5.6.11. Examination by functional magnetic resonance (fMR) was conducted according to our method [4].
RESULTS OF EXAMINATIONS
The external ocular finding was normal, including pupil reactions. The anterior segment of both eyes and the optic media were within the norm. On the fundus, the optic nerve papillae were atrophic with sharp borders and excavation of 0.7 and 0.8 respectively. The central landscape was within the norm, without foveolar reflex. The finding in the vessels was normal, as was the remainder of the retina. Vision: 1.0 without correction. Jaeger no. 1 also without correction bilaterally. Intraocular pressure (IOP) 18.9 and 18.5 mmHg respectively (according to Ocular Response Analyser). Achromatopsia. Visual fields with pronounced defects in central part, mainly in left eye unchanged during the observation period (from 10/2012 to 5/2020). The last findings are shown by Fig. 1. OCTA demonstrated pronounced reduction of VD both in the overall image (39.5 % and 35.5 % respectively) and peripapillary (40.2 % and 34.4 % respectively). We recorded a peripapillary decrease also in the RNFL (59 µm and 51 µm respectively). See Fig. 2. A similar decrease of the RNFL and GCC in the macula is demonstrated also in Fig. 3.
Fig. 1. Perimeter within range of 0-30 degrees. Above right, below left field 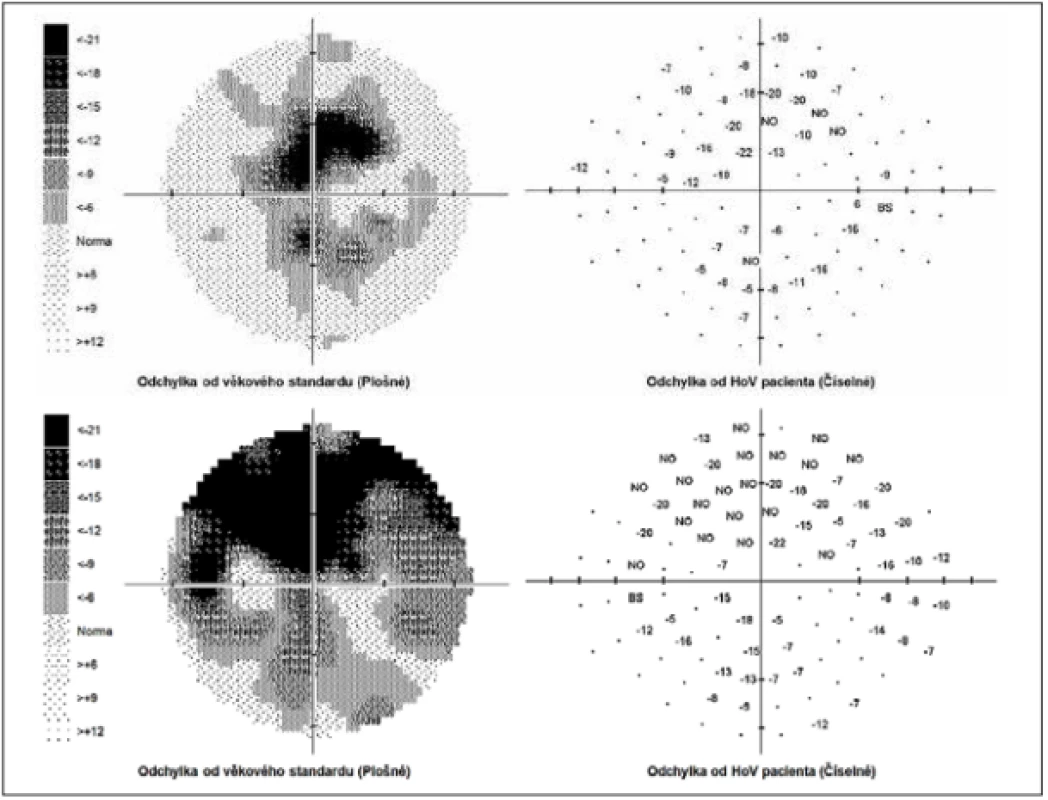
Fig. 2. Thickness of retinal nerve fibre layer (RNFL) and vessel density (VD). Above right eye, below left eye. 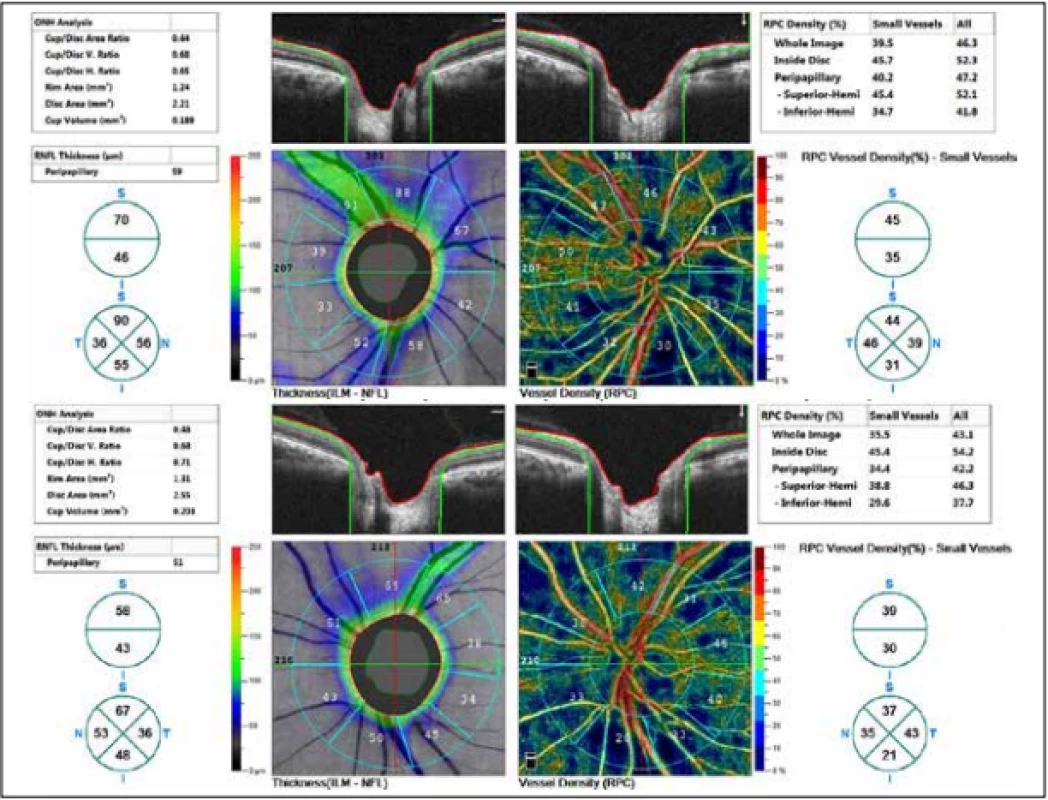
Fig. 3. Ganglion cell complex (GCC), retinal nerve fibre layer (RNFL) and optic nerve head (ONH). Above right eye, below left eye. 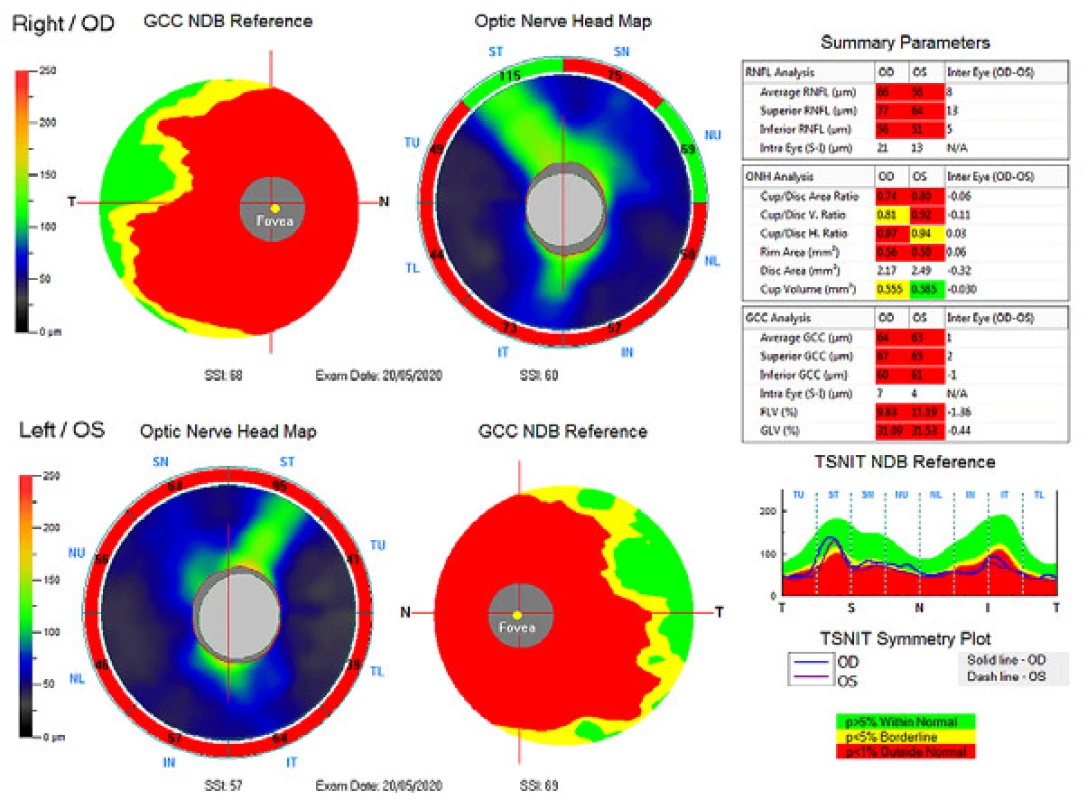
