-
Medical journals
- Career
The hepatoprotective effect of the combination of glucosamine derivatives with quercetin against methotrexate-induced liver toxicity
Authors: Kateryna V. Vietrova; Igor A. Zupanets; Tatiana S. Sakharova
Authors‘ workplace: National University of Pharmacy ; Pushkinska Str. 27, 61057 Kharkiv, Ukraine ; Department of Clinical Pharmacology and Clinical Pharmacy
Published in: Čes. slov. Farm., 2020; 69, 222-229
Category: Original article
Overview
The article presents the results of the study of the combination of aminosugars – glucosamine hydrochloride (GA h/ch) and N-acetylglucosamine – with quercetin (Q) called GlukvamineTM as a corrector of hepatotoxicity induced by methotrexate (MTX). The study was conducted on a model of MTX-induced liver damage in rats. GlukvamineTM was studied in the dose of 82 mg/kg with daily intragastric administration for 10 days compared to the substance GA h/ch, which was administered intragastrically at a dose of 50 mg/kg, and the substance of Q, which was administered intragastrically in the dose of 20.5 mg/kg. The efficiency of the drugs was assessed by the general condition of the animals, the values of the liver mass coefficient, the biochemical parameters of the blood serum and the histological analysis of the liver tissue. The GlukvamineTM effect on rats with MTX-induced liver damage caused an improvement of their general condition, recovery of the functional state of the liver by biochemical indicators, and the results of the histological analysis indicated a decrease in hepatotoxicity of MTX. At the same time, GlukvamineTM has statistically significantly exceeded the effect of GA h/ch and Q by most parameters. Thus, GlukvamineTM is a promising effective and safe drug for pharmacological correction of the hepatotoxicity of MTX.
Keywords:
glucosamine – quercetin – GlukvamineTM – hepatoprotective effect – Methotrexate
Introduction
Methotrexate (MTX) is a folic acid anti-metabolite used for the treatment of cancer (leukemia, lymphoma, osteosarcoma) and autoimmune diseases (rheumatoid arthritis, psoriasis, Crohn’s disease, etc.)1, 2). However, in addition to a wide range of clinical applications, MTX induces serious side effects, including hepatotoxicity. It is known that MTX has a direct cytotoxic effect on hepatocytes, causes the development of cholestasis, and can also lead to fibrosis and cirrhosis of the liver3). The mechanism of the hepatotoxic effect of MTX is due to direct inhibition of dihydrofolate reductase, and it leads to disruption of the synthesis of DNA, RNA and protein, as well as the ability of the cytostatic to induce the oxidative stress and activate the processes of free radical oxidation (FRO) in membranes and other components of cells4, 5).
Pharmacocorrection of the predicted hepatotoxicity of MTX is a necessary component of concomitant therapy. Currently, drugs with proven hepatoprotective activity and a high level of evidence, in particular ursodeoxycholic acid, preparations of amino acids (ademetionine, ornithine aspartate, etc.) are used for this purpose. Despite the effectiveness most of these drugs can cause dyspeptic disorders (nausea, vomiting, flatulence, diarrhea), the functional stress of the liver (ursodeoxycholic acid) significantly worsening the delayed effects of the MTX treatment and reducing the patients’ compliance with support therapy6).
Currently, compounds of natural origin, which are endo - and exogenous metabolites of the human body and, therefore, meet safety criteria, are attracting great scientific interest for solving the problem of preventing and treating side effects of drugs with proven hepatotoxicity. The pronounced hepatoprotective action can be achieved due to the combination of several substances-metabolites with a multi-vector mechanism of action aimed at protecting liver cells from damage in one drug dosage form.
The results of the numerous studies conducted at the Department of Clinical Pharmacology and Clinical Pharmacy of the National University of Pharmacy (Kharkiv, Ukraine) have proven the feasibility of the combined use of glucosamine (GA) derivatives (GA hydrochloride (GA h/ch) and the biologically active form – N-acetylglucosamine (NAG) with flavonoid quercetin (Q). It has been shown that the effect of the pharmacodynamic synergism and mutual potentiation of pharmacological effects is manifested when they are used together7). Glucosamine is a natural aminosugar, a part of biological membranes, the intercellular substance and other components of the connective tissue of living organisms. It performs the membrane-stabilizing effect, plays an important role in the mechanism of the hepatoprotective action8–-11). Quercetin is one of the most common plant bioflavonoids, an exogenous regulator of a number of metabolic processes at the cellular level. Its direct antiradical properties and the structural antioxidant function, due to which membrane-stabilizing/cytoprotective effects are implemented, are the most studied ones; thus, its protective effect during MTX therapy will be determined12–16).
