-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Acute mesenteric ischemia
Akútna mezenteriálna ischemia
Akútna mezenteriálna ischemia (AMI) je akútne život ohrozujúce ochorenie s vysokou mortalitou, vyžadujúce včasné rozpoznanie a rýchle zahájenie liečby skôr, ako dôjde k vzniku črevnej infarzácie. AMI môže byť spôsobená okluzívnou alebo neokluzívnou obštrukciou arteriálneho alebo venózneho cievneho zásobenia. Klinické prejavy môžu byť rôzne, od pomalého nástupu difúznej bolesti brucha až po náhly vznik kruhých a trvalých bolestí. Klinické podozrenie na AMI si vyžaduje včasnú rádiologickú diagnostiku použitím CT angiografie, konvenčnej angiografie alebo exploratívnej chirurgickej intervencie u pacientov s príznakmi peritoneálneho dráždenia. Cieľom liečby u pacientov s AMI je čo najrýchlejšie obnovenie krvného zásobenia čreva. Liečba spočíva v mezenteriálnej revaskularizácii a resekcii nekrotických častí čreva.
Kľúčové slová:
akútna mezenteriálna ischemia – CT angiografia – mezenteriálna revaskularizácia
Authors: K. Radwan; M. Bátovský
Authors place of work: Gastroenterologická klinika SZU a UNB Bratislava
Published in the journal: Gastroent Hepatol 2011; 65(1): 9-14
Category: Klinická a experimentální gastroenterologie: přehledová práce
Summary
Acute mesenteric ischemia (AMI) is a medical and surgical emergency. It remains a challenging diagnosis with a high mortality rate, and requires early recognition and prompt treatment before the onset of bowel infarction. Acute mesenteric insufficiency can be due to occlusive or non-occlusive obstruction of arterial or venous blood supply. Symptoms vary from the insidious onset of vague generalized abdominal pain to the sudden onset of diffuse, severe constant pain. Clinical suspicion of AMI necessitates early radiologic evaluation (computed tomographic (CT) angiography, conventional angiography) or exploratory surgery in patients with peritoneal signs. The purpose of treatment of patients with acute mesenteric ischemia is to restore intestinal blood supply as quickly as possible. Treatment options include resuscitation, mesenteric revascularization and resection of necrotic bowel.
Key words:
acute mesenteric ischemia – CT angiography – mesenteric revascularizationIntroduction
Acute mesenteric ischemia can be defined as a sudden onset of intestinal hypoperfusion, most commonly as a result of occlusion, vasospasm or reduction of the mesenteric circulation. The incidence of AMI has increased over the past 20 years due to longer life expectancies, increased awareness of ischemic syndromes, and enhanced diagnostic and therapeutic techniques. Specific risk factors include advanced age, atherosclerosis, low cardiac output states, cardiac arrhythmias, sever cardiac valvular disease, recent myocardial infarction and intra-abdominal malignancy. Clinical presentation is non-specific in most cases and can be characterized by an initial discrepancy between severe abdominal pain and minimal clinical findings. Complications such as ileus, peritonitis, pancreatitis, and gastrointestinal bleeding may also mask the initial signs and symptoms of AMI.
Practically, AMI can be divided into four different primary clinical entities [1]
acute mesenteric arterial embolus (AMAI) – 50%
acute mesenteric arterial thrombosis (AMAT) – 15 to 25%
non-occlusive mesenteric ischemia (NOMI) – 20 to 25%
mesenteric venous thrombosis (MVT) – 5 to 10%Mesenteric vascular supply
The vascular supply to the intestine includes
- Celiac artery: arises from the anterior aspect of abdominal aorta, and supplies blood to the foregut (stomach, duodenum, hepatobiliary system, and spleen).
- Superior mesenteric artery (SMA): arises approximately 1 cm below celiac artery, and supplies blood to the midgut (small intestine from the distal duodenum to the mid-transverse colon).