An electrophysiological examination – PERG demonstrated a normal response in the right eye, but an extension of N95 latency to 100.7 ms, in the left normal response but reduced by 40 % in comparison with the right eye. Similarly as in the right eye, in the left eye also there was an extension of N95 latency to 100.7 ms. PVEP amplitudes were bilaterally markedly reduced, in which there was a greater decrease on the left side. Latency of oscillation P100 was not extended.
Examination by magnetic resonance demonstrated post-malatic changes of the dorsal parts of the putamen bilaterally, with small peripheral gliosis as changes of the character of toxic leukoencephalopathy (within the framework of changes following methylalcohol poisoning). Without signs of intracranial haemorrhage, without deposits of hemosiedrin in brain tissue, without image of acute brain ischemia. Width of optic nerves and optic nerve sheaths laterolaterally 1 cm behind dorsal contour of eyeball, within limits of norm. Width of nucleus geniculatum laterale bilaterally with slight reduction of dimensions as against norm. Width of chiasma within norm, without manifest signal alteration, without expansion [5].
With the aid of functional MR after stimulation of the right eye we determined 465 voxels, left eye 388 (normal values after stimulation of right eye 7508±2018, left eye 7340±2775) [6].
DISCUSSION
Methylalcohol ranks among substances with high toxicity of intermediary metabolites. In humans and primates, methylalcohol is oxidised especially in the liver by the enzyme alcohol dehydrogenase (ADH) into formaldehyde, and further by aldehyde dehydrogenase (ALDH) into formic acid [7,8,9,10,11].
Formaldehyde does not accumulate in the blood, because its conversion into formic acid is very rapid, with a half-life of 1-2 minutes [12,13].
