-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Adhesivity of branched plasticized oligoesters
Adhezivita větvených plastifikovaných oligoesterů
Práce se zabývá vztahem mezi reologickými a adhezivními vlastnostmi větvených oligoesterových nosičů, plastifikovaných různým typem a koncentrací plastifikátorů. Oligoestery testované v této studii jsou terpolymery dipentaerythritolu, kyseliny D,L-mléčné a kyseliny glykolové, kde dipentaerythritol (D) v koncentraci 3 %, 5 % nebo 8 % působí v průběhu stupňovité polykondenzace jako větvící složka. Nosiče označené jako 3D, 5D a 8D byly plastifikovány buď triethyl citrátem (TEC), nebo methyl salicylátem (MS) nebo ethyl salicylátem (ES) v různé koncentraci. Byla měřena dynamická viskozita při teplotě 37 °C. Plastifikované systémy vykazovaly newtonský tok, jejich viskozita závisí zejména na plastifikaci, dále na molární hmotnosti a stupni větvení oligoesterového nosiče. Byla měřena síla potřebná k odtržení plastifikovaného nosiče od modelového substrátu jako míra adhezivních vlastností. Adhezivní síla testovaných nosičů byla v rozmezí 25 N až 35 N a tomu odpovídající hodnoty dynamické viskozity se pohybovaly v rozmezí 3 Pa.s až 40 Pa.s. Bylo nalezeno optimální rozmezí hodnot dynamické viskozity plastifikovaných oligoesterových nosičů zajišťující dostatečnou bioadhezi.
Klíčová slova:
větvené oligoestery – koncentrace plastifikátoru – viskozita – bioadheze
Authors: E. Šnejdrová; M. Dittrich
Authors place of work: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmaceutical Technology
Published in the journal: Čes. slov. Farm., 2009; 58, 212-215
Category: Původní práce
Summary
This paper compares the rheological and adhesive properties of branched oligoester carriers plasticized with the use of various types and concentrations of plasticizers. The oligoesters tested and reported here are star-like branched terpolymers of dipentaeythritol, D,L-lactic acid and glycolic acids, where dipentaerythritol (D) was employed as the branching agent in three concentrations (3%, 5% or 8%) in synthesis by step-by-step polycondenzation. Carriers labelled as 3D, 5D and 8D were plasticized with either triethyl citrate (TEC), or methyl salicylate (MS), or ethyl salicylate (ES) in various concentrations. Dynamic viscosity of plasticized oligoesters was measured at 37 °C. Plasticized systems behaved as Newtonian fluids, their viscosity depended mainly on plasticization, and then on the molecular weight and branching ratio of the carriers. Detachment force as the measure of the adhesiveness of the plasticized carriers was determined. Adhesive forces of the tested carriers ranged most frequently between 25 N and 35 N with the corresponding values of dynamic viscosity from 3 Pa.s to 40 Pa.s. The optimal range of dynamic viscosity of the plasticized carriers providing sufficient bioadhesion was found out.
Key words:
branched oligoesters – plasticizer concentration – viscosity – bioadhesionIntroduction
Poly(lactic acid) (PLA) and its copolymers are used in a number of medical applications such as sutures, drug delivery, orthopaedic implants, etc. They combine biodegradability and biocompatibility with easy processing, application, and storage stability 1).Low molecular weight oligoesters are preferred as drug carriers, as their hydrolysis may proceed simultaneously or just somewhat slower than drug release 2). They possess good mechanical properties and clarity in addition to their processability; however the brittleness is their major drawback for many applications. This is the reason for their blending with other polymers or with common and even non-traditional plasticizers. Low molecular weight compounds have been used as plasticizers, e.g. glycerol 3), citrate esters or triacetine 4, 5). Even very non-traditional plasticizers such as liquid drugs 6) or liquids with a potential pharmacodynamic effect, such as salicylate or pyruvate esters, can serve as plasticizers. The use of triethyl citrate as a plasticizer is very advantageous as proved by its long-term use. It is extremely efficient in lowering the glass transition temperature of aliphatic polyesters 7). Triethyl citrate is miscible with all the tested carriers and can be used without limitations.
