-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRanibizumab for the treatment of choroidal neovascularization due to cause other than age related macular degeneration
Authors: A. Stepanov; M. Středová; J. Dusová; N. Jirásková; J. Studnička
Authors place of work: Fakultní nemocnice Hradec Králové, Oční klinika, přednostka prof. MUDr. Naďa Jirásková, Ph. D., FEBO ; Univerzita Karlova, Lékařská fakulta v Hradci Králové, katedra očního lékařství
Published in the journal: Čes. a slov. Oftal., 75, 2019, No. 3, p. 138-144
Category: Původní práce
doi: https://doi.org/10.31348/2019/3/4Summary
Purpose: To evaluate the safety and clinical efficacy of ranibizumab (Lucentis) in the treatment of choroidal neovascularization (CNV) caused by diseases other than age-related macular degeneration (AMD).
Patients: 21 patients with mean age 61 17.2 years (min 16, max 85) with CNV due to causes other than AMD, in particular pathological myopia (n=11), angioid streaks (n=3), central serous chorioretinopathy (n=2), North Carolina macular dystrophy (n=1), dominant familial drusen (n=1) and idiopathic CNV (n=3).
Methods: The patients were treated at the Ophthalmology Department of the University Hospital in Hradec Kralove with three monthly initial intravitreal injections of ranibizumab 0.5 mg with subsequent treatment regimen pro re nata (PRN). The best corrected visual acuity (BCVA) was evaluated on the ETDRS optotypes (Early Treatment Diabetic Retinopathy Study), central retinal thickness (CRT) was measured by optical coherent tomography (OCT) (Zeiss, Cirrus). These parameters were evaluated before start of the study and then at 1 (BCVA only), 4, 8, and 12 months during treatment. We also evaluated the possible occurrence of ocular and systemic side effects.
Results: Statistically significant improvement in the mean of BCVA score of 11.4 letters (p<0.001) at the end of the follow-up period in patients with myopic CNV was observed. In the subgroup of CNV patients from other causes BCVA improvement was 5.9 letters (p=0.043). The mean CRT at 12 months statistically significantly decreased by 42.2 μm (p=0.028) in patients with myopic CNV and by 119.4 μm (p=0.002) in patients with CNV from other causes. We have not detected any serious ophthalmic or systemic side effects associated with ranibizumab s treatment.
Conclusions: Intravitreal ranibizumab given in the PRN regimen after the initial three monthly doses was demonstrated efficiency and safety in the treatment of CNV due to cause other than AMD during the annual follow-up.
Keywords:
ranibizumab – myopic CNV – other cause than AMD – anti-VEGF – Lucentis
INTRODUCTION
Choroidal neovascularisation (CNV) is characterised by the presence of pathological choroidal capillaries beneath the retinal pigment epithelium (RPE) or beneath the retina. The cause of occurrence of CNV is not known, although the role of angiogenic factors is demonstrated in its pathogenesis, for example vascular endothelial growth factor (VEGF) (3, 10, 14).
The most common cause of occurrence of CNV is age-related macular degeneration (ARMD), which, if untreated, leads to a significant reduction of visual acuity in patients aged over 55 years (5). Other causes of the occurrence of CNV include pathological myopia, presumed ocular histoplasmosis syndrome, angioid streaks, uveitis, choroidal rupture or trauma, central serous chorioretinopathy and central dystrophy.
Choroidal neovascularisation due to causes other than ARMD and pathological myopia is rare, and usually occurs in adults in productive age (2).
Myopia is the most common cause of deterioration of visual acuity in the general population, especially in East Asia, where it affects approximately 40% of adults at the age of over 40 years (20). Pathological myopia is the most severe form of myopia, and its definition encompasses a refractive error of minimum -6.0 dioptres or axial length of the eyeball of 26.5 mm and more, as well as degenerative changes of the sclera, choroid and retina (6, 15, 17).
Choroidal neovascularisation upon a background of pathological myopia (myopic CNV) is one of the most severe complications of pathological myopia in patients of productive age, with a prevalence of 0.04% to 0.05% within the overall population (4, 7).
In this study we shall evaluate the treatment of intravitreally administered ranibizumab m in a pro re nata (PRN) regime on patients with CNV caused by pathologies other than ARMD.
