-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Hot-melt extrusion
Extruze tavenin
Extruze tavenin, metoda původem z průmyslu plastů, se stává v současnosti velice zajímavou technologií také ve farmacii. Počet špatně rozpustných léčiv s nízkou biologickou dostupností stále stoupá. Většina organických léčiv vykazuje polymorfii. Spolu s využitím vhodných nosných a dalších farmaceutických pomocných látek představuje extruze tavenin zajímavou možnost, jak zvýšit rozpustnost špatně rozpustných léčiv nebo zabránit vzniku nežádoucích polymorfů a jak zlepšit biologickou dostupnost problematických léčiv. Extruzi tavenin lze využít také při formování lékových forem s řízeným uvolňováním léčiv, např. pelet nebo filmů. Je to relativně levná, účinná a reprodukovatelná technologie bez použití rozpouštědel, která je šetrná k léčivům. Článek je přehledem možné využitelnosti této moderní technologie ve farmacii.
Klíčová slova:
extruze tavenin, zařízení, farmaceutické pomocné látky, pelety, filmy
Authors: Jana Šalandová 1,2; Miloslava Rabišková 1
Authors place of work: Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno
Published in the journal: Čes. slov. Farm., 2012; 61, 87-92
Category: Přehledy a odborná sdělení
Summary
Hot-melt extrusion (HME), originally coming from plastic industry, becomes at present an interesting technology also in the pharmaceutical field. The number of drugs with poor solubility and also bioavailability is increasing. In addition, most of the organic drugs are exhibiting polymorphism. Together with the utilization of appropriate carriers and other excipients, HME introduces an interesting possibility how to increase the solubility of poorly soluble drugs or protect unwanted polymorphic changes and how to improve bioavailability of problematic drugs. HME can also be used in the formation of dosage forms with controlled drug release, e.g. pellets or films. It is a relatively inexpensive, efficient and reproducible technology, a solvent free and drug-friendly process. This article is an overview of possible utilization of this modern technology in the pharmaceutical field.
Keywords:
hot-melt extrusion, equipment, excipients, pellets, filmsIntroduction
Hot-melt extrusion (HME) has become a very interesting pharmaceutical technology during the recent decade. The origin of this method comes from plastic industry1–3), but it is attractive in the pharmaceutical field due to many advantages4). The formulation produced by HME can contain a drug dispersed in a matrix at the molecular level forming thus a solid dispersion, which is very beneficial for poorly soluble drugs5). The number of molecules included in the Biopharmaceutical Classification System (BCS) class II is increasing – these drugs are permeable, but not soluble6). For the drugs of this class, the limiting-rate parameter for the absorption is dissolution rate and thus improving of aqueous solubility is challenging for the formulation7). HME is a solvent free process, it does not produce residual solvents and it is not a costly method8). Drug substance is not treated by compression and during the process a minimum amount of dust is formed9, 10). The HME process is shorter and more efficient than the conventional extrusion. The process is reproducible, that is why the scale-up procedure of the medicinal preparation produced by HME is not a critical part of the development11, 12). Another challenge for formulators is developing stable solid solutions4). More than 80% of organics show polymorphism, i.e. the ability to exist in various crystalline solid forms. Polymorphs exhibit different physical and chemical properties such as morphology, melting point, density, and mainly solubility. Thus they have an influence on the drug stability and also its bioavailability. During manufacturing of a dosage form or within stability testing the polymorph can be changed, which is not required and the final product can fail the specification criteria. Development of quantitative and qualitative solid-state analytic methods of active pharmaceutical substances is very sophistical. Powder X-ray Diffraction (PXRD), Differential Scanning Calorimetry/Modified Temperature Differential Calorimetry (DSC/MTDSC), Fourier Transform Infrared Spectroscopy (FT-IR) and Microscopy are the most commonly used methods 13, 14). HME is an attractive technology for producing stable solid solutions and preventing polymorphism changes.
This review article summarizes the HME process, brings information about suitable materials and makes an overview of the large utilization of this modern technology in the pharmaceutical field. The aim of this article is to collect recent trials and experiments connected with HME as well.
