-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Alkaloids from hydrastidis canadensis and their cholinesterase and prolyl oligopeptidase inhibitory
Authors: A. Hošťálková 1; J. Kuneš 2; K. Macáková 1; M. Hrabinová 3; L. Opletal 1
Authors place of work: ADINACO Research Group, Department of Pharmaceutical Botany and Ecology, Faculty of Pharmacy in Hradec Králové, Charles University in Prague 1; Department of Inorganic and Organic Chemistry, Faculty of Pharmacy in Hradec Králové, Charles University in Prague 2; Centre of Advanced Studies, Faculty of Military Health Sciences, University of Defence 3
Published in the journal: Čes. slov. Farm., 2015; 64, 41-43
Category: Rozšířená abstrakta z konference
Introduction
Alzheimer’s disease (AD) is one of the most prevalent causes of dementia. Treatment of this neurodegenerative disease is only symptomatic and targets single pathogenetic pathways. One of therapeutic approaches is application of central cholinesterases inhibitors alleviating cholinergic deficit in the brain (galanthamine, donepezil)1). Acetylcholinesterase (AChE) cleaves acetylcholine to the basic components and maintains a metabolic balance. Cleavage of acetylcholine is an important factor for the regeneration of neuron. AChE inhibition has become a standard approach for AD treatment2). Butyrylcholinesterase (BuChE), which appears to substitute and supplement AChE function in the brain of AD patients, is a valuable target3).
Prolyl oligopeptidase (POP) is a cytosolic serine peptidase that hydrolyzes proline-containing peptides involved in the learning and memory processes, thus inhibitors could serve as supplementary treatment4).
Hydrastis canadensis L. (Ranunculaceae), also known as goldenseal, is perennial plant which grows wild in North America. The chief components are isoquinoline alkaloids, which can be divided into several structural types. The most abundant are protoberberine-type alkaloids: berberine (the main component), berberastine, canadine, corypalmine and isocorypalmine5). Further isolated alkaloids are: hydrastine, hydrastidine, isohydrastidine (phtalideisoquinoline-type), canadaline6) and canadinic acid (secoberberine-type)7).
Berberine exerts potent AChE and BuChE inhibitory effect with IC50 values ranging between 0.44–1.07 ∝M and 3.44–6.84 μM respectively8) and also inhibits POP with IC50 value of 145 μM).
In the course of screening for natural cholinesterase inhibitors, we found that rhizome extract from H. canadensis showed promising AChE and BuChE inhibition activity with IC50 value of 67.1 ± 11.3μg/ml and 78.9 ± 11.3μg/ml, respectively.
Experimental methods
General – NMR: VNMR S500 spectrometer (Varian, Palo Alto, California, USA), CDCl3 or CD3OD, 25 °C, 499.87 MHz for 1H and 125.70 MHz for 13C; MS (ESI): spectrometer LC/MS Thermo Finnigan LCQDuo (GenTech Scientific, Arcade, New York, USA) with electrospray ionization in positive mode and ion trap as analysator; optical rotatory power: polarimeter P3000 (A. Krüss Optronic, Hamburg, Germany).
Materials – Acetylthiocholine iodide (ATChI), butyrylthiocholine iodide (BuTChI), berberine chloride (> 95%), POP and its substrate, Z-Gly-Pro-p-nitroanilide (≥ 99%) were purchased from Sigma-Aldrich, galanthamine hydrobromide (> 98%) from Changsha Organic Herb Inc., and huperzine A (98%) from Tai’an zhonghui Plant Biochemical Co., Ltd. The red blood cell ghosts used as a source of AChE (HuAChE), and human plasma as a source of BuChE (HuBuChE) were prepared in our laboratory. For TLC, the development solvents used were mixtures of cyclohexane (cHx), toluene (To), chloroform (CHCl3), diethylamine (DEA) and ethanol (EtOH) (Penta, Ing. Švec, Praha, Czech Republic). Dry commercial goldenseal extract was obtained from the dealer Naturex (Wien, Austria). A voucher specimen is deposited at the Department of Pharmaceutical Botany and Ecology, Faculty of Pharmacy in Hradec Králové.