Taking into account the above the aim of our work was to experimentally substantiate the use of the combination of GA derivatives (GA h/ch and NAG) with quercetin (GlukvamineTM combination) as a possible corrector of the hepatotoxic effect of MTX in the experiment in rats.
Experimental part
Materials
The combination GlukvamineTM was used in the form of capsules for oral administration. It contains 370 mg of the capsule mass and has the following active ingredients (per 1 capsule): Q – 80 mg, GA h/ch – 125 mg, NAG – 125 mg. The composition and technology were developed at PJSC SIC Borschahivskiy CPP under the supervision of A.S. Shalamay, PhD in Chemistry. The substance of GA h/ch manufactured by Protein Chemicals (Japan) and the substance of Q by PJSC SIC Borschahivskiy CPP (Ukraine) were used as reference samples. Methotrexate from EBEWE Pharma (Austria) was used to induce pathology. The biochemical assays were performed using the commercial kits of Aspartate aminotransferase (AST) (cat. No. 10003150), Alanine aminotransferase (ALT) (cat. No. 10003151), Alkaline phosphatase (ALP) (cat. No. 10003197), Urea (cat. No. 10003079), Total protein (cat. No. 10008081) manufactured by Erba Lachema s.r.o. (Czech Republic).
Methods
Test object and its preparation
Glucosamine was used as the study object in the form of suspension in the conditionally therapeutic dose of 82 mg/kg intragastrically using a probe. The suspension was prepared with a previous grinding of the capsule mass in a mortar and using 0.9% sodium chloride solution for injections (Darnitsa Pharmaceutical Company, Ukraine).
Reference object and its preparation
The reference samples of the substances of GA h/ch and Q were administered intragastrically using a probe. The substance GA h/ch was used in the form of an aqueous solution in the conditionally therapeutic dose of 50 mg/kg. The reference sample of Q was used in the form of a suspension in the conditionally therapeutic dose of 20.5 mg/kg17). The suspension was prepared with the previous grinding of Q in a mortar and using 0.9% sodium chloride solution for injections (Darnitsa Pharmaceutical Company, Ukraine).
Experimental animals and grouping
Adult random-bred male albino rats with the body weight of 170–210 g were included in the present study. The rats (30 animals) were taken from the vivarium of the Central Research Laboratory (National University of Pharmacy, Kharkiv, Ukraine). The rats were housed under conventional laboratory conditions in standard polypropylene cages in a well-ventilated room at 25 ± 1 °C and a relative humidity 55 ± 5% with a regular 12 h light/12 h dark cycle18, 19). The animals received a standard diet and water ad libitum.
The research was conducted in accordance with the EU Directive 2010/63/EU on compliance with the laws, directives and administrative regulations of the EU countries on the protection of animals used for scientific purposes20). The study design was approved by the Bioethics Commission of the National University of Pharmacy (Protocol No. 12 of December, 17, 2014).
All animals were randomly divided into 5 experimental groups as follows:
- group 1 – intact control (healthy animals receiving the combination orally, n = 6)
- group 2 – control pathology (untreated animals receiving the combination orally (n = 6)
- group 3 – animals with the control pathology treated with GA h/сh orally in the dose of 50 mg/kg (n = 6)
- group 4 – animals with the control pathology treated with GlukvamineTM orally in the dose of 82 mg/kg (n = 6)
- group 5 – animals with the control pathology treated with Q orally in the dose of 20.5 mg/kg (n = 6)
Study design
Animals received the test samples daily for 10 days. GA h/ch was administered in the dose of 50 mg/kg corresponding to the effective dose by the hepatoprotective action21). GlukvamineTM was administered in the dose of 82 mg/kg corresponding to the effective dose in our preliminary studies17). Q was administered in the dose of 20.5 mg/kg corresponding to the dose of Q in the combination of GlukvamineTM studied. Animals in the groups of the intact control and the control pathology received an equivalent volume of 0.9% sodium chloride solution for injections (Darnitsa Pharmaceutical Company, Ukraine).
MTX in the dose of 20 mg/kg in the form of a solution for injection was administered once intraperitoneally to all groups of animals, except rats of the intact control, on the background of the introduction of objects (on the 8th day of the study)22). In 3 days after the simulation of pathology in rats (10 days after administration of the drugs) the hepatoprotective effect of the samples studied was assessed. Further, rats were taken out of the study in order to obtain their liver and blood for biochemical assays and histological examinations.