The equipment used in the hot melt extrusion process
Hot-melt extruders used in pharmaceutical industry have to be adapted to regulatory requirements, e.g., contact parts have to be inert to the medical product. The extruder consists of a feed hopper, a rotating screw inside a heated barrel, a die and heating and cooling systems. In general, there are two types of hot-melt extruders, which differ in transport mechanism and mixing ability: a single-screw and a multi-screw extruder. Screws are situated inside a stationary cylindrical barrel and contain three main parts: feeding zone, transition or melting zone and metering zone. These parts of the extruder are described in Figure 1. The single-screw extruder is simpler and cheaper, but less advantageous. The hot-melt process in a twin-screw extruder takes place due to agitation of two screw, which cause high kneading potential, shorter residence time, fewer tendencies to over-heat and large dispensing capacities. Easier utilization of twin-screw extruders is typical as well. The equipment can be adjusted to different formulations2). Material is moved between two screws to achieve a homogeneous mixture. Screws in twin-screw extruders can be adjusted in different ways. Most important for pharmaceutical industry is the co-rotating extruder, where the screws rotate in the same direction. They are required for high screw speed and high output. Counter-rotating screws have to be operated at a low screw speed, because high pressure between the screws can occur and also low output is expected.
Fig. 1. Single screw extruder (modified from <sup>17)</sup>) 
The twin-screw extruder can be also divided as either intermeshing or non-intermeshing. In addition, the configuration of the screws themselves may be varied using conveying elements, kneading blocks and other designs to achieve a particular mixing configuration15).
The size of extrusion screws is characterized by the L/D ratio, which is the length divided by the diameter. The equipment for the development has usually the size of 18–30 mm, whereas production machines exceed 50 mm2).
The shape of an extrudate is given by the die at the end of the barrel. The final step of the process-cooling-may be in the form of nitrogen, air, in water or on a steel conveyor, rolls by shaping devices, cyclic molding and else16).
The material for the extrusion is directly fed from the hopper – gravimetrically or volumetrically. The mass is conveyed in the barrel and it is mixed, compressed, melted and plasticized. Suitable homogenous plastic material continues to the metering zone, where it should achieve a uniform delivery rate through the die cavity. The drug is deaggregated and a uniform dispersion, a solid solution or a combination of both is produced18). Screw rotation and the electrical heating system are the sources of the heat.
Conditions during the process have to be set up accurately. The barrel temperature depends on glass transition, the melting temperature and molecular weight of the polymers. The combination of the optimal feed rate, screw speed together with motor load and melt pressure is required. The influence of each parameter is displayed in Table 1.
Tab. 1. Influence of parameters on the hot-melt process (modified from <sup>16)</sup>) 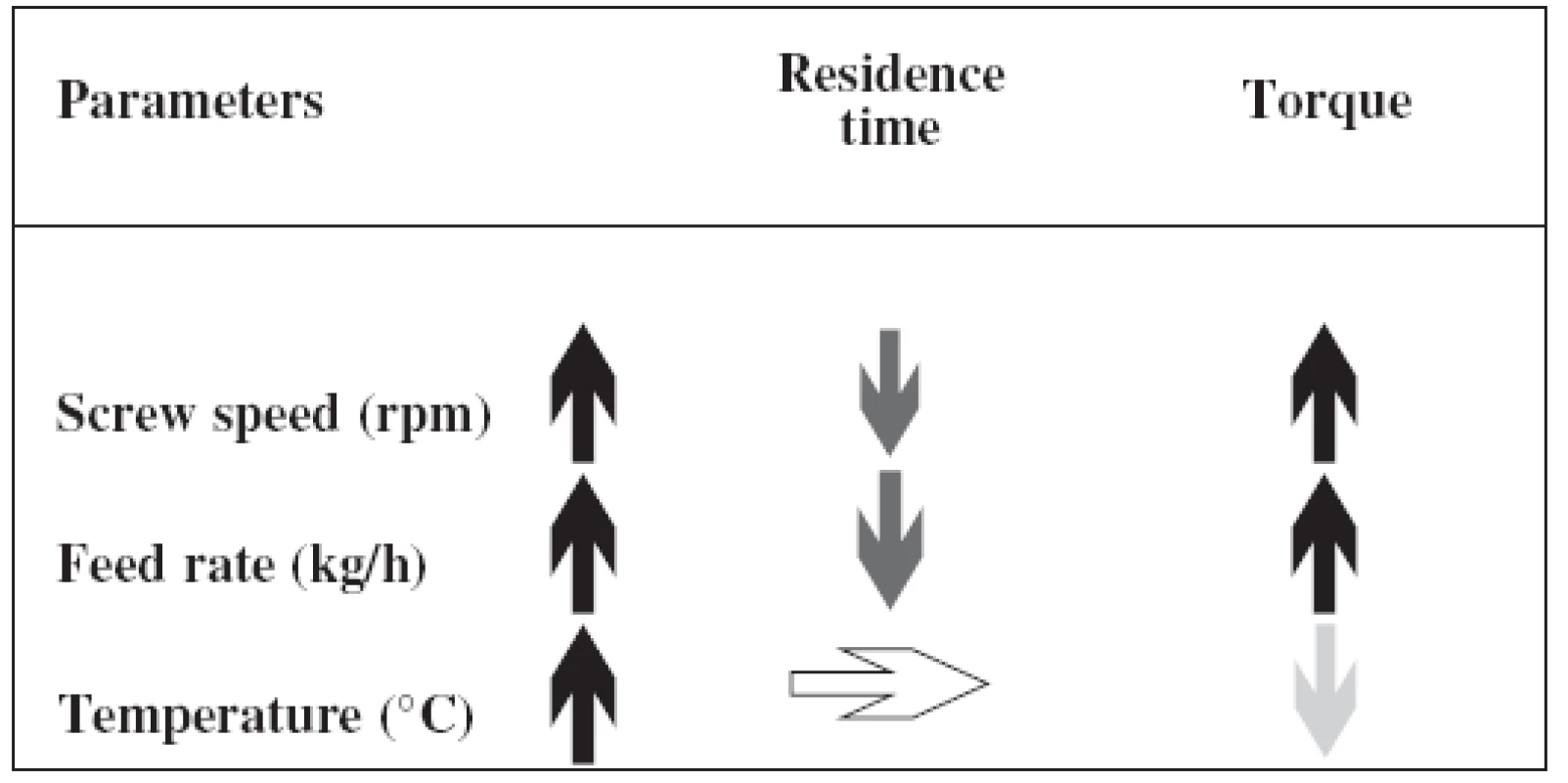
Material for hot-melt extrusion