Extraction and isolation – 1033 g dry commercial goldenseal extract was exhaustively extracted with ethanol (EtOH) (96% v/v, 2×) by boiling for 30 min under reflux and the combined extract was filtered and evaporated to dryness under reduced pressure. The crude extract (0.3 kg) was acidified to pH Δ 1.5 with 2% hydrochloric acid (5.8 l), filtered and the filtrate was defatted with diethyl ether (3 × 2 l). Solution was alkalized to pH 9–10 with 10% Na2CO3 and exhaustively extracted with CHCl3 (4× 8.6 l). The organic layer was evaporated to give 33.9 g of solid brown residue. For purification, the residue was treated with liquid-liquid extraction again. The extract (25.0 g), which was Dragendorff positive, was further fractionated by column chromatography on Al2O3, eluting with light petrol gradually enriched with CHCl3 (80 : 20 – 0 : 100), and then CHCl3 enriched with EtOH (99 : 1 – 80 : 20). Fractions of 250 ml were collected and monitored by TLC (To:CHCl3:EtOH:DEA 70 : 20 : 10 : 3; 1×⋅), yielding 129 fractions which were combined into 3 fractions.
Preparative TLC (To:CHCl3:EtOH:DEA 70 : 20 : 10 : 3; 1⋅×) of fraction I (0.976 g) gave two subfractions. Subfraction Ia was treated with preparative TLC (cHx:DEA 95 : 5; 1×) to give compound 1, crystallized from EtOH and CHCl3 mixture (white crystals; 9.3 mg). Subfraction Ib was subjected to preparative TLC (cHx:DEA 95 : 5; 2⋅×) to yield compound 2, crystallized from EtOH (yellowish crystals; 424.1 mg). Fraction II (20.734 g) repeatedly crystallized from EtOH to give compound 3 (white crystals; 16.871 g). Fraction III (0.771 g) was chromatographed by preparative TLC (cHx:DEA 90 : 10; 2⋅×), subfraction IIIb was subsequently treated with preparative TLC (To:CHCl3:DEA 75 : 25 : 5; 1⋅×) to give compound 4, which was crystallized from MeOH (yellowish crystals; 40.2 mg).
Preparation of red blood cells ghosts; Acetylcholinesterase and butyrylcholinesterase assay; Prolyl oligopeptidase assay – The same procedures were used as in our previous report10).
Results and discussion
Extensive chromatographic purification led to isolation of four isoquinoline alkaloids (Figure 1), belonging to four structural types. Their structures were determined by comparison of their MS and NMR spectra and specific optical rotations with literature data. In the present study, we report the first full 1H and 13C NMR assignments and additional physical properties or 1-(6’-allyl-1’,3’-methylenedioxybenzoyl)-3-chloro-5,6-dimethoxy-isoquinoline 1:
Fig. 1. Structures of alkaloids isolated from Hydrastis cana- densis extract 
[α]20D wasn’t determined due to absence of chiral centre. white crystals; 9.3 mg
1H NMR (500 MHz, CDCl3): δ 8,06 (1H, s, H4), 8,01 (1H, d, J = 9,4 Hz, H9), 7,37 (1H, d, J = 9,4 Hz, H8), 7,20 (1H, dd, J = 17,3 Hz, J = 11,0 Hz, H18), 7,08 (1H, s, H14), 6,83 (1H, s, H17), 6,02 (2H, s, H20), 5,57 (1H, d, J = 17,3 Hz, H19), 5,22 (1H, d, J = 11,0 Hz, H19), 4,03 (3H, s H22), 4,02 (3H, s, H21).
13C NMR (125 MHz, CDCl3): δ 193,8 (C11), 157,2 (C1), 152,1 (C7), 151,5 (C15), 146,7 (C16), 144,4 (C3), 140,8 (C6), 137,5 (C13), 135,8 (C18), 135,1 (C5), 129,2 (C12), 123,4 (C9), 121,0 (C10), 116,8 (C8), 116,2 (C19), 115,7 (CC4), 111,8 (C17), 107,5 (C14), 102,0 (20), 61,4 (C21), 56,6(C22).