Preparation and storage of biological samples
At the end of the experiment, rats were euthanized under anesthesia with ketamine/xylazine (75/10 mg/kg, i.p.)23). Animals were weighed; the liver was removed and weighed. Blood samples were collected from the inferior vena cava and centrifuged at 1500 g at +4 °C for 10 min using an Eppendorf 5702R refrigerated centrifuge (Eppendorf, Germany). All biological samples were frozen and stored at –80 °C.
Evaluation of the liver mass coefficient N. K.
The liver mass coefficient (LMC) was determined by the ratio of the weight of the liver to the weight of the animal and expressed in %24).
LMC = (Mliver/Manimal) × 100 %
where Mliver is the mass of the liver, Manimal is the mass of the animal.
Evaluation of biochemical parameters N. K.
To evaluate the parameters, biochemical assays were performed using commercial kits of the company Lachema (Czech Republic) and an Express Plus automatic biochemical analyzer (Bayer Diagnostics, Germany). The content of substances reacting with thiobarbituric acid (TBA) – TBA-reactive products (TBA-RP) in the blood serum was determined by the reaction with TBA25). The activity of AsAT and AlAT in the blood serum of animals was found by the Reitman-Frankel reaction. The activity of ALP in the blood serum of animals was determined by the reaction with 4-nitrophenylphosphate. The content of total protein was determined by the biuret reaction. The concentration of urea was studied by the reaction with diacetylmonooxime.
Histological analysis N. K.
All samples were fixed in 10% formalin solution, dehydrated in high-strength alcohols, embedded into paraffin. Tissue sections were stained with Hematoxylin-Eosin (HX&E). The micropreparations were examined using a Granum L3003 microscope (Granum, China-Ukraine), photomicrography of microscopic images was performed using a Granum DCM 310 digital camera (Granum, China-Ukraine). The photos were processed on a Pentium 2,4 GHz computer using the Toup View program. The morphological studies were carried out at the premises of the Central Research Laboratory of the National University of Pharmacy under the supervision of senior researcher Y.B. Larianovska, PhD in Biology.
Statistical analysis N. K.
The results obtained were processed by descriptive statistics and presented as the mean ± standard error of the mean (M ± SE). Statistical differences between groups were analyzed using the Student’s t test26). The computer software used included IBM SPSS Statictics v. 22 (IBM Corp.) and MS Excel 2016 (Microsoft Corp.). The level of statistical significance was considered as p ≤ 0.05.
Results
The experimental data obtained indicate the hepatotoxic effect of MTX, which is consistent with the literature data5, 27). The LMC in the group of animals receiving MTX probably increased by 1.2 times compared to the intact group (Fig.1). In the absence of significant changes in the body weight of animals, such changes in the indicator are due to an increase in the liver mass, which can be caused by the development of hemodynamic disorders and congestions in the parenchyma of the organ27, 28).
1. The liver mass coefficient in rats with MTX intoxication under the effect of GlukvamineTM and reference objects of GA h/ch and Q (n = 30)
*significant relative to the intact control group (р ≤ 0.05)
**significant relative to the control pathology group (р ≤ 0.05)
•significant relative to the reference object of Q (р ≤ 0.05)
••significant relative to the reference object of GA h/ch (р ≤ 0.05)
n – the number of animals in the experiment
As can be seen from the table, the introduction of the samples studied reduced the toxic effect of MTX. LMC in the group of animals that received GlukvamineTM against the background of MTX significantly decreased relative to the control pathology group (by 1.1 times), and this indicator significantly differed by the severity of changes from that in the groups of reference samples. In the groups of animals receiving reference samples of GA h/ch and Q, no significant changes were observed compared to the control group by the value of LMC.
The literature data analysis shows that the mechanism of MTX toxic action is based on the free-radical oxidation (FRO) induction and increased membrane permeability22). The results of the experiment showed that the process of lipid peroxidation was activated in animals with MTX-induced intoxication; it was evidenced by a significant increase in the content of ТBA-RP in the blood serum by 2.1 times relative to the intact group (Table 1).