Drug
Extrudates contain drugs as undissolved particles (solid suspension), dissolved molecules (solid solution) or as a combination of both of them. Positive effects of solid solutions on drug dissolution, absorption and therapeutic effect were described in the past. Only after some time, the technology of hot-melt extrusion is considered as a very convenient method how to reach a stable solid solution and incorporate it to a dosage form. Solid solution is thermodynamically stable and the drug is below saturation solubility16, 19). If the drug reaches saturation solubility, the system is after that only kinetically controlled and the drug can precipitate out of the polymer or crystallize, which causes a worse drug solubility profile 16. Amorphous form of drugs produced by HME can be 5–10 times more soluble in comparison with crystalline form12).
In solid dispersion, where the drug is in a hydrophilic carrier, aqueous solubility is improved. The drug can be in a microcrystalline form as a part of a eutective drug-polymer mixture or it can be located within an amorphous or crystalline polymer6). Solid dispersion should be stable during stability testing. It is suggested to store solid dispersions at the temperature of 50 °C below its glass transition temperature (Tg). Nevertheless, a positive effect of short post-heating was described in the study of nimodipine with Kollidone® VA64 (polyvinylpyrrolidone/vinylacetate copolymer), where a short action of high temperature inhibited recrystallization in this solid dispersion20).
Drug substance is exposed to high temperature during HME. A heat sensitive drug can be degraded. A plasticizer exerts an effect on decreasing Tg of a polymer, but it does not change the melting temperature of the drug. One of the possibilities how to avoid thermal degradation of a drug is cocrystals preparation. Cocrystals are homogenous single crystalline phases containing solid components. Components are interacted to the drug via noncovalent interactions such as hydrogen bonds or van der Waals interactions. Cocrystals can be prepared first and then added to the polymer to produce a solid dispersion. Another, simpler method of the production of a cocrystals solid dispersion is melting the mixture consisting of a drug, a conformer and a polymer together. In one study, the preparation of carbamazepine-nicotinamide cocrystal was investigated. An amorphous solid dispersion of that cocrystal and the polymer was in-situ produced by HME. Processing temperature was decreased and dissolution rate was increased5).
In the study described by Ghosh et al.10), another temperature-sensitive and poorly water soluble drug (Novartis compound NVS981) was tested in an HME formulation based upon 3 different types of hypromellose (HPMC), i.e. HPMC (3cps), HPMC phthalate, HPMC acetyl succinate. The composition with the latter carrier was the most chemically and physically stable solid dispersion with a satisfied drug dissolution profile.
Excipients for hot-melt extrusion
Polymers, plasticizers, antioxidants, lubricants and colorants are the typical hot-melt extrusion excipients. Polymers and plasticizers are the most indispensable. Different excipients for the modification of the drug release profile, which is common for the production of solid dosage forms, can be also used.