COHORT AND METHOD
The study conducted at the Department of Ophthalmology at the University Hospital in Hradec Králové in the period from January 2016 to November 2017 included 21 patients. Table 1 shows the basic demographic and clinical characteristics of the cohort. Fluorescence angiography (FA), fundus photography and optical coherence tomography (OCT Cirrus, Zeiss) were used for confirmation of the diagnosis. The patients were included in the study if they signed a written informed consent form and were diagnosed with CNV due to causes other than ARMD. Specifically this concerned the following diagnostic units: angioid streaks, central serous chorioretinopathy, pathological myopia (Fig. 1), North Carolina macular dystrophy, familial dominant retinal drusens and idiopathic CNV (Fig. 2). Patients with pathological myopia had a mean axial length of the eye of 29.21 mm and mean refraction was -11.67 dioptres. Treatment with ranibizumab was within a PRN dosing regime, thus after the first three monthly injections there were subsequent follow-up examinations with applicable administration of a further injection upon persistence of signs of CNV activity according to OCT (intra and subretinal fluid, RPE ablation), as well as upon a finding of fresh macular haemorrhage. Preparation of the drug and its administration took place under aseptic conditions. Application of ranibizumab (0.5 mg in 0.05 ml) was performed under local anaesthesia with the aid of a 30-gauge needle transsclerally 3.5 to 4.0 mm from the limbus.