- Inferior mesenteric artery (IMA): arises approximately 6–7 cm below the superior mesenteric artery, and supplies blood to hindgut (from transverse colon to the rectum).
Important collaterals exist between branches of the major vessels. They provide a substational protection from ischemia or infarction in the sitting of vascular occlusion.
The major collateral connections are
- celiac axis and superior mesenteric artery: communicate through the junction of the superior and inferior pancreatoduodenal artery
- superior mesenteric artery and inferior mesenteric artery: communicate through anastomose of middle colic and left colic artery
- collateralization of inferior mesenteric artery with systemic circulation occurs in rectum as superior rectal vessels merge with inferior rectal vessels
However, these collaterals are not sufficient to prevent ischemia if the SMA is acutely occluded. But in a chronic progressive atherosclerotic occlusive disease, they can steadily increase in size to prevent ischemia.
Pathophysiology
Splanchnic circulation receives approximately 25% of the resting cardiac output but may, on occasion, exceed 30%. About 70% of splanchnic inflow goes to the mucosa, which is the most metabolically active area of the gut. Intestinal blood flow is under complex regulation controlled primarily by resistance arterioles and precapillary sphincters. Numerous extrinsic and intrinsic factors influence the splanchnic circulation [2].
Ischemic injury of intestines occurs when there is insufficient supply of oxygen and nutrients required for cellular metabolism. A sudden onset of major vessel occlusion would result in almost immediately opening of collateral circulation. However, after several hours, progressive vasoconstriction develops in the obstructed bed, increasing its pressure and thereby reducing collateral flow. Vasoconstriction may remain present even after blood flow has restored leading to a continued intestinal ischemia.
Mesenteric arterial embolism
Embolism to mesenteric arteries is most frequently due to dislodged thrombus originating from the left atrium in patients with atrial fibrillation, an akinetik or aneurysmal portion of left ventricle after myocardial infarction, or less frequently from cardiac valves in patients with bacterial endocarditis. Embolization to the SMA is the most frequent cause of AMI, accounting for about half of all cases [1]. One third of patients have a history of prior embolic event. Over 20% of acute mesenteric emboli are multiple.
In response to acute occlusion, vasoconstriction may ensue, further compromising arterial perfusion and exacerbating the ischemic injury. The middle segment of the jejunum is most often involved in the ischemic process, as it is most distant from collateral circulation of the celiac and inferior arteries.
Mesenteric arterial thrombosis
Arterial thrombosis accounts for about 15% of cases of acute mesenteric ischemia. It usually occurs in patients with history of chronic intestinal ischemia from progressive atherosclerotic stenosis. Atherosclerotic occlusive lesions tend to occur at the origin or very proximal segments of the mesenteric arteries [3]. Typically, the SMA plaque slowly progresses to a critical stenosis over years and the residual lumen suddenly thromboses during a period of relative hypotension or reduced flow. Patients usually have diffuse atherosclerotic disease with prior coronary, cerebrovascular or peripheral arterial insufficiency. Although chronic atherosclerotic disease is the most common cause of acute mesenteric arterial thrombosis, other causes should be kept in mind. AMI may result from arterial dissection producing the malperfusion syndrome. Fibromuscular dystrophy and Takayasu’s arteries may be also associated with an acute mesenteric arterial occlusion. Rarely, arterial thrombosis may occur in vessels with no or minimal preexisting disease secondary to an underlying hypercoagulable state. It can also occur in the setting of abdominal trauma or infection.
Mesenteric venous thrombosis
Thrombosis of the SMV accounts for 5–10% of all cases of AMI. The clinical presentation of MVT can be acute, characterized by the sudden onset of symptoms; subacute, in which symptoms occur for days or weeks without bowel infarction; or chronic, involving portal or splenic vein thrombosis and stigmata of portal hypertension with or without variceal bleeding. The risk factors and causes of SMVT are summarized in tab. 1.