The design of hot-melt process is mostly influenced by the character of polymers. Their appropriate thermoplastic characteristics are necessary for the hot-melt process. High thermal conductivities and low melt viscosities are beneficial. During the hot-melt process polymers do not have to undergo the conditions like chain scission, depolymerization or thermal degradation. Polymers are defined by Tg or melting temperature (Tm) and melt viscosity. Bellow the glass transition temperature the molecules of the polymer are without any motility. Above Tg, the polymer becomes rubbery due to weakening of the bonds between the polymer chains. The polymer is capable of plastic or elastic deformation21). The temperature for hot-melt extrusion is usually 20–40 °C above the Tg8). That is why polymers with high Tg or Tm are not suitable as carriers. In general, typical extrusion temperature is 100–200 °C. Polymers should be low-hydroscopic and non-toxic. Their degradation temperature limit (Tdeg) should not be close to the value of Tg or Tm. Thus for instance, Soluplus® – polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol copolymer (PEG-VCap-VAc) - produced by the BASF company shows a wide range between Tg and Tdeg as shown in Figure 2 together with other commonly used polymers16).
Fig. 2. Differences of Tg (Tm) and Tdeg (modified from <sup>8)</sup>) 
Soluplus® is one of the new substances which are applied as polymer carriers. In a solid solution of a poorly soluble drug, it plays the role of an active solubilizer and also the role of a matrix. Several trials to show the ability of this polymer to the improved dissolution profile of drugs from BCS class II were described. For example, amorphization of spironolactone in the Soluplus matrix was achieved7).
Polyethylene oxide (PEO) and povidone (PVP) belong to the group of water-soluble or water-miscible materials. They can be used as carriers for insoluble drugs to form solid dispersion. Thus for instance, HME of the highly lipophilic drug bicalutamide with PEO was tested to show the influence of the polymer on the stability of a solid dispersion. The drug-polymer ratios 1 : 10 and 2 : 10 showed the drug in the amorphous form which significantly enhanced its dissolution rate. However, in the formulation containing the drug-polymer ratio 3 : 10, the recrystallization of bicalutamite resulted6).
Polymers of pH-dependent solubility should be chosen according to the required drug delivery system. A mixture of polyvinyl acetate and povidone (brand name Kollicoat® SR) is insoluble in water, which is the reason for using it for a sustained release profile of a drug. The anionic copolymer of methacrylic acid and ethyl acrylate (Kollicoat® MAE 100P) is used as an enteric matrix. Other examples of polymers intended for controlled and immediate release are presented in Table 2. Some of them are available in more grades for different utilization.
Tab. 2. Polymers for hot-melt extrusion and their trade name and utilization <sup>3, 8, 16)</sup> 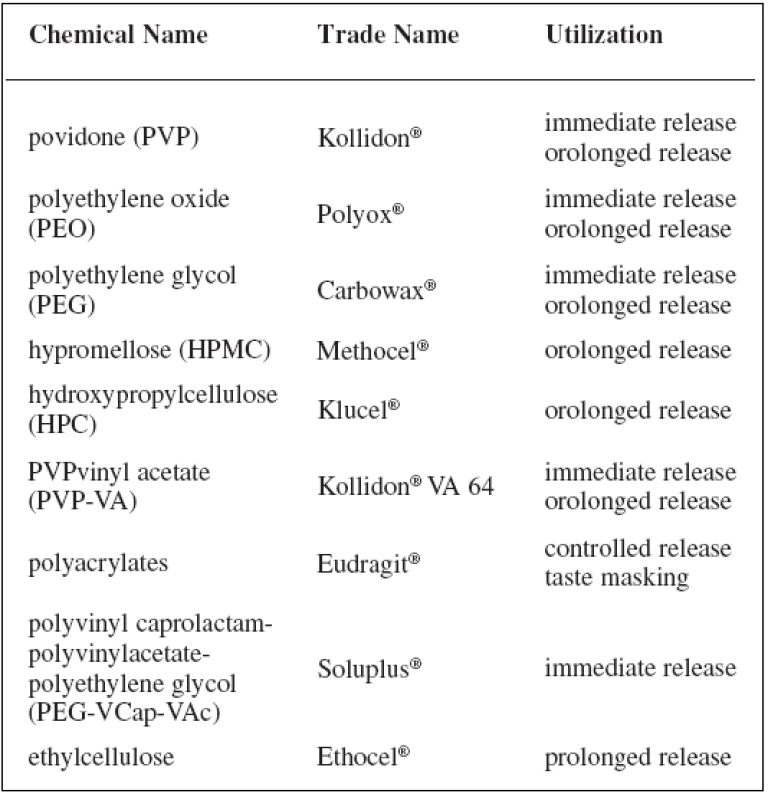
A specific polymer for a particular drug has to be identified according to its compatibility and efficacy. The poorly water-soluble drug bifendate was hot-melt extruded with several polymer carriers. Its dissolution enhancement was achieved with Eudragit® EPO (ethylacrylate, methyl methacrylate polymer), Plasdone® 360 and Kollidone® VA64 (polyvinylpyrrolidone/vinylacetate copolymer) due to the creation of a homogenous dispersion of bifendate. However, only a solid dispersion with Kollidon® VA64 as the carrier showed the ability to increase also drug relative bioavailability9).