Bioadhesives have been formulated into tablets, patches, or microparticles, typically with an adhesive polymer forming the matrix into which the drug is dispersed, or the barrier through which the drug must diffuse 8). The primary goal of bioadhesive drug delivery is to localize a delivery device within the body to enhance the drug absorption process in a site-specific manner. Potential bioadhesives are evaluated for their adhesive properties, durability and biological inertness. No standard test methods have been specifically designed for bioadhesion analysis. Thus, the data from different research papers cannot be directly compared.
Most in-vitro methods are based on the measurement of either shear or tensile stress. Two parameters, namely the work of adhesion and peak detachment force are used to study the adhesiveness of a polymer. Both provide the same information on bond strength.
The role of viscosity of bioadhesives has been investigated by several research groups with ambiguous conclusions 9, 10). Some workers take an attitude that viscosity has little to do with bioadhesion however the adhesive properties of the bioadhesives can pass through the optimal level with respect to their viscosity. This paper aims to contribute to the understanding of the relation between dynamic viscosity of plasticized branched oligoesters and their adhesive force.
EXPERIMENTAL PART
Materials
Oligoesters were synthesized at the Faculty of Pharmacy, Department of Pharmaceutical Technology. Their basic characteristics are given in Table 1. Their molecular weights were determined using a combination of size exclusion chromatography (SEC) with multi-angle light scattering (GPC-MALS). This measurement was caried out at Synpo, Pardubice, Czech Republic.
Tab. 1. Relevant characteristics of oligoester carriers 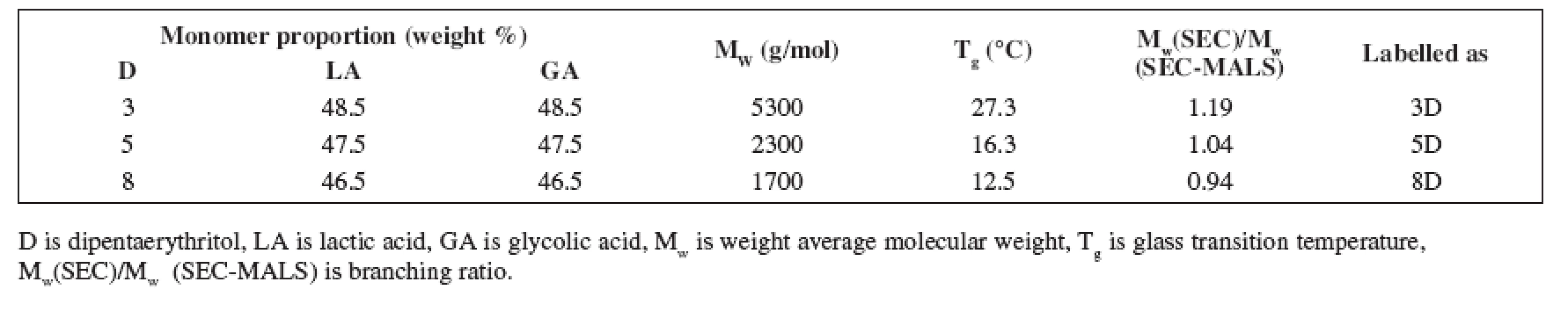
Triethyl citrate, methyl salicylate and ethyl salicylate as plasticizers were purchased from Fluka. Mucin from porcine stomach, Type III, used as a model substrate for bioadhesion, was purchased from Sigma. Co.
Preparation of plasticized oligoesters
Each tested oligoester sample in a sufficient quantity for both viscosity measurements and adhesivity tests as well was melted in a tray dryer at a temperature not exceeding 80 °C. The plasticizer in a given concentration was added immediately and then the mixture was thoroughly homogenized. If necessary, the mixture was heated repeatedly to get a perfectly homogeneous plasticized oligoester system.
Dynamic viscosity measurement
Dynamic viscosity of plasticized oligoesters was measured using a Digital Brookfield Viscometer DV-E with an adapter for small samples and a spindle 14 or 15 at 37 °C. The appropriate spindle number was chosen according to the assumed extent of dynamic viscosity. The speed of the spindle rotation was changed in the range from 0.3 RPM to 100 RPM. Under-range and over-range readings were not accorded.