1. The impact of the study objects on the biochemical parameters of the blood serum of rats with methotrexate intoxication (M ± SE, n = 30) 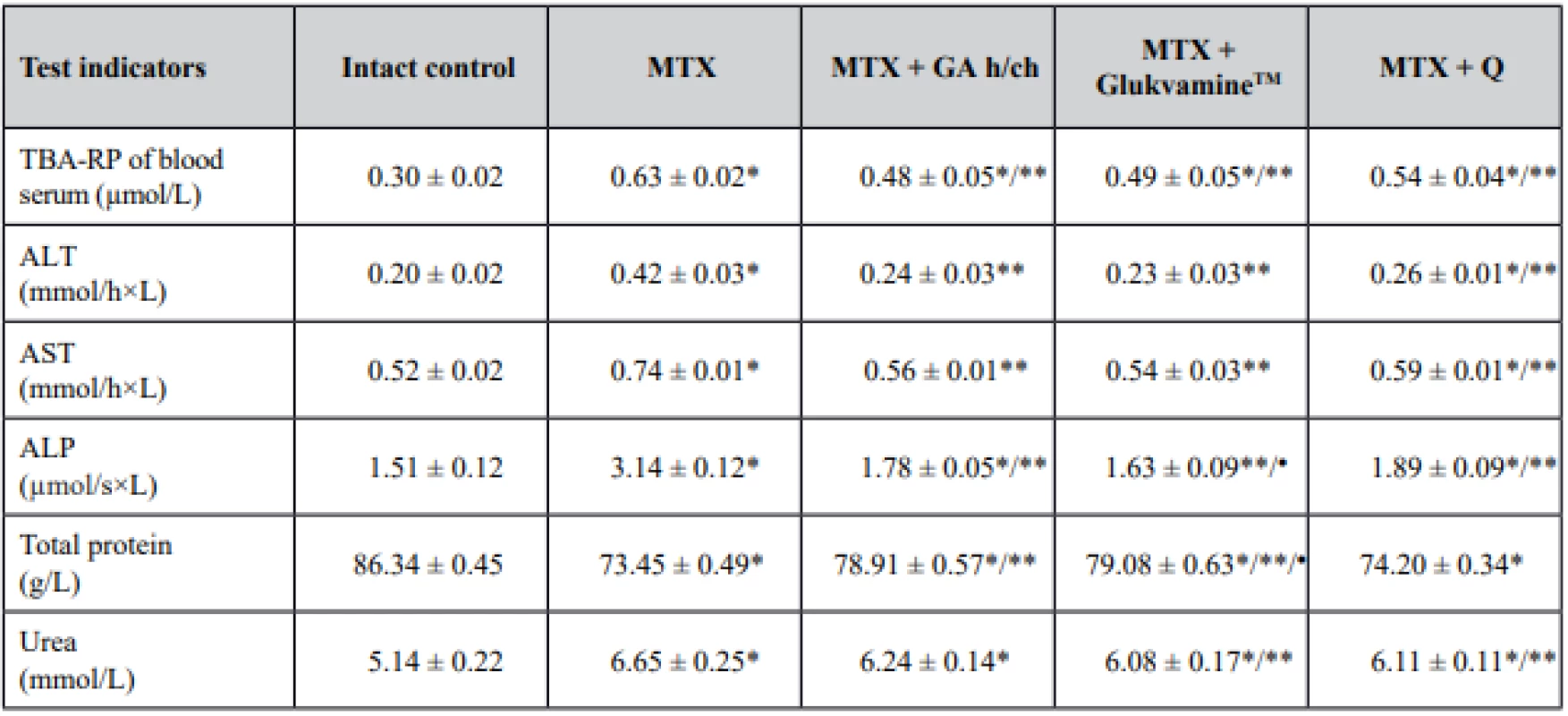
*significant relative to the intact control group (р ≤ 0.05)
**significant relative to the control pathology group (р ≤ 0.05)
•significant relative to the reference object of Q (р ≤ 0.05)
n – the number of animals in the experiment
There was also a significant increase in the activity of indicator enzymes of cytolysis and cholestasis: the activity of AST increased by 1.4 times, ALT – by 2.1 times, and alkaline phosphatase (ALP) – by 2.1 times relative to the group of intact animals (Table 1). Against the background of lipid peroxidation activation and hyperenzymemia, protein metabolism disorders were observed in animals of the control pathology group – a significant decrease in the total protein content relative to the intact group (by 1.2 times) and an increase in the urea concentration (by 1.3 times) in the blood serum of animals. It generally indicates the predominance of protein catabolism processes due to the mechanism of MTX hepatotoxicity with oxidative modification of proteins and accumulation of toxic metabolic products29, 30).