Waxes, lipids and wax-based materials are also used as carriers in HME especially for controlling the drug release from the final product22). Lipids have advantages such as biodegradability, bitter taste of drugs masking and protection for water-sensitive drugs, and they can also improve drug absorption in the gastrointestinal tract. In general, waxes have lower melting points compared to polymers23). Carnauba wax and castor wax are commonly used for HME7). However, drug release from lipophilic carriers can be very slow. An addition of hydrophilic excipients can tailor the required properties for dissolution rate. Polyethylene glycol contributes to the porous network of extrudates and drug diffusion is after that faster. In one study, microcrystalline wax was tested in HME with theophylline as a water-soluble drug. The aim of the study was to speed up the diffusion of the drug from the lipophilic inert matrix by another approach – by modification of the shape of the extrudates. A higher specific surface area is one of the other options how to reach more rapid dissolution. In that study three different helical shapes with 2, 3 and 4 blades and one classical cylindrical shape were tested in vitro. The system having helix shape with 3 blades and the composition of 70 : 30 theophylline:wax exhibited the desired in vitro theophylline release11).
Amorphous acidic drugs can be stabilized also by inorganic silicates instead of organic polymers. A blend of inorganic magnesium aluminometasilicate (Neusilin®) and the anti-inflammatory drug sulindac was extruded. The material had similar physical stability compared to previous samples produced by ball milling. However, the extrudate had a better dissolution profile24).
Plasticizers are additives to polymers due their capability of lowering glass transition temperature. They cause a weakness of cohesive intermolecular forces along the chains in a polymer. In general, plasticizers have an effect of easy workability because of increasing elongation and flexibility of polymers. The addition of plasticizers causes polymers chains movement and that is why the toughness of a polymer is reduced. Plasticizers decrease the value of Tg by the location between the chains of polymers, therefore polymers are softer and their structure is more flexible21). Usually 10% (w/w) of the plasticizers are sufficient. Some drugs can also function as plasticizers3, 10). For instance, the antiretrovital drugs zidovudine and lamivudine were found to decrease Tg of ethylcellulose25).
Another example of a positive role of plasticizers was described by Kanaujia et al.12) The dissolution of a ketoconazole solid dispersion prepared by HME was examined. During dissolution, particles can grow from the molecular form followed by the formation of nanoparticles and subsequently microparticles and the rate of drug release can be slower. Positive effects of plasticizers on the inhibition of particles growth were confirmed in the study.
Most common plasticizers for HME are polyethylene glycols, citrate esters and triacetin3, 8, 16).
The utilization of hot-melt extrusion process
During recent years, the interest in hot-melt extrusion process has been increasing because of many advantages mentioned above. Hot-melt extrusion also offers various possibilities of applications in drug delivery. It can be used for developing different kinds of dosage forms such as pellets, granules, immediate or modified release tablets, transdermal, transmucosal films and implants15, 18, 26). Many innovative approaches for drug delivery systems have been published. Nanoparticle engineering has several limitations such as poor wetability or particle aggregation. Hot-melt extrusion of itraconazole-polyvinylpyrrolidone and itraconazole-hypromellose microparticles dispersed subsequently in mixtures of Poloxamer and polyethylene oxide has been evaluated. In this study, the above-mentioned problems of nanoparticles were overcome, particles were disaggregated and wetting was enhanced. Homogenous dispersion of polymers and particles was achieved8).
In another experimental work, lamivudine and zidovudine were studied. Lamivudin and zidovudine used in the treatment of the human immune-deficiency virus are appropriate drugs for sustained release formulation. Prolonged release of these drugs decreases their plasma fluctuation. Drug side effects are reduced and dosage regimen is simplified improving thus the patients’ compliance. For this reason, HME of the formulations based on ethylcellulose together with plasticizers, i.e. triethylcitrate (TEC) and polyethylene glycol (PEG 6000) was studied. Thermal analysis (differential scanning calorimetry) showed that zidovudine forms an amorphous solid solution with ethylcellulose at studied concentrations up to 40% by weight and had a plasticizing effect on the flow properties of ethylcellulose. Lamivudine appeared to become saturated within the polymer at drug loadings above 30% by weight, forming a solid dispersion with evidence of partial crystallinity. TEC appeared to be a more suitable plasticizer to aid processing during HME than PEG 6000, especially at concentrations of 5–10% by weight. PEG 6000 was found to recrystallize upon cooling and thereby may render the formulation unstable during storage25).
