-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mortality Prediction in a Neurosurgical Intensive Care Unit
Predikce úmrtnosti na neurochirurgické jednotce intenzivní péče
Cíl:
Celosvětově je pro péči o pacienty velmi důležité, abychom byli schopni identifikovat faktory, které ovlivňují výsledky intenzivní péče, obzvláště mortalitu. Byla identifikována řada klinických proměnných, které velmi dobře predikují mortalitu na všeobecných jednotkách intenzivní péče (JIP). Použití takových prediktorů na neurochirurgických JIP nebylo zatím zkoumáno. Cílem naší studie bylo zhodnotit prediktivní schopnost proměnných spojených s mortalitou v terciární neurochirurgické JIP.Materiál a metody:
Do studie byli zapojeni všichni neurochirurgičtí pacienti přijatí na JIP během pětiměsíčního období roku 2011 (n = 258). Data byla analyzována pomocí logistické regrese a pro každý prediktor bylo vypočítáno odds ratio.Výsledky:
Zjištěna byla hospitalizační mortalita 3,49 %. Identifikovány byly čtyři prediktory mortality: tělesná teplota zvýšená o 0,1 °C OR = 1,21, 95% CI 1,02 – 1,44; zvýšení hladiny glukózy v krvi o 1 mg/ dl OR = 0,93, 95% CI 0,87 – 0,99; zvýšení o jeden bod na zrakové podstupnici Glasgow Coma Scale (GCS) OR = 0,26, 95% CI 0,07 – 0,89; a prodloužení hospitalizace předcházející přijetí na JIP o 1 den OR = 1,14 (1,05 – 1,24). Tyto prediktory byly vloženy do regresního modelu, přičemž plocha pod křivkou (AUC) činila 0,968, 95% CI 0,923 – 1,000.Závěr:
Jako prediktory nemocniční mortality na neurochirurgické JIP mohou sloužit tělesná teplota, hladina glukózy v krvi, zraková odpověď na GCS a délka hospitalizace před přijetím na JIP. Je proto třeba vyvinout nový prediktivní model.Klíčová slova:
teplota – délka hospitalizace – skóre Glasgow Coma Scale – urgentní příjem – výkon
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors: P. Akavipat 1; J. Thinkhamrop 2; B. Thinkhamrop 3; W. Sriraj 4
Authors place of work: Anesthesiology Department, Prasat Neurological Institute, Bangkok Thailand 1; Department of Obstetrics and Gynecology Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand 2; Department of Biostatistics and Demography Faculty of Public Health Khon Kaen University, Khon Kaen Thailand 3; Department of Anesthesiology Faculty of Medicine, Khon Kaen University Khon Kaen, Thailand 4
Published in the journal: Cesk Slov Neurol N 2017; 80/113(4): 451-456
Category: Krátké sdělení
doi: https://doi.org/10.14735/amcsnn2017451Summary
Aim:
The ability to estimate factors influencing intensive care outcome, especially mortality, is highly important to patient care worldwide. Several clinical variables have been identified for the general intensive care unit (ICU) setting with high mortality predictive ability. However, the application of such predictors in the neurosurgical ICU setting is not yet established. The study was aimed to assess the predictive ability of the variables associated with mortality in a tertiary neurosurgical ICU.Material and methods:
All neurosurgical patients admitted to ICU during a 5-month period in 2011 were recruited to the study (n = 258). A logistic regression model was used for data analysis and odds ratios were calculated for each predictor.Results:
The observed hospital mortality rate was 3.49%. The four resulting predictors of mortality were: increased body temperature of 0.1 °C OR = 1.21, 95% CI 1.02 – 1.44; 1 mg/ dl increase in blood glucose level OR = 0.93, 95% CI 0.87 – 0.99; one point increase on the Glasgow Coma Scale (GCS) eye subscale OR = 0.26, 95% CI 0.07 – 0.89; and 1 day increase in the length of stay prior to ICU admission OR = 1.14 (1.05 – 1.24). These predictors were put into a regression model and the area under the receiver operating characteristic curve (AUC) was 0.968, 95% CI 0.923 – 1.000.Conclusion:
The body temperature, blood glucose level, GCS eye response and the length of hospital stay prior to ICU admission may be hospital mortality predictors in a neurosurgical ICU. Accordingly, a new predictive model should bedeveloped.Key words:
temperature – length of stay – Glasgow Coma Scale score – emergency admission – performance
Chinese summary - 摘要
神经外科重症监护病房的死亡率预测
目标:
对一些影响重症监护结果(尤其是死亡率)的因素作出评估的能力,对于世界范围内的患者的治疗显得尤为重要。一些临床变量已被确定为广大重症监护病房(ICU)高死亡率预测指标。 然而,这种预测因子在神经外科ICU中的应用还没有建立。该研究旨在评估三级神经外科ICU中与死亡率有关的变量的预测能力。
材料与方法:
在2011年的5个月时间里,所有送入ICU的神经外科患者(258例)被纳入研究。使用逻辑回归模型进行数据分析,并计算每个预测因子的优势比。
结果:
实验所在医院的死亡率为3.49%。 死亡率的四个预测因子及其优势比分别是:体温升高0.1°C(OR = 1.21,95%置信区间1.02-1.44)、血糖水平升高1mg / dl(OR = 0.93,95%置信区间 0.87-0.99)、格拉斯哥昏迷量表(GCS)眼部分量表增加一分(OR = 0.26,95%置信区间0.07-0.89)、入住ICU前住院时间增加1天(OR = 1.14 95%置信区间1.05-1.24)。这些预测因子被放入回归模型,并且受试者工作特征曲线下面积(AUC)为0.968,95%置信区间0.923-1.000。
结论:
体温、血糖水平、GCS眼睛反应和送入ICU前的住院时间可能是神经外科ICU的医院死亡率预测指标。 因此,应该开发一个新的预测模型。
关键词:
体温 - 住院时间 - 格拉斯哥昏迷量表评分 -急诊入院 - 表现Introduction
There are three main considerations where practice or other differences among intensive care units’ (ICU) performance might matter: effectiveness; patient outcomes [1], efficiency; resource utilization for a given outcome, length of stay [2], and qualitative factors; complication rate, morbidity and rate of infection [3]. Nevertheless, the limitation of these factor assessments is still existed because of the unique patient variability especially in neurosurgical patient, e. g., physiologic response to surgery [4], anatomical lesion, endocrine axes path integration [5], patient phenotype [6], ventilator and pain management [7,8], etc.
In order to attenuate this pitfall; Acute Physiology and Chronic Health Evaluation (APACHE) score, Simplified Acute Physiology Score (SAPS) have been initiated. These scales can clinically simplify and categorize critically ill patient with its high mortality predictive ability as shown in the area under the receiver operating characteristic curve (AUC) of 0.76 – 0.90 [9,10] for general ICU and 0.81 – 0.89 [11 – 13] together with sensitivity/ specificity over 74% [14] for neurosurgical ICU. However, the differences of scale prediction have not been established, especially in neurosurgical patients lately.
Some articles accentuated the predictive mortality performance of the Glasgow Coma Scale score and its subscales, such as motor score, they found it less time-consuming but simpler and more effective with the AUC of 0.86 – 0.88 [15,16] and sensitivity/ specificity of over 65% [14]. Unfortunately, the use of this less complex system has been questioned because testing was conducted on a sample that included 8% of post-operative neurosurgical patients only [15].
Therefore, this retrospective cohort study was performed to identify parameters associated with hospital mortality and their predictive performance in patients of a large single tertiary neurosurgical ICU.