Tab. 1. Factors influencing the splanchnic circulation. Tab. 1. Faktory ovplyvňujúce splanchnickú cirkuláciu. 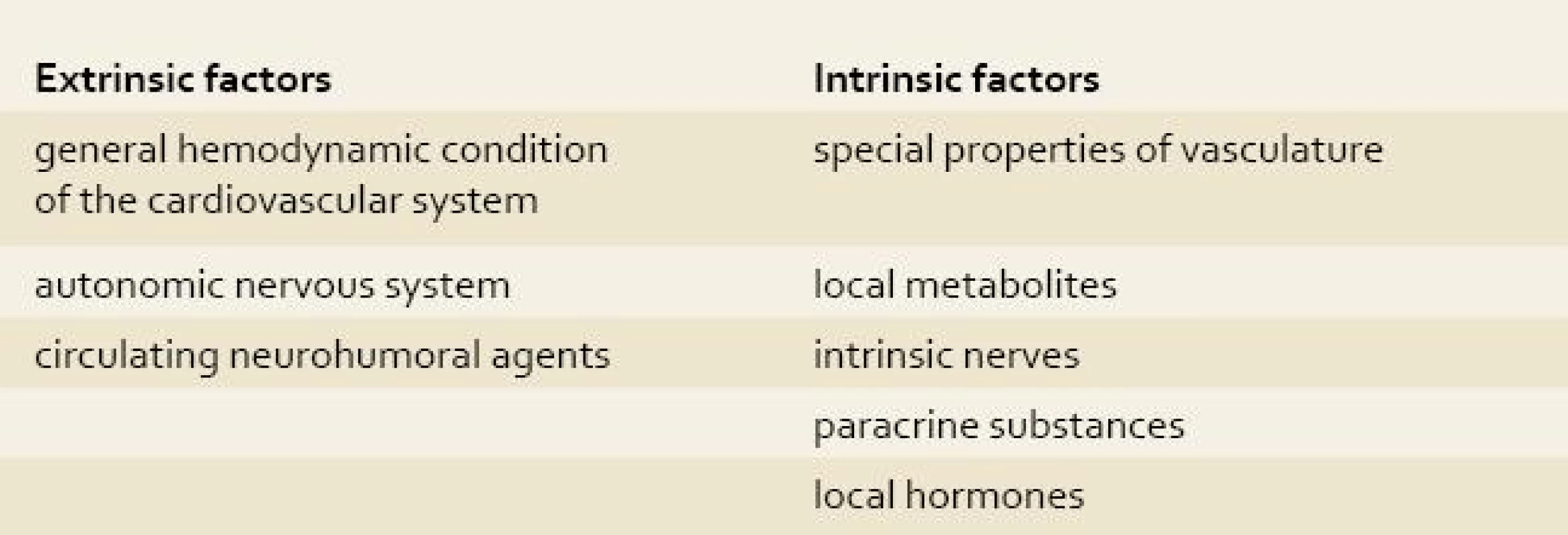
MVT leads to a resistance to mesenteric venous blood flow, with resultant bowel wall edema, fluid efflux into the bowel lumen, systemic hypotension and hemoconcentration, which can impair arterial blood flow, leading to a submucosal hemorrhage and bowel infarction [4]. Eventual focal hemorrhage and necrosis lead to loss of the gut barrier function, which allows for bacterial translocation and possible endotoxemia.
Non-occlusive mesenteric ischemia
NOMI may account for as many as 20% of all AMI cases. It occurs in the absence of anatomic arterial occlusion or venous thrombosis as a result of mesenteric vasospasm and splanchnic hypoperfusion [5]. Typically, NOMI develop in elderly patients with diffuse vascular disease, but it can also be seen in patients with vasculitis or who are on vasoconstrictions medications. Predisposing factors include conditions such as myocardial infarction with decreased cardiac output, congestive heart failure, cardiac arrhythmias, sepsis, dehydration, shock and administration of medications such as diuretics, digoxin and alpha-adrenergic agonists. Non-occlusive mesenteric ischemia has been reported following cardiac surgery, dialysis, and cocaine use [6,7]. The vasoconstriction is produced by sympathetic activity possibly mediated by vasopressin and angiotensin. This pathophysiological process can persist and perpetuate the ischemic injury even after normal flow is restored and the underlying cause is corrected.