Pellets
Multi-unit dosage forms such as pellets are able to be easily produced by HME. Dissolution rate can be controlled by particle size of pellets and by the type of excipients used. To produce pellets, pelletization of an extrudate formed from HME is necessary. For this purpose, two major pelletization techniques can be used: strand pelletization and die-face pelletization27). In strand pelletization, the melt strand produced by an extruder is drawn either via a conveyor belt or feed rolls through a cooling medium, such as water or air to the cutting knives or breaking device of the equipment. The solidified polymer melt is then cut or broken into cylindrical pieces and spheronized discontinuously. This step may involve using a heated spheronizer. In die-face pelletization, the molten extrudate is cut at the die-face and transported to the next processing stage by, for example, vacuum. Die-face pelletization offers an opportunity to produce pellets without contact to a cooling medium and the risk of strand breakage during the cooling phase. The largest advantage of die-face pelletization though is the fact that the particle swells to an almost spherical shape as a result of the viscoelasticity of the polymer melt. Further spheronizing steps become unnecessary, and a continuous production of spherical pellets is possible. However, the spectrum of polymer melts (e.g. starches) that can be processed with die-face pelletization is smaller compared with classical strand pelletizers28). In the study of Bialleck and Rein28), four different types of starches (corn starch, pea starch, potato starch and waxy corn starch – Waxylis 200® Roquette) and four different active ingredients (ibuprofen, paracetamol, phenazone and tramadol hydrochloride) were tested using die-face pelletization. The resulting pellets exhibited narrow size distribution and good mechanical properties. The particle size was determined by the used starch (starch swellability) and the die plate. Hydrophilic drugs were dissolved within the polymer during the extrusion, while the lipophilic ones were dispersed in an amorphous starch matrix. The mechanism of drug release for waxy corn starch pellets was erosion independently of the chemical properties of drugs. For the remaining starch pellet samples, the release mechanism was more complex and based on diffusion as well as relaxation of the matrix.
Pellets with prolonged release of water-soluble paracetamol were produced by HME with a hot-strand cutter as one of the kind of the down-streaming system. In this system, the mixture is deformed inside the extruder and solidified at the outlet. Calcium stearate was used in this trial as a thermoplastic carrier. Prolonged drug release was successfully achieved by HME. Solid suspension of paracetamol was prepared. Plasticizers were after that added to improve the process of extrusion and achieve a faster drug release profile. Glyceryl monostearate was found to form pores at the surface of the pellets and that is why the drug dissolution improves, but it does not have any influence on the production process itself. Tributyl citrate as a plasticizer increased drug release more significantly. Finally, both of the plasticizers contribute to tailor in-vitro release rate22).
Pellets are usually filled into hard capsules or can be also compressed to tablets. Compression of particles is quite challenging. During the compression process, pellets could not be damaged and especially specific functional coating has to be retained. HME is a possibility how to avoid these problems. Coated pellets can be incorporated into the matrix for HME29, 30).
Films, implants
In the development of transmucosal, transdermal and transungual delivery systems, the casting method is the leading method for production, where aqueous and organic solvents are used. Casting takes a long time, environmental toxic organic solvents from the film have to be removed and production based on aqueous solvents is not possible for all of the drug molecules. Hot-melt extrusion can overcome these disadvantages18). Additionally, in the case of transdermal application, the drug must be dissolved. Only a dissolved drug is able to diffuse from the polymeric patch and be absorbed. Thus, the hot-melt extrusion process can be used to produce a solid solution and from this solution in a polymeric patch the drug is able to diffuse and be absorbed. Films containing clotrimazol and different proportions of polymers such as hydroxypropyl cellulose (HPC) and polyethylenoxide (PEO) produced by hot-melt extrusion were tested. In all of the prototypes, clotrimazol stayed in the form of a solid solution for 12 month of long-term stability (25 °C, 60% RH). Increasing concentration of HPC had positive effect on physical stability of the drug and PEO in the HME films, but negative influence on their flexibility and bioadhesivity. On the other hand, films with PEO exhibited better mechanical and biodhesive properties. Films containing PEO : HPC : clotrimazol in the ratio 35 : 55 : 10 demonstrated optimum physical-mechanical, bioadhesive, and release properties. Polymers blends of HPC and PEO can be thus used to tailor drug release profile, mechanical and bioadhesive properties, and stability of HME films4).