Tab. 1. Initial demographic and clinical characteristics 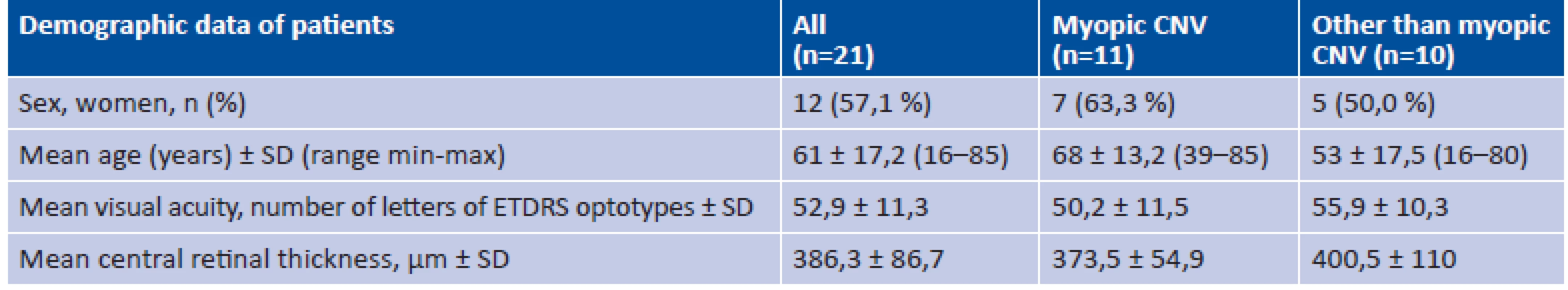
n = number of eyes; CNV = choroidal neovascularisation; ETDRS = Early Treatment Diabetic Retinopathy Study; SD = standard deviation Fig. 1. Choroidal neovascularisation in a patient with pathological myopia
A. Photograph of fundus before commencement of treatment with ranibizumab. Lesion on neovascular membrane, in nasal part subretinal haemorrhage
B-C. HD-OCT before treatment. Hyperreflective tissue, through-growing retinal pigment epithelium. Edema of neuroretina with central retinal thickness of 675 μm
D-E. HD-OCT after treatment. Reduction of edema, indication of foveolar depression
F. Photograph of fundus after treatment. Lesion bordered, haemorrhages absent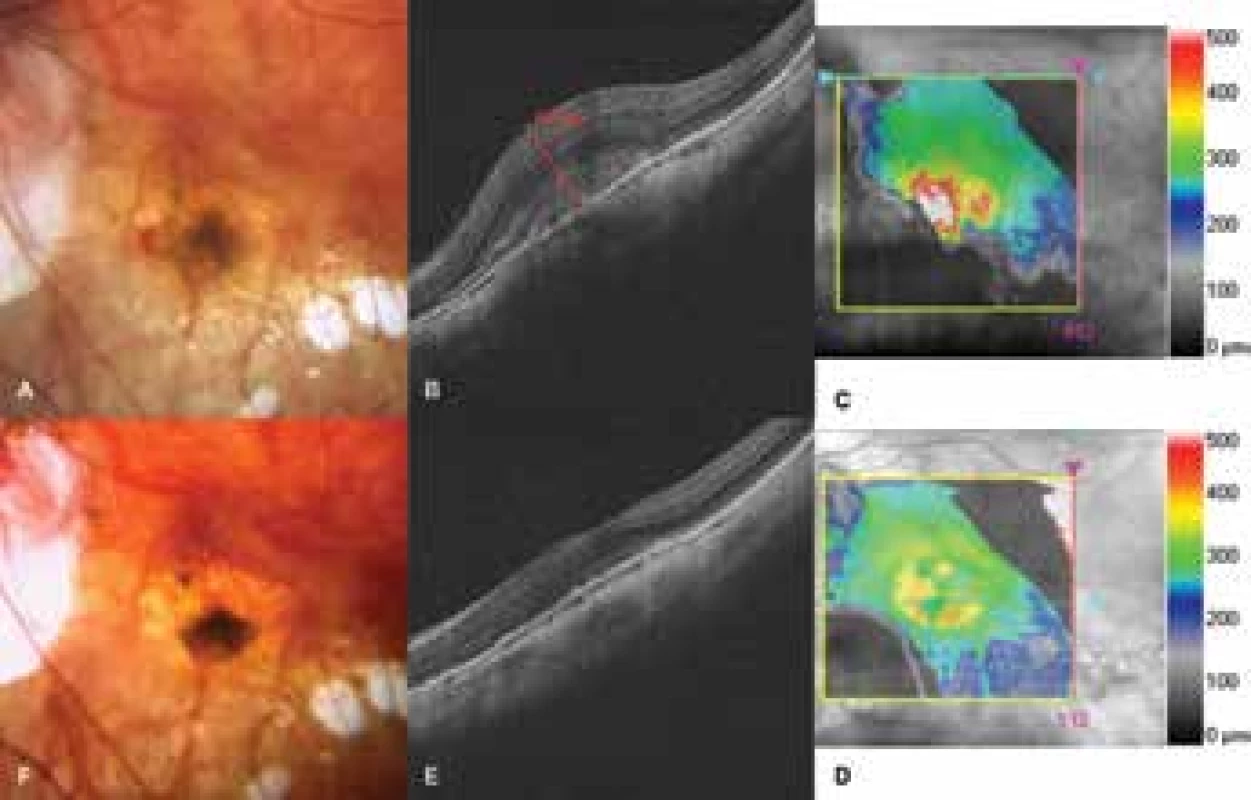
Fig. 2. Idiopathic choroidal neovascularisation
A. Photograph of fundus before commencement of treatment with ranibizumab. Lesion on neovascular membrane, subretinal haemorrhages around lesion
B. HD-OCT before treatment. Ablation and edema of neuroretina, elevation of retinal pigment epithelium.
C-D. FAG finding at first visit. Progressively grading hyperfluorescence of neovascular membrane, blockade of fluorescence due to subretinal haemorrhage
E. HD-OCT after treatment. Reduction of edema of neuroretina and retinal pigment epithelium
F. Photograph of fundus after treatment. Bordered lesion with pigment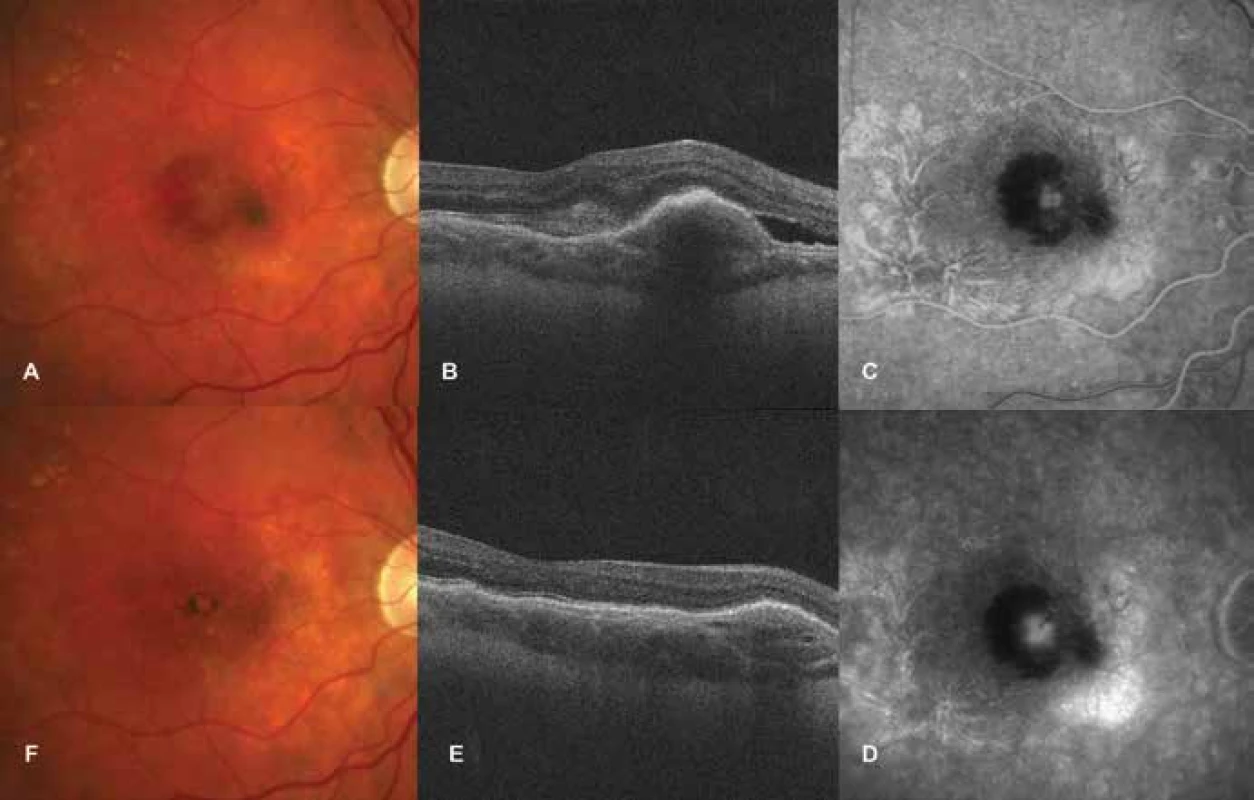