The mortality rate from NOMI is high for several reasons, including advanced patient age, comorbidities, and difficulty in making the diagnosis and revising ischemia once it has started.
Clinical presentation
Typically, patients with AMI present with severe, acute, unremitting abdominal pain out of proportion to physical findings, and are commonly manifest after embolic or thrombotic occlusion of SMA. The pain is initially colicky but becomes constant with progression of ischemia. It may be diffuse or localized to any quadrant of the abdomen. Diarrhea with blood, mucus and melena may ensue, and usually signifies severe ischemia or bowel infarction. Feculent breath may herald intestinal necrosis.
Tab. 2. Causes of superior mesenteric vein thrombosis. Tab. 2. Príčiny trombózy hornej mezenterickej žily. 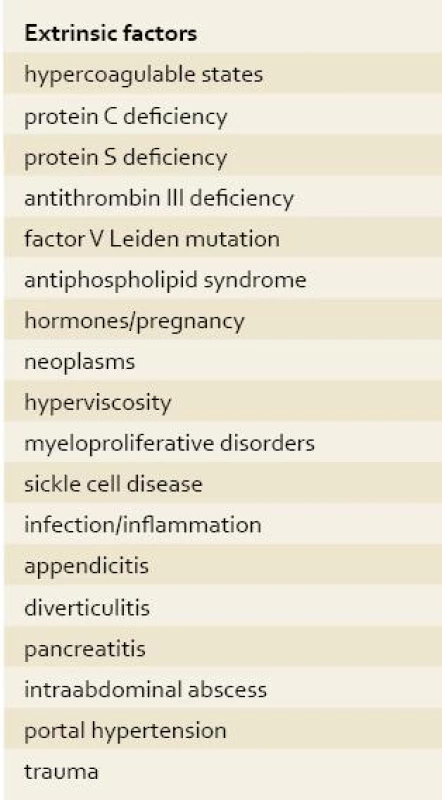
The presentation may be more insidious with mesenteric vein thrombosis in which symptoms may have been present for weeks to months (typically 5–14 days) before diagnosis [4,8]. Fever, nausea, vomiting and diminished bowel sounds are other common but non-specific manifestations of AMI. Diagnosis of NOMI is difficult because many of these patients are already hospitalized for treatment of other illnesses, and initial clinical manifestations of AMI maybe masked. Abdominal pain is absent in up to 25% of patients with NOMI. Gastrointestinal symptoms maybe non-specific and present as constipation, loss of appetite, nausea, and vomiting. Diffuse or localized abdominal tenderness, rebound and rigidity are warning signs and usually herald transmural bowel infarction. Bowel infarction also leads to hypotension from fluid loss and septicemia, decreased urine output from hypovolemia and renal hypoperfusion, and hyperventilation from hypoxia and acidosis [9]. Mental status changes can be found in one-third of elderly patients with NOMI [10]. A diagnosis of NOMI should be considered in all patients with risk factors, if the patient complains about gradually increasing abdominal discomfort with no other obvious cause.
Laboratory findings
Although laboratory studies are non--specific abnormal values maybe helpful in blustering suspicion of AMI. A complete blood count with differential, electrolyte panel, coagulation studies, live function tests and amylase levels should be drawn in any patient suspected of experiencing an acute abdominal process, including AMI. The onset of ischemia often produces a profound leukocytosis with peripheral WBC elevation in excess of 20,000. Serum lactate elevation and metabolic acidosis are later findings, and consistent with more advanced intestinal ischemia and, most likely, non-viable bowel. Elevation of serum phosphate, alkaline phosphatase levels, as well as prerenal azotemia, hypoxemia and bacteremia are associated with intestinal necrosis.