Another field of HME utilization is taste-masking. A suitable polymer can mask unpleasant taste due to the creation of a solid dispersion of bitter drug substances. Paracetamol, as the mainly used analgetic and antipyretic drug was used as a model drug for taste masking. The extrudate containing Kollidon® VA64 and 30% of paracetamol provided the best masking effect31).
Conclusion
Many advantages of HME and the large field of its utilization can be the reasons why HME has become an attractive alternative to traditional production methods. HME has been spread in the academic field and the industry has started to be interested in it more recently. Expansion of this method is definitely assumed in near future.
Conflict of interest: none.
Received 14. April 2011 / Accepted 24. April 2011
prof. PharmDr. Miloslava Rabišková, CSc.
Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno
Palackého 1–3, 612 42 Brno
e-mail: rabiskovam@vfu.cz
J. Šalandová
Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno
Zentiva, k.s
Zdroje
1. Brabander C. D., Vervaet C., Remon J. P.: Development and evaluation of sustained release mini-matrices prepared via hot melt extrusion. J. Control Release. 2003; 89, 235–247.
2. Chokshi R., Zia H.: Hot-melt extrusion technique: A review. Iranian Journal of Pharmaceutical Research. 2004; 3, 3–16.
3. Crowley M. M., Zhang F., Repka M. A., Thumma S., Upadhye S. B., Battu S. K., McGinity J. W., Martin C.: Pharmaceutical applications of hot-melt extrusion: Part I. Drug. Dev. and Ind. Pharm. 2007; 33, 909–926.
4. Prodduturi S., Urman K. L., Otaigbe J. U., Repka M. A. Stabilization of hot-melt extrusion formulations containing solid solutions using polymer blends. AAPS PharmSciTech. 2007; 8(2), 1–10.
5. Liu X., Lu M., Guo Z., Huang L., Feng X., Wu C.: Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm. Res. 2011; 29(3), 806–817.
6. Abu-Diak O. A., Jones D. S., Andrews G. P.: Understanding the performance of melt-extruded poly(etylene oxide)-bicalutamide solid dispersions: Characterisation of microstructural properties using thermal, spectroscopic and drug release methods. J. Pharm. Sci. 2012; 101(1), 200–213.
7. Nagy Z. K., Balogh A., Vajna B., Farkas A., Patyi G., Kramarics A., Marosi G.: Comparison of electrospun and extruded soluplus® -based solid dosage forms of improved dissolution. J. Pharm. Sci. 2012; 101(1), 322–332.
8. Karl M., Djuric D., Kolter K.: Pharmaceutical excipients for hot-melt extrusion. Pharm. Tech. Europe 2011; 35, 74–82.
9. Feng J., Xu L., Gao R., Luo Y., Tang X.: Evaluation of polymer carriers with the regards to the bioavailability enhancement of bifendate solid dispersions prepared by hot-melt extrusion. Drug Dev. Ind. Pharm. 2011; 1–9.
10. Ghosh I., Snyder J., Vippagunta R., Alvine M., Vakil R., Tong W., Vippagunta S.: Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using melt extruder. Int. J. Pharm. 2011; 419, 12–19.
11. Hasa D., Perissutti B., Grassi M., Zacchigna M., Pagotto M., Lenaz D., Kleinebudde P., Voinovich D.: Melt extuded helical waxy matrices as a new sustained drug delivery system. E. J. Pharm. Biopharm. 2011; 79, 592–600.
12. Kanaujia P., Lau G., NG W. K., Widjaja E., Hanefeld A., Fischbach M., Maio M., Tan R. B. H.: Nanoparticle formation and growth during in vitro dissolution of ketoconazole solid dispersion. J. Pharm. Sci. 2011; 100(7), 2876–2885.
13. Brittain H.G.: Polymorphism in pharmaceutical solids. Informa Healthcare USA, Inc., New York 1999.
14. Chieng N., Rades T., Aaltonen J.: An overview of recent studies on the analysis of pharmaceutical polymorphs. J. Pharm. Biomed. Anal. 2011; 55, 618–644.
15. Andrews G. P., Jones D. S. et al.: Hot melt extrusion: An emerging drug delivery technology. Pharmaceutical Technology Europe. 2009; 21, 24–27.