Formic acid inhibits cytochrome c oxidase in mitochondria (Ki~6 mmol/l), and thereby triggers cellular hypoxia accompanied by an accumulation of lactate and a decrease of ATP in cells [14,15,16,17,18].
Accumulation of formic acid and later also lactic acid leads to the development of severe metabolic acidosis [19,20,21].
There is a direct correlation between the concentration of formic acid in serum and fatality due to methylalcohol poisoning [22]. The most sensitive to the cytotoxic effect of formates are neurons of the retina, axons of the optic nerve and neurons of the basal ganglia of the brain [23].
Eells et al. determined that the concentration of formic acid in the ocular tissues of rats, especially in the retina and vitreous body, was 50 % higher than in the brain due to slower oxidation [24,25]. The onset of severe metabolic acidosis potentiates the effect of formic acid on the central nervous system (CNS), because it facilitates its passage through the blood-brain barrier, which leads to oedema of the brain and damage (necrosis, haemorrhage) in the region of the putamen, nucleus pallidus and subcortical white matter [26,27,28,29].
Malfunctions of vision are manifested in blurred vision, malfunction of colour vision or light flashes and feelings of dazzling, or conversely “dimming” and scotomas [30,31,32,33,34].
On the ocular fundus there is frequent presence of hyperaemia and oedema in the region of the optic nerve papilla [35,36,37].
Hyperaemia may also be the result of the vasodilation effect of methylalcohol, not only on the level of the retina, but also of the brain [38].
Retinal ganglion cells and their axons, which form the optic nerve, are very sensitive to histotoxic hypoxia caused by an inhibition of mitochondrial cytochrome c oxidase, because they have higher energy demands and a relatively low number of mitochondria [39,40,41].