MS (ESI positive) m/z (%): 398 [M+H]+ (100), 380 (11), 340 (5)
These data are available only in Chinese in the patent item11). In brief, the NMR assignments of (+)-canadaline 26), (±)-hydrastine 312) and (–)-canadine 413) have been reported previously. Chlorine-substituted benzylisoquinoline-type alkaloid 1 was already isolated from Thalictrum foliolosum (Ranunculaceae)11). An origin of this compound needs further exploration; it could be of native origin or arise from oxidation of native alkaloid in solution. Compounds 2–4 have been previously isolated from H. canadensis (Fig. 1).
All isolated compounds have been assayed for their human cholinesterase and POP inhibition activities (Table 1). Galanthamine hydrobromide, huperzine A and berberine were used as positive controls.
Tab. 1. HuAChE, HuBuChE and POP inhibition activity of the alkaloids isolated from Hydrastis canadensis extract 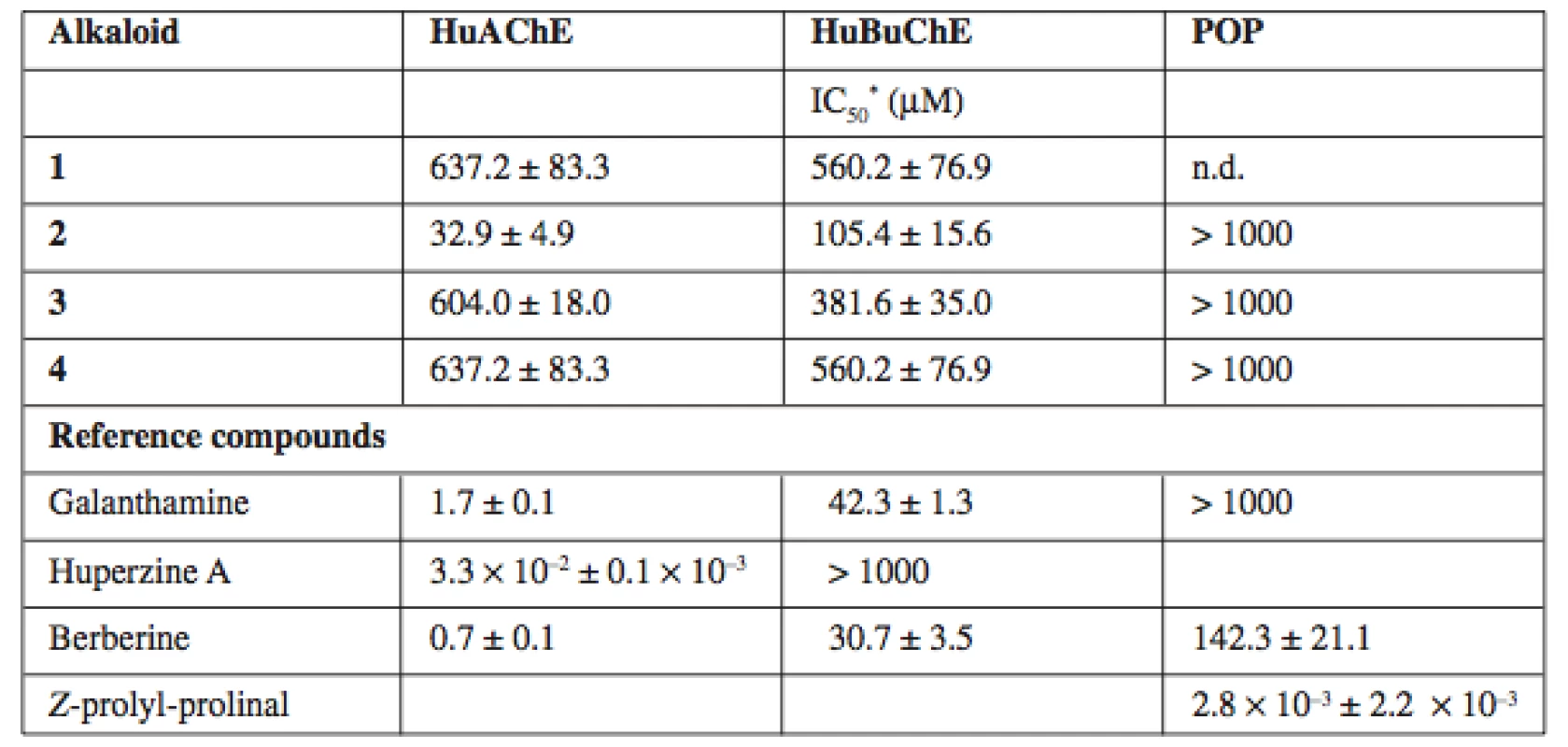
* – results are the mean of six replications; n.d. – not determined due to limited material (+)-Canadaline exerts interesting activity against HuAChE and moderate activity against HuBuChE (IC50 values 32.9 ± 4.9 ∝M and 105.4 ± 15.6 μM respectively). Any of isolated alkaloids did not inhibit POP significantly.