16. Kolter K., Karl M., Nalawade S., Rottmann N. Hot-melt extusion with BASF pharma polymers, 2nd edition. 2011.
17. Shuwisitkul D.: Biodegradable implants with different drug release profiles. Bangkok 2011, Dissertation, page 29.
18. Repka M. A., Majumdar S., Battu S. K., Srirangam R., Upajdye S. B.: Applications of hot-melt extrusion for drug delivery. Expert Opin. Drug Deliv. 2008; 5(12), 1357–1376.
19. Rabišková M.: Peletizace tavenin a kapalin. Čes. slov. Farm. 2011; 60(2), 54–60.
20. Jijun F., Lishuang X., Xiaoguang T., Min S., Mingming Z., Haibing H., Xing T.: The inhibition effect of high storage temperature on the recrystallization rate during dissolution of nimodipine-kollidon VA64 solid dispersion (NM-SD) prepared by hot-melt extrusion. J. Pharm. Sci. 2011; 100(5), 1643–1647.
21. Mahnaj T., Ahmed A. U., Plakogiannis F. M.: Evaluating the efficacy of a group of nontraditional plasticizers on the glass transition temperature of ethyl cellulose polymer. Drug Dev. Ind. Pharm. 2011; 37(3), 342–350.
22. Roblegg E., Jäger E., Hodzic A., Koscher G., Mohr S., Zimmer A., Khinast J. Development of sustained-release lipophilic calcium stearate pellets via hot melt extrusion. Eur. J. Pharm. Biopharm. 2011; 79, 635–645.
23. Liu J., Zhang F., McGinity J. W.: properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur. J. of Pharm. Biopharm. 2001; 52, 181–190.
24. Maclean J., Medina C., Daurio D., Alvarez-Nunez F., Jona J., Munson E., Nagapudi K.: Manufacture and performance evaluation of a stable amorphous complex of an acidic drug molecule and neusilin. J. Pharm. Sci. 2011; 100(8), 3332–3344.
25. Maru S. M., Matas M., Kelly A., Paradkar A.: Characterization of thermal and rheological properties of zidovudine, lamivudine and plasticizer blends with ethyl cellulose to assess their suitability for hot melt extrusion. Eur. J. Pharm. Sci. 2011; 44, 471–478.
26. Repka M. A., Battu S. K., Upadhye S. B., Thumma S., Crowley M. M., Zhang F., Martin C., McGinity J. W.: Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev. Ind. Pharm. 2007; 33, 1043–1057.
27. Case Ch. C. Melt pelletization. In: Ghebre-Sellasie I. and Martin Ch. eds Pharmaceutical extrusion technology, 1st ed. New York and Basel: Marcel Dekker Inc. 2003.
28. Bialleck S., Rein H.: Preparation of starch-based pellets by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011; 79, 440–448.
29. Schilling S. U., McGinity J. W.: Novel application of hot-melt extrusion for the preparation of monolithic matrices containing enteric-coated particles. Int. J. Pharm. 2010; 400, 24–31.
30. Shajahan A., Chandevar A. V., Jaiswal S. B.: A flexible technology for modified-release drugs: Multiple-unit pellets system (MUPS). J. Control Release. 2010; 147, 2–16.
31. Maniruzzaman M., Boateng J. S., Bonnefille M., Aranyos A., Mitchell J. C., Douroumis D.: Taste masking of paracetamol by hot melt extrusion: an in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012;80(2), 433–442.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2012 Číslo 3- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Pod ochranou sv. Dymphny – LIV. sympozium z historie farmacie a veterinární medicíny
- Modulácia leukotriénovej cesty – potenciálne ciele
- Mikrobiálne sekundárne metabolity ako inhibítory farmaceuticky významných oxidoreduktáz a transferáz
- Fenotypizace enzymů podílejících se na metabolismu léčiv
- SOLUTIO 2011
- Vysoce účinné potenciální antimykobakteriální látky proti Mycobacterium avium
-
Štúdium lokálnych anestetík – časť 197*
Vplyv xylitolu na farmaceutickú dostupnosť lidokaínu a tokové vlastnosti hydrogélov - Prof. RNDr. J. Čižmárik, PhD. – doctor honoris causa
- Doc. RNDr. Ingrid Tumová, CSc., jubiluje
- Životné jubileum docenta RNDr. Jozefa Sokolíka, CSc.
- Polyméry s molekulovými odtlačkami
- Extruze tavenin
- Inhibítory gamma-sekretázy v terapii Alzheimerovej choroby
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fenotypizace enzymů podílejících se na metabolismu léčiv
- Extruze tavenin
- Modulácia leukotriénovej cesty – potenciálne ciele
- Doc. RNDr. Ingrid Tumová, CSc., jubiluje
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