The analysis of biochemical indicators in the groups of animals studied revealed a positive dynamics towards leveling the toxic effect of MTX. Administration of GlukvamineTM and the reference samples of GA h/ch and Q to rats suppressed FRO initiated by MTX. It was shown by a significant decrease in the level of the blood serum ТBA-RP by 1.3, 1.3 and 1.2 times, respectively, relative to the control group of animals (Table 1). All the samples studied also prevented the development of cytolysis and cholestasis to varying degrees on the background of MTX toxicity (Table 1). In groups of rats that received GlukvamineTM and the reference sample of GA h/ch on the background of MTX the ALT activity significantly decreased by 1.8 times relative to the control pathology group and almost reached the same activity as in the group of intact animals (Table 1). The ALT activity in the group of animals that received the reference sample of Q on the background of MTX significantly decreased by 1.6 times relative to the control group, but did not reach the intact values. Administration of GlukvamineTM and the reference samples to animals on the background of MTX-induced intoxication also led to a significant decrease in the activity of the AST enzyme in the blood serum of rats: in the group of GlukvamineTM – by 1.4 times, and in the groups of GA h/ch and Q – by 1.3 times relative to animals in the control pathology group (Table 1). The AST activity in the groups of GlukvamineTM and the reference sample of GA h/ch did not significantly differ from the similar value in the intact group.
Against the background of the toxic effect of MTX the combination of GlukvamineTM studied showed the ability to reduce the phenomena of cholestasis, which manifested in reduction of the ALP activity by 1.9 times in the control group of animals. In addition, the ALP activity in this group of animals significantly differed from that in the group of the Q reference sample and did not significantly differ from the intact group. Under the effect of the reference samples of GA h/ch and Q the ALP activity significantly decreased by 1.8 and 1.7 times, respectively, compared to the group of rats treated with the cytostatic, but did not reach the values of the group of intact animals. On the background of MTX-induced intoxication the combination studied also reduced hypoproteinemia and within certain limits normalized the concentration of urea in the blood serum of the treated rats (Table 1). The total protein content in the blood serum of animals that received GlukvamineTM on the background of MTX significantly increased by 1.1 times compared to the control group of rats, and this indicator significantly differed from that in the group of the Q reference sample. In the group of the GA h/ch reference sample the total protein content significantly increased by 1.1 times compared to the control group of rats, while in the Q group it did not significantly differ from that in the control group of animals.
In animals that received GlukvamineTM and the Q reference sample on the background of MTX the urea concentration significantly decreased by 1.1 times (p ≤ 0.05) relative to the control pathology group. In the group of rats that received the reference sample of GA h/ch the urea concentration did not significantly change compared to the control group. It should be noted that in any of the rat groups the urea concentration did not reach the value of the group of intact animals.
The macroscopic examination of livers from the intact control group showed a normal red-brownish, smooth and shiny appearance. On the contrary, livers obtained from the MTX-induced damage group were enlarged and yellowish. Most of livers taken from the GlukvamineTM group had a normal color and appearance like in the intact control group, while most of livers from protective groups GA h/ch and Q showed a normal color, but were somewhat enlarged.
The microscopic examination of the samples of liver tissues of the intact control showed the normal hepatic architecture. Each hepatic lobule consisted of anastomosing radially distributing hepatocytes. Hepatocytes were of characteristic shape and size with well-defined boundaries, the cytoplasm was acidophilic, evenly colored, optically dense, and did not contain inclusions. The nuclei of hepatocytes were normochromic, centrally located and contained 1, sometimes 2 nucleoli. Kupffer cells were common. The hepatic portal tracts were seen at the periphery of the lobule. Portal tracts had branches of the portal vein, hepatic artery and bile duct (Fig. 2A).