Materials and methods
This study had been registered by the Thai Clinical Trials Registry with the identification number TCTR 20151012001. Approval for the study (No. 10/ 2555) was received from the Prasat Neurological Institutional Ethics Committee (Chairman: Suchart Hanchaipiboonkul) on Feb 8, 2012, and written informed consent was obtained from all patients or legal relatives in case of unconsciousness. All post-operative neurosurgical patients who met ICU admission criteria [17] by having impaired level of consciousness, impaired airway protection postoperatively, progressive respiratory impairment or the need for mechanical ventilation, seizures, clinical or other evidence of raised intracerebral pressure, threatening intraoperative medical complications, or the need for monitoring in a neurosurgical intensive care unit at Prasat Neurological Institute, Bangkok, during February 1 – July 31, 2011 were included consecutively. Demographics and parameters including body temperature, mean arterial pressure, heart rate, respiratory rate, arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), arterial pH, serum sodium, serum potassium, BUN, creatinine, hematocrit, white blood cell count, Glasgow Coma Scale score (scored 1 on the verbal subscale if the patient was intubated), 24-hour urine output recorded until the time of assessment, blood glucose, albumin, bilirubin and duration of hospital stay before ICU admission were collected at baseline within 30 min after admission by certified neurosurgical registrar nurses. The clinical parameters were measured by a patient monitoring system (Drager, Infinity Delta XL, Germany, 2011) and laboratory testing was done with a standardized automatic machine (Beckman Coulter hematology analyzer, XLH 780, USA 2011; Beckman Coulter chemistry analyzer, Unicel DXC 800, USA, 2010). Hospital death was derived from medical records with the Glasgow Outcome Scale at the day of discharge.
For demographic data, descriptive statistics were analyzed and reported as mean, stanard deviation (SD), median, minimum-maximum, 95% confidence interval (95% CI), number and percent. Logistic regression model was calculated using Stata software version 13.1 (Texas, USA, 2013) to determine the association between the measured parameters and mortality. The values werepresented as odds ratio and 95% CI. The area under the receiver operating characteristic curve (AUC) was analyzed to demonstrate the performance of the parameters and hospital mortality.
Results
258 patients fulfilling the admission criteria were enrolled. The mean age was 47.90 ± 15.11 years while the APACHE II score was 16.54 ± 5.85. The mean duration of surgery was 192.23 ± 87.57 min and the procedures were done as followed: craniotomy with lesion removal in 187 (72.48%) cases, craniotomy with vascular clipping in 15 (5.81%) cases, spinal surgery in 15 (5.81%) cases and other procedure in 41 (15.89%) cases. Overall mortality was 9 (3.49%) and there were no deaths during ICU admission. The mean ± SD and median (min. – max.) duration of patient stay in ICU was 2.36 ± 2.19 days and 2 (1 – 25) days. The median (min. – max.) length of hospitalization before ICU admission was 3 (0 – 97) days. Patient demographics and characteristics are shown in Tab. 1.
Tab. 1. Demographics and patient characteristics. 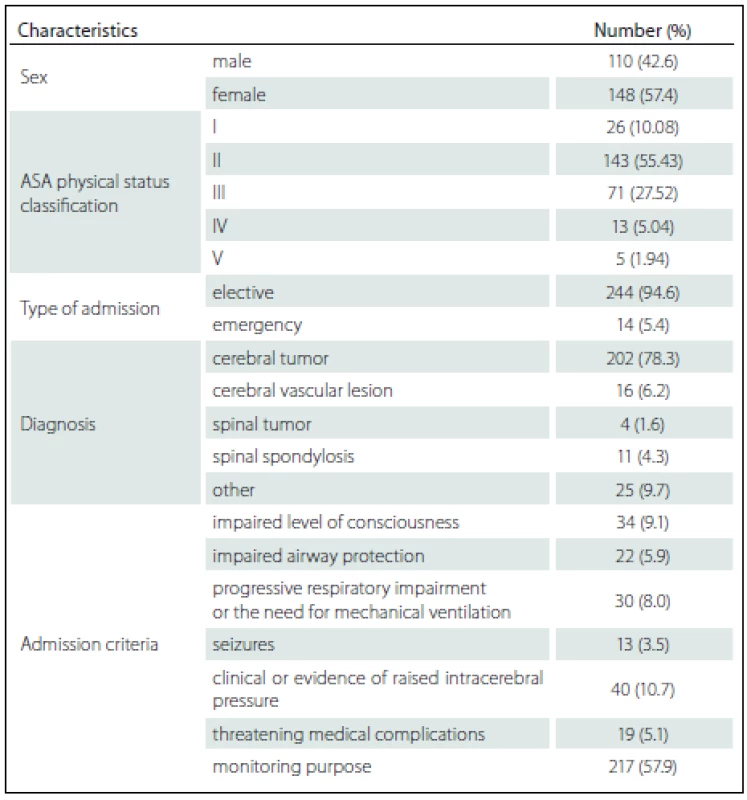
ASA – American Society of Anesthesiologists. Among the 258 patients, the incidences of comorbidities, i.e. renal failure, acquired immune deficiency syndrome (AIDS), hepatic failure, lymphoma, metastatic cancer, leukemia, compromised immune system and cirrhosis were unidentified. The bivariate and multivariate analysis of parameters that possibly affected hospital mortality are presented in Tab. 2 and Tab. 3, resp. Crude odds ratio (95% CI) for type of admission (elective vs. emergency cases) was 2.27 (0.26 – 19.53; p = 0.497) and it was 0.87 (0.46 – 1.65; p = 0.660) for the comparison between diagnoses (cerebral tumor vs. other).