Adhesion test
The adhesive force measurement of the plasticized oligoesters was reported previously by the present authors 11). Maximal force Fmax in Newtons needed for detachment of the tested material from the model substrate using a Material Testing Machine Zwick/Roel T1-FR050TH.A1K was measured. Unlike the previous tests, porcine stomach mucin gel as a model substrate was used. Consolidation force of 5 N, contact time of 60 s and detachment rate of 100 mm/min were applied during the adhesive test. The adhesive force measurement of each sample was repeated five times. Statistical analysis was performed using the Student’s unpaired t-test.
RESULTS AND DISCUSSION
Oligoester carriers tested in this study were synthesized in the workplace of the authors from an equimolar portion of D,L-lactic acid and glycolic acid using polycondensation reaction. Dipentaerythritol as the branching agent in concentrations of 3%, 5% or 8% was used in the synthesis. The process led to the formation of the molecules with different molecular weights and branching ratio (Table 1). The products differ in hydrophilicity, course of swelling and erosion as well.
The table shows the molecular weight Mw determined by conventional SEC with calibration on polystyrene standards and its ratio to Mw determined by SEC-MALS expressing the branching degree of the oligoester carriers. The higher value of the ratio Mw(SEC)/Mw(SEC-MALS) the lower degree of branching the carrier possess. The value of Mw(SEC)/Mw (SEC-MALS) for linear poly-DL-lactic acid equals 1.50.
The results signify that molecular weight of the carriers decreases with increasing concentration of the branching agent (i.e. dipentaerythritol). This is caused by a rising portion of the hydroxyl groups offered for polycondenzation reaction. Simultaneously the branching of the carriers increases with increasing concentration of the dipentaerythritol in the reactive blend as well.
The mechanical properties of oligoester carriers can be improved by blending with a plasticizer. Table 2 shows the values of dynamic viscosity of the plasticized carriers. Dynamic viscosity of the non plasticized carriers at 37 °C was not measurable in our circumstances.
Tab. 2. Dynamic viscosity of the oligoester carriers η (Pa.s) 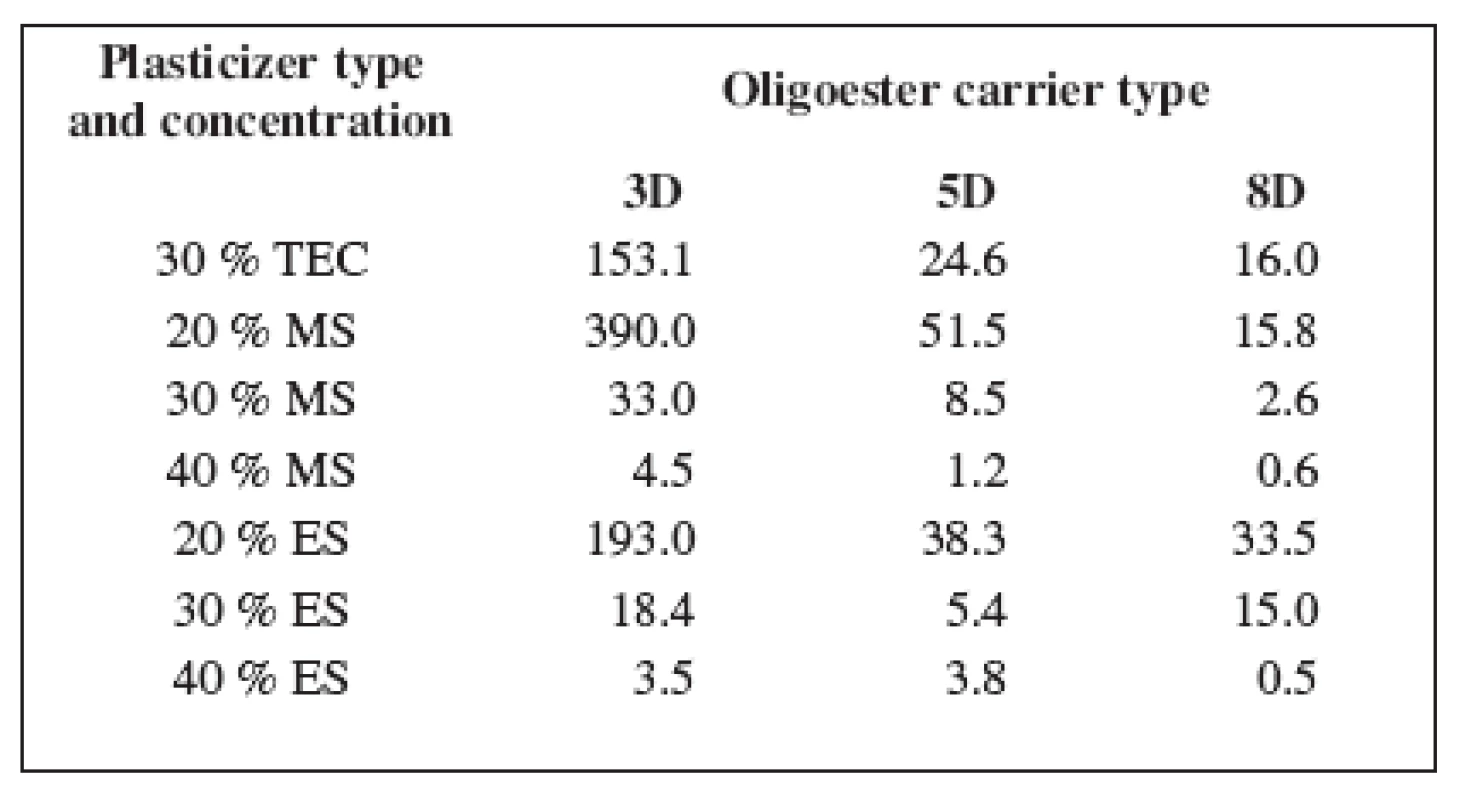
Despite of plasticizer type and concentration, carrier 3D possessed the highest viscosity, carrier 5D came after and the lowest viscosity was found in carrier 8D. Dynamic viscosity of the oligoester carriers is influenced by both molecular weight and degree of branching. The highest viscosity of carrier 3D with the highest value of molecular weight and the lowest branching of the chainis related to the lower random coil density and higher radius of gyration.