Nevertheless, biochemical and morphological changes caused by the toxic effect of formic acid are present also in other types of cells: in photoreceptors, in Müller cells etc. [42,43,44].
Symptoms of affliction of sight in patients with acute methylalcohol poisoning are manifested with a certain latency of 8-48 hours. The latency period depends on the quantity of consumed methylalcohol, ratio of ethanol and methanol in the toxic beverage, and other factors [45,46,47].
In a range of cases, full recovery takes place over the course of a number of weeks, with normalisation of the finding on the ocular fundus and normalisation of visual functions [35,48,49].
However, long-term damage to sight may persist in 10-30 % of surviving individuals [37,46,50,51,52,53,54].
Although our patient has visual acuity within the norm, we recorded severe changes in the perimeter, which were virtually unchanged from the first examination in 2012, as well as achromatopsia.
The long-term consequences of poisoning on the visual analyser include constriction of the visual field, scotomas, reduced visual acuity and contrast sensitivity, malfunctions of colour sensitivity to complete blindness.
In a retrospective study conducted by Galvez-Ruiz et al. [46,50], out of 50 patients examined after the subsidence of acute optic neuropathy, excavation of the optic nerve papilla was present in 22 patients. All the patients from the cohort had signs of atrophy of the optic nerve and defects of the visual field. The authors of the study came to the conclusion that excavation following acute methylalcohol poisoning may have a higher prevalence than previously assumed. Excavation of the papilla is a manifestation of the toxic effect of formic acid on axons and glial cells in the prelaminar, laminar and retrolaminar regions.
Eight years after the acute damage, our patient also had excavation of papillae of 0.7 and 0.8 respectively. Its deepening is not only the result of acute direct loss of axons of retinal ganglion cells [55], but also by the long-term effect of glutamate on VD. This is documented not only by the pronounced loss of VD in our patient, but also the experimental demonstration of the influence of glutamate on the retinal capillaries [56]. Damage to the myelin sheath of the optic nerve was described in a study by Sharpe in four patients who died of acute methylalcohol poisoning. Sharpe observed demyelination of the retrolaminar part of the optic nerve, without disruption of axons [41].
Upon acute toxic neuropathy, swelling of the myelin sheath leads to compression of the axons and disruption of conductivity or complete blockage [57].
Acute demyelination of the optic nerve caused by the direct toxic effect of formic acid may lead to axonal degeneration as a consequence of loss of trophic support from myelin, and disruption of the normal interaction of axons with myelin [41,57].
Damage to the myelin sheath of the axons of the optic nerve could have taken place only in the acute stage of poisoning. Extension of P100 latency of visual evoked potential was recorded at a follow-up examination on a patient 10 months after discharge from hospital. In the following years the finding was normalised. In our case report, at the examination in 2020 we did not record any signs of demyelination of white brain matter.
The influence of methylalcohol on retinal potentials was investigated in an animal model by Lee et al. and Garner et al., who determined a lower amplitude of wave b on ERG [58,59].
Similar changes in ERG were recorded by Cigánek et al. on 9 patients after consumption of methylalcohol, who had changes in the visual fields. They determined a reduction of amplitude of wave b in 30 % of cases. Wave a was reduced in 100 % of cases [2].
In our patient a PERG examination demonstrated a lower response of the photoreceptors, central retinal structures and ganglion cells in the left eye (in comparison with the right eye the decrease was by 40 %). In the right eye the finding was normal. However, there was an extended N95 latency of oscillation bilaterally (to 102 and 107 ms respectively), which demonstrates damage to the axons of the retinal ganglion cells.
Extended latency of VEP reflects the degree of demyelination of individual axons of the optic nerve (range of demyelinated regions) and the following shortening of latency provides information about the process of remyelination [60].
In our patient, VEP amplitudes in the right eye were reduced by 50 %, in the left eye the response was virtually absent. Extension of P100 latency was recorded only at the first examination after discharge, in the following years and at the time of the last examination we did not record this alteration.
Acute toxic neuropathy of the optic nerve may lead to axonal degeneration, both as a consequence of the direct effect of formic acid, and indirectly, as a consequence of a myelinoclastic effect [41,45].
We demonstrated damage to the RNFL both peripapillary (with the aid of OCTA) and on the papilla, and also in the macular region, which was verified by a pronounced drop in VEP amplitudes and extended latency of N95 PERG.
However, in the case of acute toxic neuropathy caused by formic acid, histotoxic hypoxia may trigger a range of biochemical and genetically conditioned mechanisms leading to neuronal apoptosis after the subsidence of acute poisoning [61].