Tab. 2. Bivariated analysis of study variables categorized in tertiles on hospital mortality. 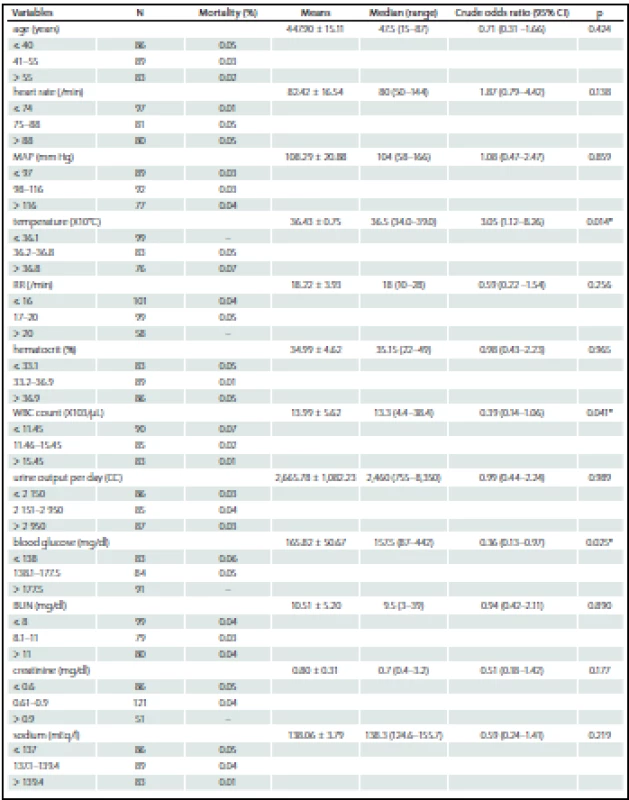

N – number, MAP – mean arterial pressure, RR – respiratory rate, GCS – Glasgow coma score, GCSe – eye subscale in Glasgow coma scale score, GCSv – verbal subscale in Glasgow coma scale score, GCSm – motor scale in Glasgow coma scale score, ICU – intensive care unit. *Statistical signifi cance at p < 0.05. Tab. 3. Multivariated analysis of study variables on hospital mortality. 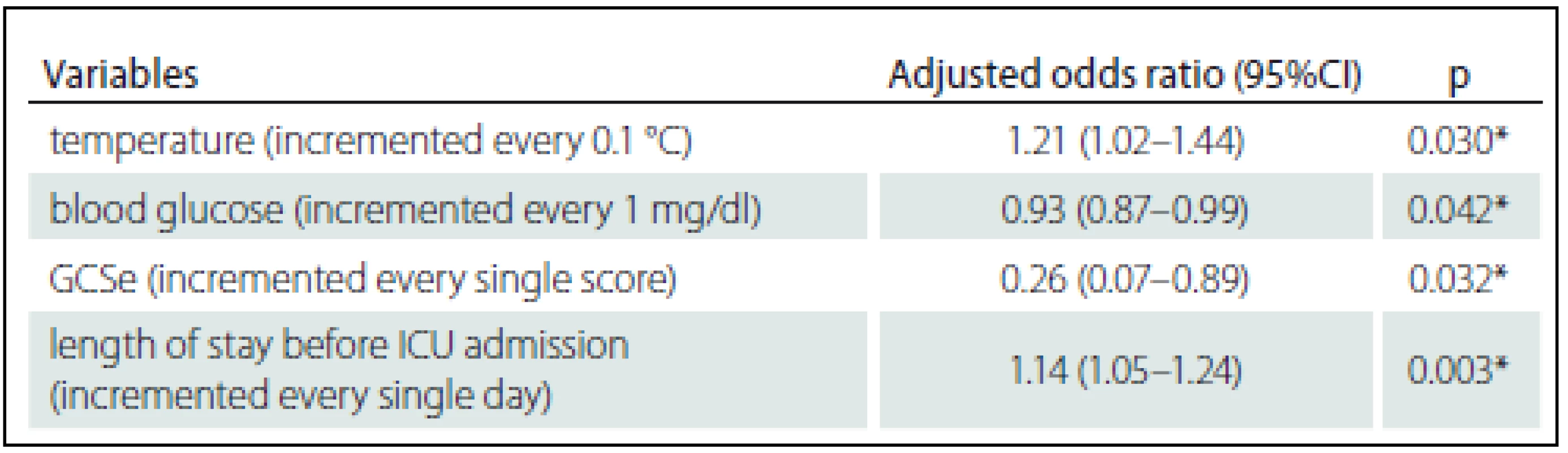
GCSe – Eye subscale in Glasgow Coma Scale score, ICU – intensive care unit. * Statistical significance at p < 0.05. The AUC (95% CI) for the impact of body temperature, blood glucose, eye subscale of the Glasgow Coma Scale and duration of hospitalization prior to ICU admission on hospital mortality was 0.968 (0.923 – 1.000) as shown in Graph 1.
Graph. 1. The receiver operating characteristic curve of the model containing body temperature, blood glucose, eye subscale in Glasgow coma scale score and length of stay before ICU admission to the hospital mortality. 
Discussion
Mortality prediction models for neurosurgical patients have not yet been established. Various scores have good discrimination in neurosurgical patients but have limited usage in this specific group of patients. The study variables included in our analysis were selected from the process of critical literature reviews that included both neurological and non-neurological searches. The radiologic finding was the abundance for data collection because of the time limited for primary evaluation. Together with the length of ventilator days and pathological report, radiological examination was initially considered for inclusion in the predictive model but the evidence showed to be insufficiently robust [18].
In this study, body temperature, duration of hospitalization prior to ICU admission, blood glucose and Glasgow Coma Scale eye subscale were demonstrated as the parameters affected to the mortality. The AUC statistics were not as strong as that for the complex scoring system but these can represent specifically well for the post-operative neurosurgical patients.