(+)-Canadine isolated from Corydalis cava is significantly more potent HuAChE inhibitor14) then (–)-canadine from Hydrastis canadensis. These tetrahydroderivatives of berberine are less potent cholinesterase inhibitors. The structural requirements concerning cholinesterase are in connection with the planarity and rigidity of molecules in this case15).
Compound 1 was not tested for its POP inhibition activity due to the isolation of insufficient amount. BuChE plays an important role in the late AD stages, when the level of AChE is declined by up 85% and BuChE represents the predominant cholinesterase in the brain16).
Conclusions
Isolated compounds were tested for their inhibitory activity against human cholinesterases and POP for first time. Compound 1 was tested for biological activity for second time so far.
In conclusion, the findings of this study indicate that major quarternary alkaloid berberine is probably responsible for cholinesterase inhibition of alkaloidal extract in the preliminary tests and there also was not any other alkaloid which would be more effective in POP inhibition.
Isoquinoline alkaloids are important for their wide spectrum of biological activities and still are of interest, although tertiary alkaloids from H. canadensis do not dispose inhibitory potency in term of human cholinesterases and POP.
Acknowledgements
This work was supported by grants SVV 260 063, Charles University grant Nr.204026/2012/UNCE, the European Social Fund and the state budget of the Czech Republic: TEAB Nr. CZ.1.07/2.3.00/20.0235 and the Grant Agency of the Czech Republic Nr. P303/11/1907.
Conflicts of interest: none.
HOŠŤÁLKOVÁ A.1, KUNEŠ J.2, MACÁKOVÁ K.1, HRABINOVÁ M.3, OPLETAL L.1
1ADINACO Research Group, Department of Pharmaceutical Botany and Ecology, Faculty of Pharmacy in Hradec Králové, Charles University in Prague
2Department of Inorganic and Organic Chemistry, Faculty of Pharmacy in Hradec Králové, Charles University in Prague
3Centre of Advanced Studies, Faculty of Military Health Sciences, University of Defence
e-mail: hosta4aa@faf.cuni.cz
Zdroje
1. Jirák R. Farmakoterapie demencí. Prakt. Lékáren. 2008; 4, 286–290.
2. Lleó A. Current therapeutic options for Alzheimer’s disease. Curr. Genomics 2007; 8, 550–558.
3. Orhan I. E. Current concepts on selected plant secondary metabolites with promising inhibitory effects against enzymes linked to Alzheimer’s disease. Curr. Med. Chem. 2012; 19, 2252–2261.
4. García-Horsman J. A., Männistö P. T., Venäläinen, J. I. On the role of prolyl oligopeptidase in health and disease. Neuropeptides 2007; 41, 1–24.
5. McKenna D. J., Plotnikoff G. A. Goldenseal (Hydrastis canadensis). In: Coates, P. M. ed. Encyclopedia of Dietary Supplements. 1st ed. New York: Marcel Dekker, Inc. 2005, 379–390.
6. Messana I., La Bua R., Galeffi C. The alkaloids of Hydrastis canadensis L. (Ranunculaceae). Two new alkaloids: hydrastidine and isohydrastidine. Gazz. Chim. Ital. 1980; 110, 539–543.
7. Galeffi C., Cometa M. F., Tomassini L., Nicoletti M. Canadinic acid. An alkaloid from Hydrastis canadensis. Planta Med. 1997; 63, 194.
8. Hyun Ah J., Byung-Sun M., Takato Y., Je-Hyun L., Yeong Shik K., Jae Sue C. Anti-alzheimer and antioxidant activities of coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009; 32, 1433–1438.