2. Photomicrographs of samples of liver tissues of intact control rats (A), rats treated with MTX (B, C): a focus of hepatocyte necrosis (В) (× 250), vacuolar degeneration of hepatocytes (C) (HX&E × 200) 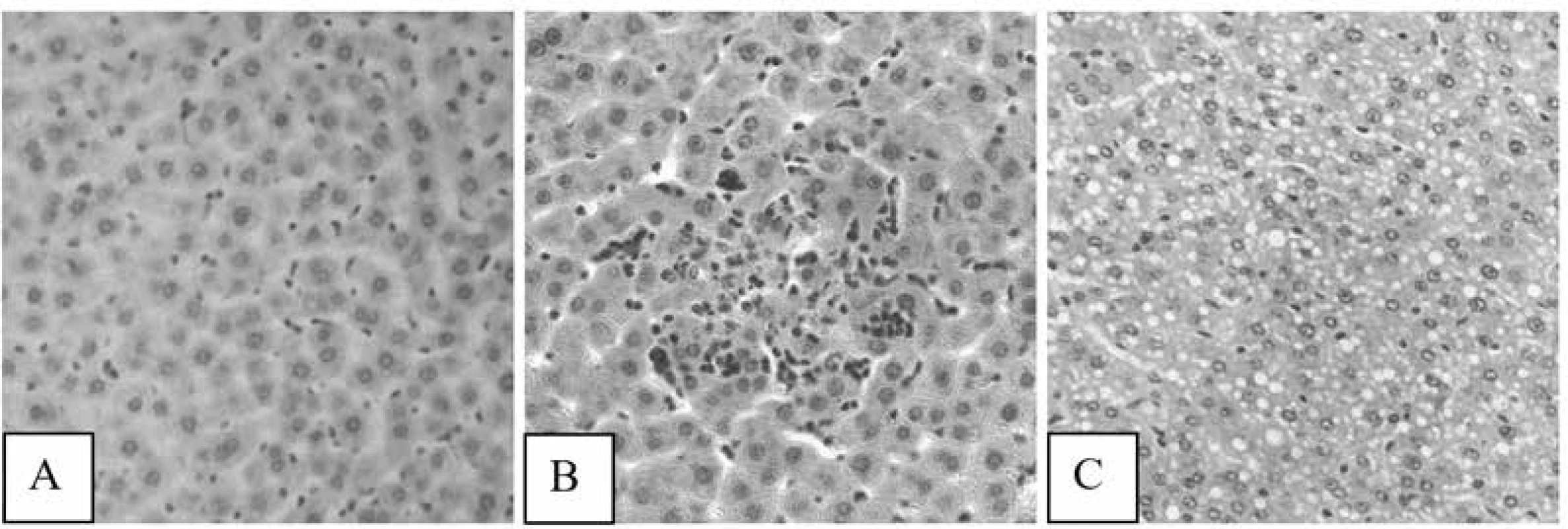
The samples of liver tissues of rats exposed to methotrexate showed signs of local hemodynamic disorders, and it confirmed the increase of LMC. Erythrocyte stasis and thrombosis were observed in the collecting veins and in the surrounding sinusoidal capillaries. The inflammatory cell infiltrations were detected around the region of portal triads. The foci of hepatocyte necrosis of different sizes were seen quite often (Fig. 2B). Vacuolar degeneration of hepatocytes of varying severity was observed (Fig. 2C). The number and size of areas of extramedullary hematopoiesis was increased. Activation of Kupffer cells and their small proliferates was observed, and it was a marker of the excess antigen elimination. The proliferation of the bile ductules was observed.
There was a marked improvement of liver tissues when examining the liver samples in the GlukvamineTM group. Dystrophic changes in hepatocytes and foci of cell necrosis were not observed (Fig. 3A). Сongestion of collecting veins and blood vessels of the triads was normal. The number of binucleated hepatocytes was significantly increased. The number of areas of extramedullary hematopoiesis was decreased. The inflammatory cell infiltrations were not observed. Kupffer cells remained in an activated state, but cell proliferation was not seen.
3. Photomicrographs of samples of liver tissues of rats treated with GlukvamineTM (A), GA h/ch (B) and Q (C): A – the normal arrangement of hepatocytes, B, C – small foci of hepatocyte necrosis (HX&E × 200) 
Significant hemodynamic disorders and dystrophy of hepatocytes of the samples of liver tissues in the GA h/ch group were not observed. The inflammatory cell infiltrations were not observed. The number of areas of extramedullary hematopoiesis was decreased. However, small foci of hepatocyte necrosis in different places were observed. The number of binucleated hepatocytes was not increased. Kupffer cells were activated (Fig. 3B).
There was a weak vacuolar degeneration of hepatocytes in 50% samples of liver tissues in the Q group. Small foci of hepatocyte necrosis and moderate inflammatory cell infiltrations were observed. Kupffer cells were activated. The number of binucleated hepatocytes was decreased (Fig. 3C).
Discussion
Hepatotoxicity is caused by the impact of various pathogenic factors (chemical, biological, etc.) and is manifested by degeneration of liver cells, necrosis and changes in the main functions of the liver. Thus, hepatotoxicity is one of the serious side effects of the MTX treatment with a cytostatic of the antimetabolite group of folic acid antagonists31). After distribution in tissues, high concentrations of MTX in the form of polyglutamate are found in various organs (and in particular, in the liver) where the cytostatic can be retained for several weeks or even months. Studies of many scientists suggest that the occurrence of toxic effects in relation to the liver is due to the activation of cytostatic processes of FRO, oxidative modification of proteins, nucleic acids and the accumulation of toxic metabolic products3–5, 27–31). Since the hepatotoxic effect of MTX can be a significant limiting factor for its clinical use, today it is of interest to search and study drugs with hepatoprotective properties to reduce the negative effect of cytostatics on the liver. This study evaluated the effect of the combination of derivatives of GA with Q called GlukvamineTM on MTX-induced hepatotoxicity in rats.