We evaluated the incidence of ocular and systemic adverse effects, the percentage of patients who gained 15 and more letters of best corrected visual acuity (BCVA) in ETDRS optotypes, and also the development of central retinal thickness (CRT) according to OCT. The examinations were conducted at an interval of 1 (BCVA only), 4, 8 and 12 months from the commencement of treatment.
Statistical analysis
The statistical analysis was conducted with the aid of the software IBM SPSS Statistics 23. Quantitative data is expressed by the mean and scope. The values of BCVA and CRT were analysed by a Kolmogorov-Smirnov normality test. Changes of BCVA and CRT were evaluated with the aid of a Friedman test. Statistical significance was defined as p<0.05.
RESULTS
The most common cause of CNV in our study was pathological myopia, which was diagnosed in approximately one half of the treated patients (table 2). In the entire group the average number of injections applied to one patient was 4.8 ± 2.1 (range 3-10) according to the therapeutic protocol of PRN. The average number of applied injections for patients with pathological myopia was 4.1 (range 3-7 injections), for patients with central serous chorioretinopathy 8.0 (range 7-9 injections), in patients with angioid streaks 6.0 (range 4-7 injections), and in patients with idiopathic CNV 8.0 (range 3-10 injections). The patient with North Carolina macular dystrophy and the patient familial dominant retinal drusens were treated with three injections.