Normal D-dimer levels may help to exclude acute intestinal ischemia, but elevated levels are less helpful for its diagnosis. Elevated D-dimer levels can also be associated with a variety of conditions such as in patients with acute pancreatitis and those with an abdominal aortic aneurysm [11]. Levels of serum alpha-glutathione S-transferase (alpha-GST) and intestine fatty acid-binding protein (I-FABP) levels have been detected in a few patients with AMI. However, there are no reliable data on sensitivity and specificity of these serum markers [12].
Imaging studies
Plain abdominal radiographs should be obtained early in the elevation of patients with suspected AMI. They are most helpful to exclude other potential causes of abdominal pain, such as a perforate viscus, small or large obstruction, or gallstones. In about 25% of patients with AMI, no abnormalities have been reported at all [13].
Suggestive findings include the presence of an ileus with distended loops of bowel, “thumbprinting”, or bowel wall thickening resulting from edema or hemorrhage, and/or pneumatosis intestinalis. Intraluminal barium studies are contraindicated because residual contrast can limit visualization on angiography. Doppler-flow ultrasonography may be of some benefit in visualizing flow in SMA and celiac axis. It can detect proximal stenosis or complete occlusion of SMA and celiac axis with high specificity (92–100%) but low sensitivity (70–89%) [13]. However, the test is technically limited by the presence of air-filled loops of distended bowel. Computed tomography (CT) of the abdomen has an important role in the evaluation of patients with acute abdominal pain. It can detect focal or segmental bowel wall thickening or intestinal pneumatosis with portal vein gas. Among patients with AMI secondary to arterial thrombosis or embolism, however, the CT scan may be normal or non-diagnostic. In a series of 39 patients, the findings of at least one of number of signs, including arterial or venous thrombosis, intramural gas, portal venous gas, lack of bowel enhancement, or liver and spleen infarcts on dynamic scanning, resulted in a sensitivity of 64% and specificity of 92% [14]. In other studies, the sensitivity of CT for the diagnosis of mesenteric venous thrombosis was approximately 90% [15]. The superior mesenteric or portal vein appears large, with a central area of low attenuation, suggestive of thrombus.
Magnetic resonance angiography (MRA) and the newest generation of CT angiography, known as multidetector row CT (MDCT), provide more detailed information about mesenteric vessels and small bowel. Multidetector row CT provides even a better chance of detecting mesenteric ischemia, thanks to increased spatial resolution, the high quality three dimensional (3D) reconstructions, and the shorter examination times. It has thus confirmed a fundamental role for CT in the diagnosis of AMI, achieving a sensitivity of 80–96% and specificity of 94–98% [16,17]. Selective catheter angiography has been the gold standard diagnostic methodology in the evaluation of the patient with AMI. Sensitivities of angiography in 5–6 studies have ranged between 90–100% [18]. Although early angiography will produce a number of negative results, it is essential if diagnoses are not to be made early enough to improve survival [18,19]. On the other hand, angiography may not be as helpful in confirming the diagnosis of MVT, especially if there is segmental venous thrombosis. Perhaps most importantly, arteriography can exclude acute arterial thrombosis, embolism and NOIM.
However, prompt laparotomy should be performed in patients with suspected AMI, in whom expeditious angiography is not available.