Mortality in the majority of neurosurgical patient is the consequence of primary neuronal damage but secondary insults from cascade events including brain edema, ischemia, excitotoxicity and dysregulation of homeostasis leading to cell death may occur [19]. Hyperthermia has for many years been known to have this harmful effect [20,21]. The odds ratio of 0.43 was reported for the association between lower body temperature and favorable outcome in severe stroke patient [22]. Additionally, many authors suggest that body temperature as well as any other vital signs, oxygenation and ventilation should be monitored and managed as part of immediate intensive care in traumatic brain injury patients, particularly hemorrhage stroke and cerebral infarction subgroups [23 – 25]. Interestingly, in another study of neurosurgical patients, duration of hospital stay before ICU admission and duration of ICU stay in non-survivors were longer than that of survivors [26]. The severe grading derangement in physiology and pathology which possibly lead to acute clinical deterioration among the critically ill patients needed more time to optimize, correct and manage, were explained. However, the unavoidable morbidity associated with patients’ condition might occur and result in mortality in the non-survivors group.
In our study, blood glucose level was a factor affecting mortality but had lower predictive power than in other subgroups of traumatic brain injury, or intracerebral hemorrhage with severe brain injury [27 – 29]. The impact of low mortality rate has previously been mentioned. Despite the statistically significant difference in blood glucose levels between survivors (168 ± 73.6 mg/ %) and non-survivors (192 ± 101.6 mg/ %) reported by Ramesh et al., multivariable analysis did not provide statistically significant findings [30]. However, the report of Natarajan et al which studied in neuromedical ICU especially in acute stroke patient, the initial blood glucose of over 115 mg/ % was the main predictor of death and poor outcome at 90 days after discharge [31].