9. Tarrago T., Kichik N., Segui J., Giralt E. The Natural Product Berberine is a Human Prolyl Oligopeptidase Inhibitor. Chem. Med. Chem. 2007; 2, 354–359.
10. Cahlíková L., Hrabinová M., Kulhánková A., Benešová N., Chlebek J., Jun D., Novák Z., Macáková K., Kuneš J., Kuča K., Opletal L. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase,butyrylcholinesterase and prolyloligopeptidase activities. Nat. Prod. Commun. 2013; 8, 1541–1544.
11. Hua H., Su Y., Ma D., Li S., Li D., Li J., Guo J. Two chlorine-substituted benzyl isoquinoline alkaloids with antitumor activity separated from Thalictrum foliolosum. Faming Zhuanli Shenqing 2014; CN 103772366. apud Chemical Abstract 160, 730241.
12. Herath W. H. M. W., Ferreira D., Khan I. A. Microbial transformation of the phthalideisoquinoline alkaloid, (–)-ββ-hydrastine. Nat. Prod. Res. 2003; 17, 269–274.
13. Janssen, R. H. A. M., Wijkens P., Kruk C., Biessels H. W. A., Menichini F., Theuns H. G. Assignments of proton and carbon-13 NMR resonances of some isoquinoline alkaloids. Part 2. Phytochemistry 1990; 29, 3331–3319.
14. Chlebek J., Macáková K., Cahlíková L., Kurfürst M., Kuneš J, Opletal L. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava Schweigger. & Korte (Fumariaceae). Nat. Prod. Commun. 2011; 6, 607–610.
15. Correché E. R., Andujar S. A., Kurdelas R. R., Gómez Lechón M. J., Freile M. L., Enriz, R. D. Antioxidant and cytotoxic activities of canadine: Biological effects and structural aspects. Bioorg. Med. Chem. 2008; 16, 3641–3651.
16. Greig N. H., Lahiri D. K., Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002; 14, 77–91.
Štítky
Farmacie Farmakologie
Článek Účinnost fytoterapie v podpůrné léčbě diabetes mellitus typu 2 Borůvka černá (Vaccinium myrtillus)Článek Jewish pharmacists in pharmacies of interwar Czechoslovakia and their lives during World War IIČlánek Podpora aktivní účasti jednoho člena České farmaceutické společnosti na kongresu FIP v DüsseldorfuČlánek Jedová stopa.
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 1-2- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Vliv velikosti otvoru kónické testovací násypky na parametry rovnice sypání sorbitolu a jeho velikostních frakcí
- Příprava a hodnocení 5-amino-N-fenylpyrazin-2-karboxamidů jako potenciálních antiinfektiv
- Alzheimerova nemoc: význam nových léků a národní strategie pro snížení nákladů na léčbu
- Účinnost fytoterapie v podpůrné léčbě diabetes mellitus typu 2 Borůvka černá (Vaccinium myrtillus)
- Užívání vybraných OTC přípravků: srovnání Řecko a Česká republika
- 5. postgradual and 3. postdoctoral conference Faculty of Pharmacy UK
- Jewish pharmacists in pharmacies of interwar Czechoslovakia and their lives during World War II
- Computational approach to search for novel antituberculostics
- Alkaloids from hydrastidis canadensis and their cholinesterase and prolyl oligopeptidase inhibitory
- K životnému jubileu pani doc. RNDr. Zuzany Vitkovej, PhD.
- Podpora aktivní účasti jednoho člena České farmaceutické společnosti na kongresu FIP v Düsseldorfu
- Výskum a vývoj liekových foriem v súčasnosti
- Zdravotnícke pomôcky. Legislatíva a regulácia.
- Alois Borovanský, farmaceutický chemik par excellence
- Jedová stopa.
- Antitrombotiká v klinickej praxi.
- Biologická dostupnost léčiva a možnosti jejího ovlivňování
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Biologická dostupnost léčiva a možnosti jejího ovlivňování
- Účinnost fytoterapie v podpůrné léčbě diabetes mellitus typu 2 Borůvka černá (Vaccinium myrtillus)
- Užívání vybraných OTC přípravků: srovnání Řecko a Česká republika
- Jedová stopa.
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