Tab. 2. Etiology of choroidal neovascularisation and number of injections of ranibizumab 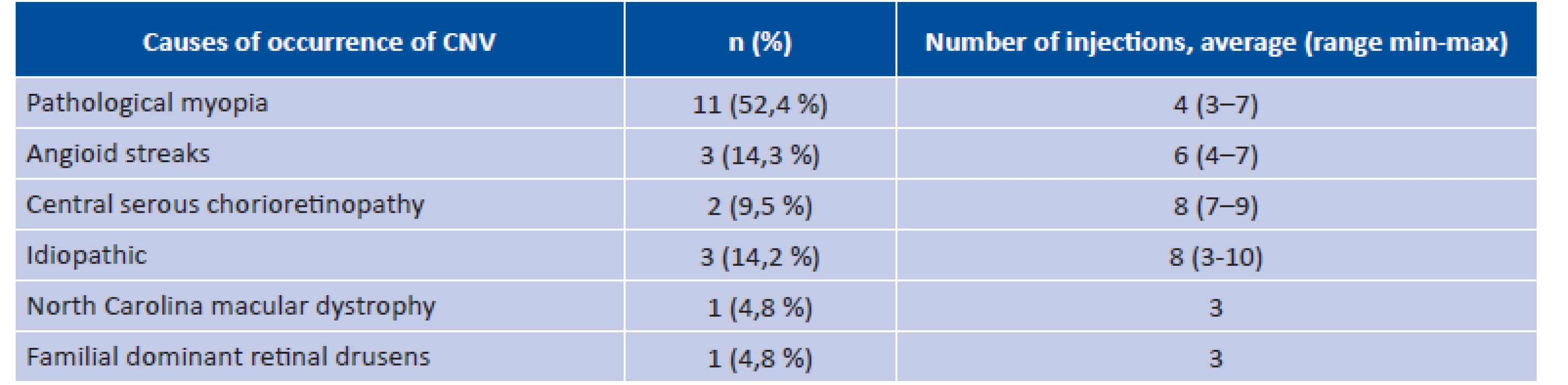
n = number of eyes; CNV = choroidal neovascularisation Change of BCVA
In the full group of 21 patients, 5 patients (23.8%) gained 15 letters or more of BCVA after the first injection of ranibizumab in comparison with the baseline values, and after 12 months 6 patients (28.6%) gained 15 or more letters (Graph 1).
Graph 1. Change of best corrected visual acuity during the course of one-year observation in the subgroup of myopic choroidal neovascularisation and choroidal neovascularisation due to other causes in the observed cohort 
In the subgroup of patients with myopic CNV we recorded a statistically significant improvement of BCVA by 14.2 letters of ETDRS optotypes (p<0.001) immediately after the 1st injection of ranibizumab, in the subgroup of patients with CNV due to other causes the improvement by 5 letters was also statistically significant (p=0.003). In the period of 1 month to 8 months from the commencement of treatment we determined a slight statistically insignificant decrease of BCVA in the case of myopic CNV by 2.4 letters of ETDRS optotypes (p=0.167) and in the case of CNV due to other causes by 0.6 letters (p=0.402). In the observation period of 8-12 months from the commencement of treatment we documented a further insignificant decrease of BCVA by 0.7 letters of ETDRS optotypes (p=0.353) in patients with myopic CNV and a significant improvement of BCVA by 1.5 letters (p=0.045) in patients with CNV due to other causes (Graph 1).
After one year of observation we determined an improvement of visual acuity in comparison with the values before treatment in all eyes except two. In one patient with idiopathic CNV a decrease of BCVA by 1 row of ETDRS optotypes was determined, even despite the application of three initial injections of ranibizumab. In the second patient with CNV on a background of retinal angioid streaks 7 injections were applied, but despite this treatment BCVA deteriorated by 7 letters of ETDRS optotypes. With regard to irreversible fibrous changes in the macula in these patients, the treatment was terminated. After one year of treatment in 4 patients (36.4%) there remained signs of activity of CNV, and as a result treatment with ranibizumab was continued for these patients. The other patients remain under our observation.
Anatomical results
A significant improvement of CRT was recorded in all patients immediately following the first 3 injections of ranibizumab, with an average statistically significant reduction of CRT by 59.9 µm from the baseline values (p<0.001) in the group of patients with myopic CNV, and by 59.9 µm (p<0.001) in the group of CNV due to other causes.
From the 4th to the 8th month of observation there was a statistically insignificant increase of CNV by 17.3 µm (p=0.19) in the patients with myopic CNV and a further reduction of CRT by 5.5 µm in the group with CNV due to other causes (p=0.41).
In the observation period of 8-12 months from the commencement of treatment we documented a further statistically insignificant increase of CRT by 0.4 µm (p=0.56) in the patients with myopic CNV and by 7.4 µm (p=0.231) in the patients with CNV due to other causes (Graph 2).
Graph 2. Change of central retinal thickness during the course of one-year observation in the subgroup of myopic choroidal neovascularisation and choroidal neovascularisation due to other causes in the observed cohort 
Safety of treatment
No serious complications such as endophthalmitis, retinal crack or detachment, vitreous haemorrhage, glaucoma, active uveitis or systemic adverse effects were observed. In one patient temporary intraocular hypertension was recorded after the application of ranibizumab, which responded well to local hypotensive therapy with Cosopt gtt. The most frequent ocular adverse effect during the one year of treatment was 3 cases of subconjunctival haemorrhage.