Management
General measures
The purpose of treatment of patients with AMI is to restore intestinal blood flow as quickly as possible. Initial management is aimed at resuscitation, which should include aggressive hemodynamic monitoring and support with fluids to replace intravascular fluid loss, and it can be guided by a placement of urinary drainage catheter, as well as central venous catheter or Swang-Ganz catheter in patients with significant cardiac disease. Adequate hydration and optimization of cardiac function are particularly important in NOMI because hypovolemia and hypotension exacerbate mesenteric vasoconstriction. Medications that cause vasoconstriction, such as vasopressin or digitalis, should be avoided if possible, since they can exacerbate mesenteric ischemia. If vasopressors are required, dobutamin or low-dose dopamine are preferred because, in low doses, it may act as a mesenteric vasodilators, and in higher doses, it produces less severe mesenteric vasoconstriction than the latter agents. Electrolyte abnormalities and metabolic acidosis should be corrected. Broad spectrum antibiotics should be administered intravenously because intestinal ischemia promotes bacterial translocation and sepsis. A placement of nasogastric tube is required for gastric decompression because increased intraluminal pressure may decrease mucosal perfusion. Measurement of arterial oxygen concentration will guide the need for oxygen supplementation or mechanical ventilation. The role of anticoagulation is dependent on the etiology of the AMI. Systemic anticoagulation with heparin is acutely indicated to prevent thrombus formation or progression unless patients are actively bleeding. Most of patients will require long-term anticoagulation with warfarin, especially if an underlying hypercoagulability disorder is uncovered [20,21].
Treatment of mesenteric arterial embolism and thrombosis
Various therapeutic options have been proposed for mesenteric arterial ischemia. Patients with signs of peritonitis or clinical suspicion of perforation or gangrene require emergent laparotomy, after hemodynamic stabilization, to restore mesenteric blood flow and resect non-viable bowel. Patients who are hemodynamically stable should undergo angiography to diagnose obstructive lesions and begin treatment with vasodilators, such as papaverine, which when infused directly into mesenteric vessels can help to increase blood flow by revising vasospasm. Once an obstructing lesion is confirmed with angiography, patients can undergo either surgical revascularization (by aorto-mesenteric bypass grafting, embolectomy or thromboendarterectomy), or endovascular revascularization (using balloon dilation and angioplasty with or without stenting, or catheter-directed thrombolytic therapy in selected cases). Long term management is aimed at the prevention of future embolic and thrombotic events, typically with the use of warfarin [4].
Treatment of mesenteric venous thrombosis
The treatment of acute mesenteric venous thrombosis depends on whether intestinal infarction has occurred or it is strongly suspected. Standard treatment for acute MVT includes heparin anticoagulation, laparotomy and resection of infracted bowel.
In patients with clinical and radiologic evidence of MVT, but no infarction, and with a good mesenteric blood flow demonstrated by angiography, conservative management can be attempted using anticoagulation therapy (i.e. heparin). Anticoagulation is usually continued for 6 months or longer if a thrombophilic state has been identified.
The use of thrombolytic agents such as streptokinase, urokinase, and tissue plasminogen activator has been reported in a small number of patients [22].
Treatment of non-occlusive mesenteric ischemia
Management of NOMI is essentially pharmacological, and includes hemodynamic resuscitation, antibiotics, and intra-arterial continuous infusion of vasodilators such as papaverine, which reverses vasoconstriction and restores mesenteric blood flow [1]. Subsequent management is directed by patient’s clinical response to vasodilator therapy. Papaverine infusion is usually continued for approximately 24 hours. Angiogram should be then performed to document the relief of vasospasm. Depending on the clinical course, and the presence or absence of vasoconstriction in the angiogram, papaverine is discontinued or maintained for another 24 hours. Repeated angiograms are performed at 24 hours intervals until radiographic signs of vasoconstriction have disappeared. Antiplatelet agents may be beneficial in the long term management of patients with NOMI [1].
Prognosis
Acute mesenteric ischemia is associated with mortality rate exceeding 60%. Early diagnosis and prompt intervention with angiography, or surgery, or both, are of critical importance for improving outcomes for patients with AMI. Reviewing outcomes of 21 patients with SMA embolism, Lobo Martínez and colleagues found that intestinal viability was 100% in patients whose symptoms duration were less than 12 hours, a 56% when symptoms lasted 12–24hours, and only 18% when symptoms lasted more than 24 hours [11]. Survival was 90% in the setting of early angiography in patients with no peritoneal signs. However, mortality was about 80% among patients, in whom intestinal infarction had already occurred.