With the aid of PERG, we demonstrated alteration of the retinal ganglion cells in our patient only in the left eye. We explain the pronounced reduction of voxel activity of the visual cortex mainly with reference to direct toxic damage caused by methylalcohol.
Bilateral necrosis of the basal ganglia, in particular putamen, and haemorrhagic lesions of subcortical white matter are typical findings on CT or MRI of the brain in patients who have survived severe methylalcohol poisoning [62,63,64,65,66,67].
Other, less common findings include necrotic changes and haemorrhagic lesions in the globus pallidus, nucleaus caudatus in the thalamus, cerebellum and brain stem [68,69,70,71,72,73].
Our patient also had post-malatic changes of the dorsal parts of the putamen bilaterally upon magnetic resonance examination.
Results of fMR in patients surviving methylalcohol poisoning are not available in the literature. Our patient manifested markedly lower voxel activity (depending on functional and morphological changes) in the visual paradigm. Damage to the ganglion cells and visual cortex was most probably caused by metabolites of methanol. The lower average of CGL indicates alteration also of this subcortical visual structure.
CONCLUSION
In this case report on a patient eight years after consumption of methylalcohol, with the aid of MR we did not determine any changes in the brain which are typical of a demyelinating disease. We also did not determine changes in the size (width) of the optic nerves or chiasma. CGL was also reduced. We recorded a pronounced decrease of voxel activity upon fMR examination depending on changes in the visual fields. An electroretinography examination (PERG) demonstrated a normal finding in both eyes. However, in the left eye the response was 60 % in comparison with the right eye. The response of the axons of the ganglion cells was altered bilaterally. VEPs did not demonstrate demyelination of the visual pathway, but showed severe damage thereto.
We registered substantial pathological changes in VD, which are most probably responsible also for deepening excavation and the further loss of nerve fibres of the retinal ganglion cells, which to date have not been described in the literature.
According to these findings, we may consider direct damage to the nerve structures and vessels by toxic products of the metabolism of methylalcohol in the acute stage. In the subsequent period we then observe their reparation processes [74].
The authors declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company. The study has not been submitted to any professional journal or printed elsewhere.
Zdroje
1. Votočková J. Novější terapie otrav methyalkoholem. [Newer therapies for methyl alcohol poisoning]. Cesk. Oftalmol. 1954;10 : 404-409. Czech.
2. Cigánek L, Vladyková J, Svěrák J, Peregrin J, Ernest J. Oční nálezy při hromadné otravě metylalkoholem. [Ocular findings in a mass poisoning with methyl alcohol]. Cesk Oftalmol. 1986;42 : 56-65. Czech.
3. Nurieva O, Diblik P, Kuthan P. et al. Progressive chronic retinal axonal loss following acute methanol-induced optic neuropathy: four-year prospective cohort study. Am J Ophthalmol. 2018;191 : 100-115.
4. Lešták J, Tintěra J. Funkční magnetická rezonance u vybraných očních onemocnění. [Functional Magnetic Resonance Imaging in Selected Eye Diseases]. Cesk Slov Oftalmol. 2015;71 : 127-133. Czech.
5. Lešták J, Kynčl M, Svatá Z, Rozsíval P. Lateral Geniculate Nucleus in Hypertensive and Normotensive Glaucoma. J Clin Exp Ophthalmol. 2013;4 : 269. doi:10.4172/2155-9570.1000269)
6. Lestak J, Tintera J, Rozsival P. Fmri and Ocular Dominance. IJSR – International journal of scientific research. 2014;3 : 293-296.
7. Eells JT, McMartin KE, Black K, Virayotha V, Tisdell RH, Tephly TR. Formaldehyde poisoning. Rapid metabolism to formic acid. JAMA. 1981a;246 : 1237-1238.
8. Eells JT, Makar AB, Noker PE, Tephly TR. Methanol poisoning and formate oxidation in nitrous oxide-treated rats. J Pharmacol Exp Ther. 1981b;217 : 57-61.
9. Jacobsen D, Jansen H, Wiik-Larsen E, Bredesen JE, Halvorsen S. Studies on Methanol Poisoning. Acta Med Scand. 1982;212 : 5-10.
10. McMartin KE, Makar AB, Martin G, Palese M, Tephly TR. Methanol poisoning. I. The role of formic acid in the development of metabolic acidosis in the monkey and the reversal by 4-methylpyrazole. Biochem Med. 1975;13 : 319-333.