DISCUSSION
The benefit of treatment with VEGF blockers for patients with CNV with a cause other than ARMD has been demonstrated in a range of clinical trials. The RADIANCE study, which was the first in the world to evaluate the effectiveness of treatment with ranibizumab in the case of myopic CNV in 116 eyes in a PRN regime, demonstrated a significant improvement of BCVA by 14.4 letters of ETDRS optotypes at the end of one year observation, with an average number of 3.5 injections of ranibizumab (19). In our study we recorded a gain of 11.4 letters 12 months after the commencement of treatment with an average number of 4.1 injections during the course of one year of treatment in the subgroup of myopic CNV. We attribute the smaller gain to the small number of patients in our cohort, as well as to the worse baseline values of BCVA (50.2 letters of ETDRS optotypes as against 55.8 letters in the RADIANCE study). The average reduction of CRT in the RADIANCE study at the end of one year of observation was -71.3 µm against a baseline value of 373.1 µm. In our cohort we recorded an improvement of CRT by 42.2 µm from an original value of 373.5 µm, which we consider comparable.
The MINERVA study evaluated the effectiveness of treatment by ranibizumab in 119 patients with CNV due to causes other than ARMD and in pathological myopia within a PRN regime of treatment (13). The results of the evaluation of BCVA show that during the course of one year of observation there was an improvement by 9.5 letters of ETDRS optotypes upon an average number of 5.8 injections of ranibizumab (baseline values of BCVA were 62.4 letters). In our cohort we demonstrated an improvement by 5.9 as against the baseline value of BCVA of 55.9 letters in the subgroup of patients with CNV due to causes other than ARMD and pathological myopia. The average number of injections of ranibizumab over the course of one year in this subgroup of patients was 5.6. We attribute the smaller gain of BCVA in our cohort to the small number of patients and also to the worse baseline values of BCVA in comparison with the MINERVA study. The average reduction of CRT in the MINERVA study at the end of one year of observation was -77.0 µm as against the baseline value of 392.5 µm. In our cohort we recorded a reduction of CRT by 119.4 µm from an original 400.5 µm, which we consider comparable.
Troutbeck et al. observed a cohort of 41 patients with secondary CNV over the course of one year, including 15 patients with myopia, 7 with multifocal choroiditis and 8 patients with idiopathic CNV, and demonstrated the effectiveness of treatment with ranibizumab (19). The average improvement of BCVA during the course of observation was by 10 letters of ETDRS optotypes within the subgroup of myopic CNV (average 3.5 injections per year) and by 12 letters in the subgroup of CNV due to other causes (average 5.6 injections per year). The reduction of CRT was on average by 76 µm from a baseline value of 334 µm in the case of myopic CNV and by 132 µm from baseline 405 µm in the group of patients with CNV due to other causes. Our results are comparable with the study by Troutbeck et al. A similar proportional division of patients in both groups of our study (myopic CNV and CNV due to other causes) demonstrated a clinically significant improvement of BCVA, in which none of the patients recorded a clinically significant decrease of BCVA (here defined as a deterioration by 15 letters) during the course of one year of observation.
Chrapek et al. evaluated the effectiveness of treatment by ranibizumab over the course of one year in 6 patients with idiopathic CNV (9). The average improvement of BCVA over the course of the observation was by 11 letters of ETDRS optotypes, the reduction of CRT was on average by 233 µm from a baseline value of 480 µm (average 3.0 injections per year). We explain the smaller gain of BCVA and reduction of CRT in our group of patients with CNV due to other causes by the fact that we observed a heterogeneous population of patients, in whom idiopathic CNV was recorded in only 3 patients (14.2%).
An increase of BCVA by 15 or more letters of ETDRS optotypes was attained by 28.6% of eyes in our cohort after one year of observation. These results are slightly worse in comparison with the study by Carneiro et al., who state that in cases of CNV in connection with angioid streaks, inflammatory chorioretinal pathologies, central serous chorioretinopathy and idiopathic CNV there was an improvement of visual functions by 15 or more letters of ETDRS optotypes in 43% of eyes after one year of treatment (1). We attribute the smaller gain of BCVA to the smaller number of patients in our cohort (11 as against 21 in the study by Carneiro et al.), as well as the worse baseline values of BCVA (50.2 letters of ETDRS optotypes as against 54.5 letters in the cohort of Carneiro et al.).