The analysis of the data shows that under the effect of a single intraperitoneal administration of MTX in the dose of 20 mg/kg a full-scale picture of the experimental intoxication corresponding to the known literature data develops in rats16, 27, 31). Lethality was not observed in the control pathology group of rats, but the condition of the animals was quite severe as evidenced by their lack of mobility, loss of appetite, and the occurrence of diarrhea. Against the background of the introduction of MTX there was an increase in LMC (1.2 times (p ≤ 0.05), which indicated the development of dystrophic and necrobiotic processes in the parenchyma of the organ31). As it is known, the mechanism of MTX-induced hepatotoxicity plays an important role in increasing the level of reactive oxygen intermediate (ROI) and free radicals as a result of oxidative stress and activation of the process of lipid peroxidation. Since ТBA-RP is one of the end products of the process of lipid peroxidation, its content was used as a marker to determine the extent of tissue damage. Under the effect of cytostatics the content of ТBA-RP in the blood serum increased by 2.1 times (p ≤ 0.05), and it confirmed the activation of the process of lipid peroxidation5, 27, 31). The development of distinct hyperfermentemia against the background of the MTX administration (the AST activity increased by 1.4 times, ALT and ALP – by 2.1 times (p ≤ 0.05)) indicated the structural integrity disturbance of the liver28–30). Disorders of metabolic processes against the background of the toxic effect of MTX with a predominance of decomposition processes were reflected by a decrease in the total protein content by 1.2 times and an increase in the urea concentration by 1.3 times (p ≤ 0.05). These changes are obviously associated with the disturbance of protein structures and formation of protein adducts with ROI under the effect of MTX, as well as with the manifestation of the nephrotoxic action of the cytostatic (the loss of protein by the renal route is possible)29). Administration of MXT caused a marked deterioration of the histological architecture of the liver tissue compared to the intact control group. There were histological changes in the samples of liver tissues, such as inflammatory cell infiltration, vacuolar degeneration of hepatocytes, foci of hepatocyte necrosis, activation of Kupffer cells, proliferated bile ductules16, 28).
The results of the study indicate that under the effect of GlukvamineTM the manifestations of MTX-induced hepatotoxicity in rats were reduced. The general condition of animals that received GlukvamineTM improved: the rats did not lose the desire to eat, drink and did not differ in appearance, behavior from the animals of the intact group, and they did not have diarrhea in contrast to the control group. Administration of GlukvamineTM contributed to the reduction of congestion in the liver parenchyma as evidenced by a decrease in LMC by 1.1 times (p ≤ 0.05). A probable decrease in the level of the blood serum ТBA-RP by 1.3 times indicated the inhibition of the activity of the process of lipid peroxidation against the background of the experimental combination introduction. Administration of GlukvamineTM reduced the cytolytic processes and phenomena of cholestasis, and it was reflected by a significant decrease in the activity of the corresponding marker enzymes (AST – by 1.4 times, ALT – by 1.8 times and ALP – by 1.9 times). A probable decrease in hypoproteinemia and normalization of the urea concentration relative to the control pathology group indicated the restoration of liver metabolic functions under the effect of the combination. The treatment with GlukvamineTM improved the structural and functional state of the liver. There was a decrease in dystrophic changes and foci of necrosis, an increase in activity of regeneration processes in the liver.
Compared to the reference samples of Q and GA h/ch groups by the value of LMC, a number of the biochemical parameters (ALP activity, urea concentration and total protein content), as well as the results of histological analysis, the use of GlukvamineTM was more effective. The hepatoprotective activity of GlukvamineTM under conditions of the toxic effect of MTX can be attributed to the antioxidant, anticytolytic, membrane-stabilizing, anabolic, and anti-inflammatory action. This is due to the properties of its individual active components – derivatives of GA and Q.
According to the results of the studies previously conducted at the Department of Clinical Pharmacology and Clinical Pharmacy of the National University of Pharmacy (Kharkiv, Ukraine) it has been found that when taken orally amino sugars (GA h/ch and NAG) increase the bioavailability and enhance the prolongation of the pharmacokinetic profile of Q (the absorption intensity of Q increases by 2.5 times compared to the pure substance). It has been also proven that there is no pharmaceutical interaction between derivatives of GA and Q, which excludes irreversible chemical interaction between them. In addition, the combination of GlukvamineTM is safe due to the harmlessness of its monocomponents7).