There were some studies showed the evidence of satisfaction of Glasgow Coma Scale score for the mortality prediction because of its simple and effective [11,15] but the authors discovered the inferiority in this study. The pitfall has been mentioned about its exclusion of the clinical brainstem indicators, furthermore, in some situation the score may possibly be obscured by intubation, aphasia or even language barrier [32] similar to our finding. The confrontation and the utilization with this issue should be concerned.
The limitations of our study to be realized were the low mortality rate in this specific group of patients even the study was performed in a largest neurosurgical institute of Thailand. By the way, the multicenter study may strengthen more appropriated model to this prediction. Secondly, most of the patients recruited were diagnosed cerebral tumor, only few were intracranial vascular lesion and none was traumatic brain injuries. Therefore the physiology, pathology, criteria of ICU admission and the withdrawal of treatment policy difference should be taken to account before generalized these results to any circumstances.
In conclusion, it should be emphasized that the accurate mortality prediction tools in critically ill neurosurgical patient is still needed. Body temperature, length of hospitalization before ICU admission, blood glucose and GCS eye subscale are the acceptable predictive variables for hospital mortality. The ongoing improvement of evidence based and economic concerned for the mortality predictor as well as the appropriated models should be continued to counterclaim the effectiveness and efficacy in patient care worldwide.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Phuping Akavipat, MD, FRCAT, MSc
Anesthesiology Department
Prasat Neurological Institute
312 Rajvithee road
104 00 Bangkok
Thailand
e-mail: ppakvp@hotmail.com
Accepted for review: 22. 9. 2016
Accepted for print: 13. 12. 2016
Zdroje
1. Barie PS, Ho VP. The value of critical care. Surg Clin North Am 2012;92(6):1445 – 62. doi: 10.1016/ j.suc.2012.09.001.
2. Burns GB, Hogue V. WellStar Paulding Hospital intensive care unit case study: achieving a research-based, patient-centered design using a collaborative process. Crit Care Nurs Q 2014;37(1):93 – 102. doi: 10.1097/ CNQ.0000000000000008.
3. Wetzel RC, Sachedeva R, Rice TB. Are all ICUs the same? Paediatr Anaesth 2011;21(7):787 – 93. doi: 10.1111/ j.1460-9592.2011.03595.x.
4. McClain CD, Soriano SG. Anesthesia for intracranial surgery in infants and children. Curr Opin Anaesthesiol 2014;27(5):465 – 9. doi: 10.1097/ ACO.0000000000000112.
5. Chowdhury T, Prabhakar H, Bithal PK, et al. Immediate postoperative complications in transsphenoidal pituitary surgery: a prospective study. Saudi J Anaesth 2014;8(3):335 – 41. doi: 10.4103/ 1658-354X.136424.
6. Ibrahim GM, Morgan BR, Macdonald RL. Patient phenotypes associated with outcomes after aneurysmal subarachnoid hemorrhage: a principal component analysis. Stroke 2014;45(3):670 – 6. doi: 10.1161/ STROKEAHA.113.003078.
7. Souter MJ, Manno EM. Ventilatory management and extubation criteria of the neurological/ neurosurgical patient. Neurohospitalist 2013;3(1):39 – 45. doi: 10.1177/ 1941874412463944.
8. Klimek M, Ubben JF, Ammann J, et al. Pain in neurosurgically treated patients: a prospective observational study. J Neurosurg 2006;104(3):350 – 9.
9. Qiao Q, Lu G, Li M, et al. Prediction of outcome in critically ill elderly patients using APACHE II and SOFA scores. J Int Med Res 2012;40(3):1114 – 21.
10. Fadaizadeh L, Tamadon R, Saeedfar K, et al. Performance assessment of Acute Physiology and Chronic Health Evaluation II and Simplified Acute Physiology Score II in a referral respiratory intensive care unit in Iran. Acta Anaesthesiol Taiwan 2012;50(2):59 – 62. doi: 10.1016/ j.aat.2012.05.004.