Acute mesenteric ischemia is a life-threatening medical and surgical emergency that requires early diagnosis and intervention to adequately restore mesenteric blood flow and to prevent bowel necrosis and patient death. Delay in diagnosis is associated with high mortality. Specific risk factors include advanced age, atherosclerosis, low cardiac output states, cardiac arrhythmias, sever cardiac valvular disease, recent myocardial infarction and intra-abdominal malignancy. Patients often present with abdominal pain out of proportion to physical examination. Complications such as ileus, peritonitis, pancreatitis and gastrointestinal bleeding my also mask the initial signs and symptoms of AMI. The diagnosis of AMI depends upon a high clinical suspicion, especially in patients with known risk factors. However, early signs and symptoms of mesenteric ischemia are nonspecific, and definitive diagnosis often requires invasive testing, exposing the patients who typically have several comorbidities to risk. As a result, the diagnosis is often delayed. Angiography is the diagnostic procedure of choice and is potentially therapeutic. Multidetector-row CT angiography appears to be an acceptable alternative in settings where obtaining early angiography is impractical, or there is only a moderate suspicion for acute intestinal ischemia, based upon the patient‘s clinical presentation. The diagnosis of AMI should be considered in patients > 50 years, who present with sudden onset of severe abdominal pain lasting > 2 hours, especially if a history of cardiovascular disease (congestive heart failure, myocardial infarction, arrhythmia), or predisposing condition (i.e. heritable or acquired hypercoagulable states) is present, and in patients with diffuse atherosclerotic disease under condition of hemodynamic stress (hypotension), in the setting of vasoconstrictive agents (vasopressin, cocaine), and in patients with vasculitis [23]. The goal of treatment of patients with acute mesenteric ischemia is to restore intestinal blood flow as rapidly as possible after initial management that includes aggressive hemodynamic monitoring and support, correction of metabolic acidosis, initiation of broad spectrum antibiotics, and placement of a nasogastric tube for gastric decompression. Interventional infusion therapy may improve survival.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.Khaled Radwan, MD
Gastroenterologic Clinic SMU and UH Bratislava
St Cyril and Method´s University Hospital
Antolská 11, 851 07 Bratislava, SK
batovsky@pe.unb.sk
Zdroje
1. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am 1997; 77(2): 307–318.
2. Cappell MS. Intestinal (mesenteric) vasculopathy. I. Acute superior mesenteric arteriopathy and venopathy. Gastroenterol Clin North Am 1998; 27(4): 783–825.
3. Eldrup-Jorgensen J, Hawkins RE, Bredenberg CE. Abdominal vascular catastrophes. Surg Clin North Am 1997; 77(6): 1305–1320.
4. Ottinger LW. Mesenteric ischemia. N Engl J Med 1982; 307(9): 535–357.
5. Reinus JF, Brandit LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19(2): 319–343.
6. Gearhart SL, Delaney CP, Senagore AJ et al. Prospective assessement of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69(4): 324–329.
7. Gennaro M, Ascer E, Matano R et al. Acute mesenteric ischemia after cardiopulmonary bypass. Am J Surg 1993; 166(2): 231–236.
8. Diamond S, Emmett M, Henrich WL. Bowel infarcion as a cause of death in dialysis patients. JAMA 1986; 256(18): 2545–2547.
9. Wiesner W, Hauser A, Steinbrich W. Accuracy of multidetector row computed tomography for the diagnosis of acute bowel ischemia in a non-steady population. Eur Radiol 2004; 241 : 729.