All plasticizers used in this study have good plasticization efficiency on tested oligoester carriers. The plasticization efficiency is related to the concentration and molecular weight of the plasticizer 12). The highest effectiveness in viscosity lowering was achieved using 40% of MS and 40% of ES. Dynamic viscosity of all tested carriers did not exceed 5 Pa.s. The plasticizer concentration of 20% in the case of both MS and ES, and in the case of TEC in a concentration of 30% might be critical, since bellow this concentrations no sufficient plasticizing effect could be reached. The effectiveness of plasticization is decreased with increasing molar mass of the plasticizer. So the effective concentration of TEC was found to be ten percent greater in comparison with MS or ES.
Adhesiveness of plasticized oligoesters was measured as the maximum force required for the detachment of tested materials from porcine mucin as the model substrate. The adhesion strength should be sufficient to keep the adhesive material in adhesion, but not so aggressive as to damage the biological tissue in the site of administration. As shown in Figure 1, the highest adhesive force was found in carriers plasticized with 20% of MS. An extremely high adhesive force in the case of carrier 3D plasticized with 20% of MS is probably the result of the complex effect of the highest molecular weight, the highest branching degree and very high dynamic viscosity. There is a significant influence of the plasticizer concentration on the adhesivity of all the carriers tested. The higher concentration of the plasticizer is applied, the lower dynamic viscosity and lower adhesive force was found out. This is in accord with the wetting theory of bioadhesion 13). The adhesive material with lower viscosity has the higher ability to spread onto a surface as a prerequisite for the development of adhesion.
Fig. 1. The adhesive force of branched polyesters plasticized by various plasticizers 
The main focus of this study was to find out the correlation between dynamic viscosity of the carriers plasticized with plasticizers in various concentrations and the adhesiveness of these carriers. The measured values of the dynamic viscosity of three plasticized oligoesters and the corresponding values of the adhesive force are shown in Figure 2. It is evident that the adhesive forces of the tested carriers ranged most often between 25 N and 35 N. The corresponding values of dynamic viscosity are bellow 40 Pa.s but higher than about 3 Pa.s. It is possible to establish that carrier 5D with 40% of MS, carrier 8D plasticized with ES in a concentration of 30% or 40%, and MS in a concentration of 30% do not possess a sufficient adhesive force due to low dynamic viscosity. On the other hand, the adhesive forces of carrier 3D plasticized with the use of 20% of MS, 20% of ES, or 30% of TEC with extremely high viscosity above 150 Pa.s exceeded the adhesive forces of 40 N. The optimal range of dynamic viscosity of the plasticized carriers providing sufficient bioadhesion was thus found.