11. Zali AR, Seddighi AS, Seddighi A, et al. Comparison of the acute physiology and chronic health evaluation score (APACHE) II with GCS in predicting hospital mortality of neurosurgical intensive care unit patients. Glob J Health Sci 2012;4(3):179 – 84. doi: 10.5539/ gjhs.v4n3p179.
12. Moon BH, Park SK, Jang DK, et al. Use of APACHE II and SAPS II to predict mortality for hemorrhagic and ischemic stroke patients. J Clin Neuroscience 2014;22(1):111 – 5. doi: 10.1016/ j.jocn.2014.05.031.
13. Su Y, Wang M, Liu Y, et al. Module modified acute physiology and chronic health evaluation II: predicting the mortality of neuro-critical disease. Neurol Res 2014;36(12):1099 – 105. doi: 10.1179/ 1743132814Y.0000000395.
14. Zhao XX, Su YY, Wang M, et al. Evaluation of neurointensive care unit performance in China: predicting outcomes of Simplified Acute Physiology Score II or Glasgow Coma Scale. Chin Med J (Engl) 2013;126(6):1132 – 7.
15. Ting HW, Chen MS, Hsieh YC, et al. Good mortality prediction by Glasgow Coma Scale for neurosurgical patients. J Chin Med Assoc 2010;73(3):139 – 43. doi: 10.1016/ S1726-4901(10)70028-9.
16. Dalgic A, Ergungor FM, Becan T, et al. The revised Acute Physiology and Chronic Health Evaluation System (APACHE II) is more effective than the Glasgow Coma Scale for prediction of mortality in head-injured patients with systemic trauma. Ulus Travma Acil Cerrahi Derg 2009;15(5):453 – 8.
17. Howard RS, Kullmann DM, Hirsch NP. Admission to neurological intensive care: who, when, and why? J Neurol Neurosurg Psychiatry 2003;74 Suppl 3:iii2 – 9.
18. Kiphuth IC, Schellinger PD, Kohrmann M, et al. Predictors for good functional outcome after neurocritical care. Crit Care 2010;14(4):R136.
19. Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries: Positive or negative effects. Neural Regen Res 2013;8(28):2677 – 86. doi: 10.3969/ j.issn.1673-5374.2013.28.010.
20. Campos F, Blanco M, Barral D, et al. Influence of temperature on ischemic brain: basic and clinical principles. Neurochem Int 2012;60(5):495 – 505.
21. Stetler RA, Leak RK, Gan Y, et al. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol 2014;114 : 58 – 83. doi: 10.1016/ j.pneurobio.2013.11.005.
22. Bill O, Zufferey P, Faouzi M, et al. Severe stroke: patient profile and predictors of favorable outcome. J Thromb Haemost 2013;11(1):92 – 9. doi: 10.1111/ jth.12066.
23. Seo W, Oh H. Comparisons of acute physiological parameters influencing outcome in patients with traumatic brain injury and hemorrhagic stroke. Worldviews Evid Based Nurs 2009;6(1):36 – 43. doi: 10.1111/ j.1741-6787.2008.00139.x.
24. Wijdicks EF, Sheth KN, Carter BS, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke 2014;45(4):1222 – 38. doi: 10.1161/ 01.str.0000441965.15164.d6.
25. Devos D, Sevin M, De Gaalon S, et al. Management of ischemic stroke in the hyperacute phase. Panminerva Med 2013;55(1):59 – 78.
26. Witiw CD, Ibrahim GM, Fallah A, et al. Early predictors of prolonged stay in a critical care unit following aneurysmal subarachnoid hemorrhage. Neurocrit Care 2013;18(3):291 – 7. doi: 10.1007/ s12028-013-9815-4.
27. Nelson DW, Rudehill A, MacCallum RM, et al. Multivariate outcome prediction in traumatic brain injury with focus on laboratory values. J Neurotrauma 2012;29(17):2613 – 24. doi: 10.1089/ neu.2012.2468.
28. Olivecrona M, Koskinen LO. The IMPACT prognosis calculator used in patients with severe traumatic brain injury treated with an ICP-targeted therapy. Acta Neurochir 2012;154(9):1567 – 73. doi: 10.1007/ s00701-012-1351-z.