10. Kirkpatrick ID, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experiance. Radiology 2003; 229(1): 91–98.
11. Block T, Nilsson TK, Bjorck M et al. Diagnostic accuracy of plasma biomarkers for intestinal ischemia. Scand J Clin Lab Invest 2008; 68(3): 242–248.
12. Acosta S, Alhadad A, Svensson P et al. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg 2008; 95(10): 1245–1251.
13. Lobo Mrtínez E, Mero NO Carvajosa E et al. Embolectomy in mesenteric ischemia. Rev Esp Enferm Dig 1993; 83(5): 351–354.
14. Boley SJ, Brandt LJ, Sammartano RJ. History of mesenteric ischemia. The evolution of a diagnosis and management. Surg Clin North Am 1997; 77(2): 275–288.
15. Wilcox MG, Howard TJ, Plaskon LA. Current theories of pathogenesis and treatment of non-occlusive mesenteric ischemia. Dig Dis Sci 1995; 40(4): 709–716.
16. Font VE, Hermann RE, Longworth DL. Chronic mesenteric venous thrombosis: difficult diagnosis and therapy. Cleve Clin J Med 1989; 56(8): 823–828.
17. Kanda T, Fujii H, Tani T et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110(2): 339–343.
18. Smerud MJ, Johnson CD, Stephens DH. Diagnosis of bowel infarction: a comparision of plain films and CT scans in 23 cases. AJR Am J Roentgenol 1990; 154(1): 99–103.
19. Bowersox JC, Zwolak RM, Walsh DB et al. Duplex ultrasonography in the diagnosis of celiac and mesenteric artery occlusive disease. J Vasc Surg 1991; 14(6): 780–786.
20. Taourel PG, Deneuville M, Pradel JA. Acute mesenteric ischemia: Diagnosis with contrast-enhanced CT. Radiology 1996; 199(3): 632–636.
21. Rhee RY, Gloviczki P. Mesenteric venous thrombosis. Surg Clin North Am 1997; 77(2): 327–338.
22. Kaufman SL, Harrington DP, Siegelman SS. Superior mesenteric artery embolization an angiographic emergency. Radiology 1977; 124(3): 625–630.
23. Clark RA, Gallant TE. Acute mesenteric ischemia: angiographic spectrum. AJR Am J Roentgenol 1984; 142(3): 555–562.
24. Brandit LJ, Boyle SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118(5): 954–968.
25. Gallego AM, Ramirez P, Rodriguez JM, et al. Role of urokinase in the superior mesenteric artery embolism. Surgery 1996; 120(1): 111–113.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek Dynamic scintigraphy of the oesophagus in the diagnostics of gastro-oesophageal reflux diseaseČlánek Successful use of OTSC® (Over-The-Scope Clip) in the treatment of iatrogenic colon perforationČlánek Is it possible to reintroduce original biological therapy in a relapsed Crohn’s disease patient?Článek Not only NOTES in BarcelonaČlánek No country for young men
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2011 Číslo 1- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Dynamic scintigraphy of the oesophagus in the diagnostics of gastro-oesophageal reflux disease
- Autoimmune form of chronic pancreatitis and IgG4 positive mastitis
- Cytomegaloviral colitis in organ transplant recipients
- Successful use of OTSC® (Over-The-Scope Clip) in the treatment of iatrogenic colon perforation
- Is it possible to reintroduce original biological therapy in a relapsed Crohn’s disease patient?
- What should be done in patients with inflammatory bowel diseases those lose a response to biological therapy?
- Editor-in-chief´s lead article
- Not only NOTES in Barcelona
- Kalendář gastroenterologických akcí 2011
- No country for young men
- Acute mesenteric ischemia
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegaloviral colitis in organ transplant recipients
- Is it possible to reintroduce original biological therapy in a relapsed Crohn’s disease patient?
- Acute mesenteric ischemia
- Autoimmune form of chronic pancreatitis and IgG4 positive mastitis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