Fig. 2. The relation between dynamic viscosity and adhesive force of plasticized carriers 
This study was supported by the grant MSM 0021620822.
Received 29 October 2009 / Accepted 4 November 2009
Address for correspondence:
PharmDr. Eva Šnejdrová, Ph.D.
Department of Pharmaceutical Technology, Faculty of Pharmacy
Heyrovského 1203, 500 05 Hradec Králové
e-mail: eva.snejdrova@faf.cuni.cz
Zdroje
1. Rutot, D., Duquesne, E., Ydens, I., Degée, P., Dubois, P.: Polym. Degrad. Stab., 2001; 3, 561–566.
2. Wang, N., Qui, J. S., Wu, X. S.: ACS Symp. Ser., 1998; 709, 242–253.
3. Martin, O., Averous, L.: Polymer, 2001; 42, 6209–6219.
4. Labrecque, L. V., Kumar, R. A., Davé, V., Gross, R. A., McCarthy, S. P.: J. Appl. Polym. Sci., 1997; 66, 1507–1513.
5. Ljungberg, N., Wesseln, B.: J. Appl. Polym. Sci., 2002; 86, 1227–1234.
6. Wu, Ch., Mc Ginity, J. W.: Int. J. Pharm., 1999; 177, 15–27.
7. Schade, A., Niwa, T., Takeuchi, H., Hino, T., Kawashima, Y.: Int. J. Pharm.; 1995, 117, 209–217.
8. Ahuja, A., Khar, R. P., Ali, J.: Drug Dev. Ind. Pharm., 1997; 23, 489–515.
9. Lehr, C. M., Bouwstra, J. A., Tukker, J., Junginger, H. E.: STP Pharma, 1989; 5, 857–862.
10. Mansour, M., Mansour, S., Mortada, N. D.: Drug Dev. Ind. Pharm., 2008; 34, 744–752.
11. Šnejdrová, E., Dittrich, M.: Čes. slov. Farm., 2006; 55, 262–266.
12. Qussi, B., Suess, W. G.: Drug Dev. Ind. Pharm., 2006; 32, 403–412.
13. Smart, J. D.: Adv. Drug Deliv. Rev., 2005; 57, 1556–1568.
Štítky
Farmacie Farmakologie
Článek Pandemic (H1N1) 2009Článek Comparison of renal accumulation of [DOTA0, 1-Nal3]-octreotide labelled with selected radiometalsČlánek OpravaČlánek AUTORSKÝ REJSTŘÍKČlánek VĚCNÝ REJSTŘÍK
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2009 Číslo 5-6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Methods of preparation of microparticles in pharmaceutical technology
- Pandemic (H1N1) 2009
- Hypolipidemic effect of amaranth constituents
- Antimycobacterial activity of novel derivatives of arylcarbonyloxyaminopropanols
- Comparison of renal accumulation of [DOTA0, 1-Nal3]-octreotide labelled with selected radiometals
- Adhesivity of branched plasticized oligoesters
- Influence of formulation technology on theophylline release from chitosan-based pellets
- Monitoring of surface cytotoxic drugs in the environment of hospital pharmacies in the Czech Republic
- Contributions to the development of advertising in pharmacy I
- A contribution to the histories of the pharmacies of the Brethren of Mercy in the territory of contemporary Slovakia in the first decades of the 20th century
- Examination of selected quality parameters of the parenteral nutrition AIO – a pilot study
- Oprava
- K životnému jubileu doc. RNDr. Zuzany Vitkovej, PhD.
- XXV. lékárnické dny Litoměřice, 3.–5. října
- AUTORSKÝ REJSTŘÍK
- VĚCNÝ REJSTŘÍK
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Methods of preparation of microparticles in pharmaceutical technology
- Contributions to the development of advertising in pharmacy I
- Examination of selected quality parameters of the parenteral nutrition AIO – a pilot study
- Hypolipidemic effect of amaranth constituents
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