11. McMartin KE, Martinamat G, Makar AB, Tephly TR. Methanol poisoning. Role of formate metabolism in monkey. J Pharmacol Exp Ther. 1977;201 : 564-572.
12. McMartin KE, Martin-Amat G, Noker PE, Tephly TR. Lack of a role for formaldehyde in methanol poisoning in the monkey. Biochem Pharmacol. 1979;28 : 645-649.
13. McMartin KE, Ambre JJ, Tephly TR. Methanol poisoning in human subjects. Role for formic acid accumulation in the metabolic acidosis. Am J Med. 1980;68 : 414-418.
14. Cook RJ, Champion KM, Giometti CS. Methanol toxicity and formate oxidation in NEUT2 mice. Arch Biochem Biophys. 2001;393 : 192-198.
15. Erecinska M, Wilson DF. Inhibitors of cytochrome c oxidase. Pharmacol Ther. 1980;8 : 1-20.
16. Seme MT, Summerfelt P, Neitz J, Eells JT, Henry MM. Differential recovery of retinal function after mitochondrial inhibition by methanol intoxication. Invest Ophthalmol Vis Sci. 2001;42 : 834-841.
17. Drangsholt E, Vangstad M, Zakharov S, Hovda KE, Jacobsen D. The hypothesis of circulus hypoxicus and its clinical relevance in patients with methanol poisoning - an observational study of 35 patients. Basic Clin Pharmacol Toxicol. 2018;123 : 749-755.
18. Tong TG. The alcohols. Crit Care Q. 1982;4 : 75-85.
19. Aabakken L, Johansen KS, Rydningen EB, Bredesen JE, Ovrebo S, Jacobsen D. Osmolal and anion gaps in patients admitted to an emergency medical department. Hum Exp Toxicol. 1994;13 : 131-134.
20. Smith SR, Smith SJ, Buckley BM. Combined formate and lactate acidosis in methanol poisoning. Lancet. 1981;2 : 1295-1296.
21. Smith I, Kumar P, Molloy S, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001;27 : 74-83.
22. Brent J, McMartin K, Phillips S, Aaron C, Kulig K. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344 : 424–429.
23. Sivilotti ML, Burns MJ, Aaron CK, McMartin KE, Brent J. Reversal of severe methanol-induced visual impairment: no evidence of retinal toxicity due to fomepizole. J Toxicol Clin Toxicol. 2001;39 : 627-631.
24. Eells JT, Salzman MM, Lewandowski MF, Murray TG. Formate-induced alterations in retinal function in methanol-intoxicated rats. Toxicol Appl Pharmacol. 1996;140 : 58-69.
25. Eells JT, Henry MM, Lewandowski MF, Seme MT, Murray TG. Development and characterization of a rodent model of methanol-induced retinal and optic nerve toxicity. Neurotoxicology. 2000;21 : 321-330.
26. Blanco M, Casado R, Vázquez F, Pumar JM. CT and MR imaging findings in methanol intoxication. Am J Neuroradiol. 2006;27 : 452-454.
27. Feany, MB, Anthony DC, Frosch MP, Zane W, De Girolami U. August 2000: two cases with necrosis and hemorrhage in the putamen and white matter. Brain Pathol. 2001;11 : 121-122.
28. Gaul HP, Wallace CJ, Auer RN, Fong TC. MR findings in methanol intoxication. Am J Neuroradiol. 1995;16 : 1783-1786.
29. Zakharov S, Kurcova I, Navratil T, Salek T, Pelclova D. Is the measurement of serum formate concentration useful in the diagnostics of acute methanol poisoning? A prospective study of 38 patients. Basic Clin Pharmacol Toxicol. 2015;116 : 445–451.
30. Hovda KE, Hunderi OH, Tafjord AB, Dunlop O, Rudberg N, Jacobsen D. Methanol outbreak in Norway 2002–2004: epidemiology, clinical features and prognostic signs. J Intern Med. 2005a;258 : 181-190.
31. Hovda KE, Urdal P, Jacobsen D. Increased serum formate in the diagnosis of methanol poisoning. J Anal Toxicol. 2005b;29 : 586-588.
32. Coulter CV, Isbister GK, Duffull SB. The pharmacokinetics of methanol in the presence of ethanol: a case study. Clin Pharmacokinet. 2011a;50 : 245-251.
33. Coulter CV, Farquhar SE, McSherry CM, Isbister GK, Duffull SB. Methanol and ethylene glycol acute poisonings - predictors of mortality. Clin Toxicol. 2011b;49 : 900-906.
34. Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol. 2015; 53 : 589-595.
35. Desai T, Sudhalkar A, Vyas U, Khamar B. Methanol poisoning: predictors of visual outcomes. JAMA Ophthalmol. 2013;131 : 358-364.
36. Hassanian-Moghaddam H, Pajoumand A, Dadgar SM, Shadnia Sh. Prognostic factors in methanol poisoning. Hum Exp Toxicol. 2007;26 : 583-586.
37. Sanaei-Zadeh H. Is high-dose intravenous steroid effective on preserving vision in acute methanol poisoning? Optom Vis Sci. 2012;89 : 244.
38. Hayasaka Y, Hayasaka S, Nagaki Y. Ocular Changes After Intravitreal Injection of Methanol, Formaldehyde, or Formate in Rabbits. Pharmacol Toxicol. 2001;89 : 74-78.
39. Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23 : 53-89.
40. Nicholls P. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochim Biophys Acta. 1976;430 : 13-29.
41. Sharpe JA, Hostovsky M, Bilbao JM, Rewcastle NB. Methanol optic neuropathy - a histopathological study. Neurology. 1982;32 : 1093-1100.
42. Garner CD, Lee EW, Louisferdinand RT. Muller cell involvement in methanol-induced retinal toxicity. Toxicol Appl Pharmacol. 1995;130 : 101-107.
43. Seme MT, Summerfelt P, Henry MM, Neitz J, Eells JT. Formate-induced inhibition of photoreceptor function in methanol intoxication. J Pharmacol Exp Ther. 1999;289 : 361-370.
44. Treichel JL, Henry MA, Skumatz CMB, Eells JT, Burke JM. Formate, the toxic metabolite of methanol, in cultured ocular cells. Neurotoxicology. 2003;24 : 825-834.
45. Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol, 2002; 40(4): 415-446.
46. Galvez-Ruiz A, Elkhamary SM, Asghar N, Bosley TM. Visual and neurologic sequelae of methanol poisoning in Saudi Arabia. Saudi Med J. 2015a;36 : 568-574.
47. Zakharov S, Pelclova D, Urban P, et al. Long-term visual damage after acute methanol poisonings: longitudinal cross-sectional study in 50 patients. Clinical Toxicology. 2015;53 : 884-892.
48. Sanaei-Zadeh H, Zamani N, Shadnia S. Outcomes of visual disturbances after methanol poisoning. Clin Toxicol. 2011;49 : 102-107.
49. Sharma R, Marasini S, Sharma AK, Shrestha JK, Nepal BP. Methanol poisoning: ocular and neurological manifestations. Optom Vis Sci. 2012;89 : 178-182.
50. Galvez-Ruiz A, Elkhamary SM, Asghar N, Bosley TM. Cupping of the optic disk after methanol poisoning. Br J Ophthalmol. 2015b;99 : 1220-1223.
51. Hovda KE, Hunderi OH, Tafjord AB, Dunlop O, Rudberg N, Jacobsen D. Methanol outbreak in Norway 2002–2004: epidemiology, clinical features and prognostic signs. J Intern Med. 2005a;258 : 181-190.
52. Naraqi S, Dethlefs RF, Slobodniuk RA, Sairere JS. An outbreak of acute methyl alcohol intoxication. Aust N Z J Med. 1979;9 : 65-68.
53. Paasma R, Hovda KE, Jacobsen D. Methanol poisoning and long term sequelae - a six years follow-up after a large methanol outbreak. BMC Clin Pharmacol. 2009;9 : 5; doi: 10.1186/1472-6904-9-5.
54. Sanaei-Zadeh H. Is high-dose intravenous steroid effective on preserving vision in acute methanol poisoning? Optom Vis Sci. 2012;89 : 244.
55. Hayreh SS. Pathogenesis of cupping of the optic disc. Br J Ophthalmol. 1974;58 : 863-876.
56. Tsuda Y, Nakahara T, Ueda K, Mori A, Sakamoto K, Ishii K: Effect of nafamostat on N-methyl-D-aspartate-induced retinal neuronal and capillary degeneration in rats. Biol Pharm Bull. 2012;35 : 2209-2213.
57. Hantson P, de Tourtchaninoff M, Simoens G, et al. Evoked potentials investigation of visual dysfunction after methanol poisoning. Crit Care Med. 1999;27 : 2707-2715.