During the course of our observation, differences in changes of BCVA and CRT were perceptible during the course of treatment in the groups of patients with myopic CNV and CNV due to other causes, which can be attributed to cases in which a patient responded rapidly to the treatment. For example, a patient with familial dominant retinal drusens gained 22 letters of ETDRS optotypes after the first injection of ranibizumab, and this increase was subsequently stable throughout the entire observation period. In one of three patients with idiopathic CNV there was a reduction of CRT by 351 µm after the initial three saturating doses of ranibizumab, in which the improvement remained stable throughout the entire observation.
The variability of the individual reactions to ranibizumab in this study is not surprising, with regard to the fact that this concerns a heterogeneous population of patients with various causes of the occurrence of CNV.
It is noteworthy that patients with myopic CNV received 26.8% fewer injections (on average 4.1) than patients in the group of CNV due to other causes (on average 5.6) during the course of the 12 month period. Our results in this respect are nevertheless in accordance with the results of other studies (11, 12, 13, 16, 18, 19). We can explain this by the fact that myopic CNV has a predominantly classic composition (type 2 CNV), unlike CNV on a background of ARMD and CNV due to other causes, which responds more quickly to treatment by VEGF inhibitors (21).
In our study we demonstrated that the application of ranibizumab is a safe method of treatment for patients with myopic CNV and CNV due to other causes. The SUSTAIN study evaluated the safety profile of intravitreally administered ranibizumab within a PRN regime in patients with CNV, with the following conclusion – in 18.5% of patients there was a deterioration of BCVA, in 7.2% of patients an enlargement of the surface of intra/subretinal haemorrhage was documented, in 7.0% a temporary increase of intraocular pressure in the studied eye was recorded, and 5.5% of patients had subconjunctival suffusion following injection (8). None of the patients in our cohort recorded a development of sight threatening complications in connection with treatment by ranibizumab during the observation period. In two patients (9.5%) we recorded a deterioration of BCVA on the basis of irreversible fibrous changes in the macula, in three patients (14.3%) there was a progression of subconjunctival suffusion and one patient (4.8%) recorded a temporary increase of intraocular pressure, which we successfully cured by local administration of Cosopt gtt. The safety profile of treatment with ranibizumab in our cohort is comparable with the SUSTAIN study.
The limitations of this study include the relatively small cohort of patients, as well as the lack of a control group.
CONCLUSION
In conclusion it is possible to state that to date treatment of CNV occurring as a consequence of causes other than ARMD has been very limited. The clinical benefit of ranibizumab demonstrated in large clinical trials on patients with CNV on a background of ARMD has enabled its use also in the case of CNV due to other causes. Although this concerns a small group of patients, the results of our study demonstrate that intravitreal treatment with ranibizumab for patients with CNV due to causes other than ARMD is effective and safe.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
MUDr. Alexandr Stepanov, Ph.D., FEBO
Oční klinika Fakultní nemocnice Hradec
Králové, Sokolská 581,
500 05 Hradec Králové
Received: 14. 1. 2019
Accepted: 21. 2. 2019
Available on-line: 19. 10. 2019
Zdroje
1. Carneiro, AM., Silva, RM., Veludo, MJ., et al.: Ranibizumab treatment for choroidal neovascularization from causes other than age-related macular degeneration and pathological myopia. Ophthalmologica, 225(2); 2011 : 81-8.
2. Cohen, SY., Laroche, A., Leguen, Y., et al.: Etiology of choroidal neovascularization in young patients. Ophthalmology, 103(8); 1996 : 1241–1244.
3. Cui, JZ., Kimura, H., Spee, C., et al.: Natural history of choroidal neovascularization induced by vascular endothelial growth factor in the primate. Graefes Arch Clin Exp Ophthalmol, 238(4); 2000 : 326 –33.
4. Curtin, BJ.: The prevalence of myopia. In: The Myopias: Basic Science and Clinical Management. Philadelphia, PA: Harper & Row 1985 : 39–59.
5. Klein, R., Chou, CF., Klein, BE., et al.: Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol, 129(1); 2011 : 75-80.