29. Li Q, Qin XY, Zhang JH, et al. Prognosis study of 324 cases with spontaneous intracerebral hemorrhage in Chongqing, China. Acta Neurochir Suppl 2011;111 : 399 – 402. doi: 10.1007/ 978-3-7091-0693-8_68.
30. Ramesh VJ, Umamaheswara Rao GS, Kandavel T, et al. Predictive model for survival among neurosurgical intensive care patients. J Neurosurg Anesthesiol 2011;23(3):183 – 7. doi: 10.1097/ ANA.0b013e31821cb9ec.
31. Natarajan SK, Dandona P, Karmon Y, et al. Prediction of adverse outcomes by blood glucose level after endovascular therapy for acute ischemic stroke. J Neurosurg 2011;114(6):1785 – 99. doi: 10.1097/ ANA.0b013e31821cb9ec.
32. Akavipat P, Sookplung P, Kaewsingha P, et al. Prediction of discharge outcome with the full outline of unresponsiveness (FOUR) score in neurosurgical patients. Acta Med Okayama 2011;65(3):205 – 10.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek AtaxiaČlánek EditorialČlánek Analýza dat v neurologiiČlánek Recenze knihČlánek Výbor Cerebrovaskulární sekce ČNS spolupořádal v Praze European Stroke Organisation ConferenceČlánek Comment of Article The Effect of Different Occupational Therapy Techniques on Post-stroke Patients
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2017 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Ataxia
- Editorial
- Patient with Hemiplegia Should be Transported Right to the Cerebrovascular Center
- Patient with Hemiplegia Should not be Transported Right to the Cerebrovascular Center
- Should be Patient with Hemiplegia Transported Right to the Cerebrovascular Center?
- Cognitive Functions in Low-grade Glioma Patients – a Systematic Review
- Clinical Importance of Radiological Parameters in Lumbar Spinal Stenosis
- Neurosonological Markers Predict ing Cognitive Deterioration
- Czech National Guillain-Barré Syndrome Registry
- The Role of Drug-induced Sleep Endoscopy in Treatment (Surgical and Non-surgical) in Patients with Obstructive Sleep Apnea
- Prevalence of Martin-Gruber Anastomosis – an Electrophysiological Study
- Nerve Injuries in Supracondylar Humeral Fractures in Children
- A Comprehensive Nationwide Evaluation of Stroke Centres in the Czech Republic Performing Mechanical Thrombectomy in Acute Stroke in 2016
- Mortality Prediction in a Neurosurgical Intensive Care Unit
- Clinical View of the Otorhinolaryngologist and Radiologist on the Classification of Fractures of the Temporal Bone
- The Effect of Different Occupational Therapy Techniques on Post-stroke Patients
- Comment of Article The Effect of Different Occupational Therapy Techniques on Post-stroke Patients
- Dissection of All Four Cervical Arteries in a Patient with Fibromuscular Dysplasia – a Case Report
- Intravenous Thrombolysis after Dabigatran Reversal with a Specific Antidote Idarucizumab
- Experience with using the RevoLix Jr thulium laser – Case Reports
- The Czech Pneumological and Physiological Society and the Czech Society for Paediatric Pulmonology Guidelines for Long-term Home Treatment Using the CoughAssist Machine in Patients with Serious Cough Disorders
- Analýza dat v neurologii
- Recenze knih
- Výbor Cerebrovaskulární sekce ČNS spolupořádal v Praze European Stroke Organisation Conference
- Cena za přínos péči o cévní mozkové příhody doc. MUDr. Robertu Mikulíkovi, Ph.D., FESO
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Czech National Guillain-Barré Syndrome Registry
- Clinical View of the Otorhinolaryngologist and Radiologist on the Classification of Fractures of the Temporal Bone
- The Czech Pneumological and Physiological Society and the Czech Society for Paediatric Pulmonology Guidelines for Long-term Home Treatment Using the CoughAssist Machine in Patients with Serious Cough Disorders
- Nerve Injuries in Supracondylar Humeral Fractures in Children
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