58. Lee EW, Garner CD, Terzo TS. A Rat Model Manifesting Methanol-Induced Visual Dysfunction Suitable for Both Acute and Long-Term Exposure Studies. Toxicol Appl Pharmacol. 1994;128 : 199-206.
59. Garner CD, Lee EW, Terzo TS, Louis-Ferdinand RT. Role of Retinal Metabolism in Methanol-Induced Retinal Toxicity. J Toxicol Environ Health. 1995;44 : 43-56.doi: 10.1080/15287399509531942.
60. Jones SJ, Brusa A. Neurophysiological evidence for long-term repair of MS lesions: implications for axon protection. J Neurol Sci. 2003;206 : 193–198.
61. Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol. 2000;62 : 215-249.
62. Jain N, Himanshu D, Verma SP, Parihar A. Methanol poisoning: characteristic MRI findings. Ann Saudi Med. 2013;33 : 68-69.
63. Halavaara J, Valanne L, Setala K. Neuroimaging supports the clinical diagnosis of metanol poisoning. Neuroradiology. 2002 : 44 : 924-928.
64. Taheri MS, Moghaddam HH, Moharamzad Y, Dadgari S, Nahvi V. The value of brain CT findings in acute methanol toxicity. Eur J Radiol. 2010;73 : 211-214.
65. Thirunavukkarasu S, Nair PP, Wadwekar V. Acute bilateral putaminal haemorrhagic necrosis in methanol poisoning. BMJ Case Rep. 2013 Nov 8. doi: 10.1136/bcr-2013-201026.
66. Vaneckova M, Zakharov S, Klempir J, et al. Methanol Intoxication on Magnetic Resonance Imaging. Cesk Slov Neurol N. 2014;77/110 : 235-239.
67. Vaneckova M, Zakharov S, Klempir J, et al. Imaging findings after methanol intoxication (cohort of 46 patients). Neuro Endocrinol Letters. 2015;36 : 737–744.
68. Bhatia R, Kumar M, Garg A, Nanda A. Putaminal necrosis due to methanol toxicity. Pract Neurol. 2008 : 8:386-387.
69. Chiò A, Herrero Hernandez E, Mora G, Valentini C, Discalzi G, Pira E. Motor neuron disease and optic neuropathy after acute exposure to a methanol-containing solvent mixture. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5 : 188-191.
70. Karayel F, Turan AA, Sav A, Pakis I, Akyildiz EU, Ersoy G. Methanol intoxication: pathological changes of central nervous system (17 cases). Am J Forensic Med Pathol. 2010; 31 : 34-36.
71. Lee SM, Moon JM, Chun BJ, Song KH. Unusual intracranial hemorrhage in severe methanol intoxication. Am J Emerg Med. 2015;33 : 1717.e1-2.
72. Sefidbakht S, Rasekhi AR, Kamali K, et al. Methanol poisoning: acute MR and CT findings in nine patients. Neuroradiology. 2007 : 49 : 427-435.
73. Server A, Hovda KE, Nakstad PH, Jacobsen D, Dullerud R, Haakonsen M. Conventional and diffusion-weighted MRI in the evaluation of methanol poisoning. Acta Radiol. 2003;44 : 691-695.
74. Lešták J. Neurotransmission in visual analyser and bionic eye. Cesk Slov Oftalmol. 2020;76: in press
Štítky
Oftalmologie
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2020 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Familiární středomořská horečka
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Možnosti využití přípravku Desodrop v terapii a prevenci oftalmologických onemocnění
- Selektivní laserová trabekuloplastika nesnižuje nitroční tlak více než argonová laserová trabekuloplastika
-
Všechny články tohoto čísla
- Uveal Melanoma Biopsy. A Review
- VISUAL FUNCTIONS AFTER IMPLANTATION OF ACRYSOF MONOFOCAL INTRAOCULAR LENSES.
- Evaluation of Patients Presenting to the Ophthalmology Department of a Tertiary Hospital for Nonemergency Reasons During the Covid-19 Pandemic
- Changed Eye Functions and Quality of Life of Seniors with Diabetic Retinopathy
- Can Visual Function Be Affected by an Open Foramen Ovale?
- Late Functional and Morphological Findings after Methylalcohol Poisoning
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Can Visual Function Be Affected by an Open Foramen Ovale?
- VISUAL FUNCTIONS AFTER IMPLANTATION OF ACRYSOF MONOFOCAL INTRAOCULAR LENSES.
- Late Functional and Morphological Findings after Methylalcohol Poisoning
- Uveal Melanoma Biopsy. A Review
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