6. Fredrick, DR.: Myopia. BMJ, 324(7347); 2002 : 1195–9.
7. Grossniklaus, HE., Green, WR.: Pathologic findings in pathologic myopia. Retina, 12(2); 1992 : 127–33.
8. Holz, FG., Amoaku, W., Donate, J., et al.: SUSTAIN Study Group. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology, 118(4); 2011 : 663-71.
9. Chrapek, O., Vostrovská, Z., Šínová, I., et al.: Léčba idiopatické choroidální neovaskulární membrány ranibizumabem – naše zkušenosti. Cesk Slov Oftalmol,XX (XX); 2019: XXX
10. Ishibashi, T., Hata, Y., Yoshikawa, H., et al.: Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol, 235(3); 1997 : 159–67.
11. Konstantinidis, L., Mantel, I., Pournaras, JA., et al.: Intravitreal ranibizumab (Lucentis) for the treatment of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol, 247(3); 2009 : 311–8.
12. Lai, TY., Chan, WM., Liu, DT., et al.: Intravitreal ranibizumab for the primary treatment of choroidal neovascularization secondary to pathologic myopia. Retina, 29(6); 2009 : 750–6.
13. Lai, TYY., Staurenghi, G., Lanzetta, P., et al.: MINERVA study group. Efficacy and safety of ranibizumab for the treatement of choroidal neovascularization due to uncommon cause: Twelve-Month Results of the MINERVA Study. Retina. 38(8); 2018 : 1464-1477.
14. Martin, G., Schlunck, G., Hansen, LL., et al.: Differential expression of angioregulatory factors in normal and CNVderived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol, 242(4); 2004 : 321–6.
15. Miller, DG., Singerman, LJ.: Natural history of choroidal neovascularization in high myopia. Curr Opin Ophthalmol, 12(3); 2001 : 222–4.
16. Mones, JM., Amselem, L., Serrano, A., et al.: Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond), 23(6); 2009 : 1275 – 80.
17. Neelam, K., Cheung, CM., Ohno-Matsui, K., et al.: Choroidal neovascularization in pathological myopia. Prog Retin Eye Res, 31(5); 2012 : 495–525.
18. Troutbeck, R., Bunting, R., van Heerdon, A., et al.: Ranibizumab therapy for choroidal neovascularization secondary to non-age-related macular degeneration causes. Clinical and Experimental Ophthalmology, 40(3); 2012 : 67–72.
19. Wolf, S., Balciuniene, VJ., Laganovska, G., et al.: RADIANCE Study Group. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology, 121(3); 2014 : 682-92.e2.
20. Wong, TY., Foster, PJ., Hee, J., et al.: Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci, 41(9); 2000 : 2486–94.
21. Wong, TY., Ohno-Matsui, K., Leveziel, N., et al.: Myopic choroidal neovascularisation: current concepts and update on clinical management. Br J Ophthalmol, 99(3); 2015 : 289-96.
Štítky
Oftalmologie
Článek 23. Zimný kongres ESCRSČlánek Kongres SOE
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2019 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Familiární středomořská horečka
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Normotenzní glaukom: prevalence a zásady terapie
- Léčba chronické blefaritidy vyžaduje dlouhodobou péči
-
Všechny články tohoto čísla
- Influence of cornea on intraocular pressure measurement by ICARE PRO and ORA
- The use of optical coherence tomography in chiasmal compression
- Methods of improving the quality of life in patients with stable maculopathy-pilot results of a new study
- Ranibizumab for the treatment of choroidal neovascularization due to cause other than age related macular degeneration
- Pupillary abnormalities in childhood – 2 case presentations
- Selective angiography with the possibility of thrombolysis in patients with central retinal artery occlusion
- Doc. MUDr. Karel Kuběna, CSc. - zemřel
- 23. Zimný kongres ESCRS
- Kongres SOE
- Očná klinika Lekárskej fakulty Univerzity Komenského v Bratislave oslavuje 100-té výročie činnosti – 2. časť
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Pupillary abnormalities in childhood – 2 case presentations
- Influence of cornea on intraocular pressure measurement by ICARE PRO and ORA
- Methods of improving the quality of life in patients with stable maculopathy-pilot results of a new study
- Ranibizumab for the treatment of choroidal neovascularization due to cause other than age related macular degeneration
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