Therefore, all the above data and the research results substantiate the feasibility of further study of GlukvamineTM as an effective and safe agent for correcting the hepatotoxic effects of MTX.
Conclusion
The introduction of GlukvamineTM has a pronounced protective effect in relation to the rat liver under conditions of MTX-induced intoxication. The hepatoprotective effect of GlukvamineTM statistically significantly exceeds the similar effect of the reference samples (GA h/ch and Q) under conditions of MTX-induced intoxication in rats. The results obtained serve as an experimental substantiation for further in-depth preclinical study of the pharmacodynamics and pharmacology of safety of GlukvamineTM as a modifier of the toxic effect of MTX to optimize the support therapy during the treatment of diseases accompanied by excessive cell proliferation.
Conflict of interest: none.
Assistant Kateryna V. Vietrova, Ph.D. (∗) • Igor A. Zupanets • Tatiana S. Sakharova
Department of Clinical Pharmacology and Clinical Pharmacy
National University of Pharmacy
Pushkinska Str. 27, 61057 Kharkiv, Ukraine
e-mail: vkv_katya@ukr.net
Sources
1. EMA/414775/2019. New measures to avoid potentially fatal dosing errors with methotrexate for inflammatory diseases. https://www.ema.europa.eu (23.08.2019).
2. Mohammad K. Z., Tooba S., Syed S. A., Haseeb A., Fahim H. K. Understanding the binding interaction between methotrexate and human alpha-2-macroglobulin: Multi-spectroscopic and computational investigation. Archives of Biochemistry and Biophysics 2019; 675, Article 108118. https://www.sciencedirect.com.
3. LiverTox: Clinical and research information on drug induced liver injury. methotrexate. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.ncbi.nlm.nih.gov/books/NBK548219/ (19.02.2020).
4. Fouad A. A., Hafez H. M., Hamouda A. Hydrogen sulfide modulates IL-6/STAT3 pathway and inhibits oxidative stress, inflammation, and apoptosis in rat model of methotrexate hepatotoxicity. Hum. Exp. Toxicol. 2020; 39(1), 77–85.
5. Hoshyar R., Sebzari A., Balforoush M., Valavi M., Hosseini M. The impact of Crocus sativus stigma against methotrexate-induced liver toxicity in rats. J. Complement. Integr. Med. 2019; 17(2), Article 20190201. https://www.degruyter.com
6. Hempfling W., Dilger K., Beuers U. Systematic review: ursodeoxycholic acid – adverse effects and drug interaction. Aliment. Pharmacol. Ther. 2003; 18(10), 963–972.
7. Zupanets K. O. The experimental foundation of the combined application of glucosamine derivatives with quercetine in different variants of osteoarthritis. Kharkiv: Ph.D. Thesis 2011, 183 p (in Ukrainian).
8. Seyed H. M., Elham B., Azar H., Khadijeh J. Protective effects of glucosamine and its acetylated derivative on serum/glucose deprivation-induced PC12 cells death: Role of reactive oxygen species. Res. Pharm. Sci. 2018; 13(2), 121–129.
9. El-Horany H. E., El-Latif R. N., ElBatsh M. M., Emam M. N. Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson’s disease: Modulating Autophagy (Quercetin on Experimental Parkinson’s Disease). J. Biochem. Mol. Toxicol. 2016; 30(7), 360–369.
10. Tahia H. S., Nagwa A. E., Mohammed H. H., Nahed A. M., Nashwa A. M. M., Emaad A., Azza S. T. Comparative protective effects of n-acetylcysteine, N-acetyl methionine, and N-acetyl gucosamine against pracetamol and phenacetin therapeutic doses–induced hepatotoxicity in Rats. Int. J. Hepatol. 2018; 2018, Article 7603437. https://www.hindawi.com
11. Ying-Jen C., Yuahn-Sieh H., Jiann-Torng C., Yi-Hao C., Ming-Cheng T., Ching-Long C., Chang-Min L. Protective effects of glucosamine on oxidative-stress and ischemia/reperfusion-induced retinal injury. Investig. Ophthalmol. Vis. Sci. 2015; 56, 1506–1516.
12. Fernández-Palanca P., Fondevila F., Méndez-Blanco C., Tuñón M. J., González-Gallego J., Mauriz J. L. Antitumor effects of quercetin in hepatocarcinoma in vitro and in vivo models: a systematic review. Nutrients 2019; 11, Article 2875. https://www.mdpi.com/journal/nutrients
13. Wei C. B., Zhou L. D., Zhang J. W., Zhang Q. H., Tao K. Protective effects of quercetin against the triptolide induced liver injury and relevant mechanism study. Sichuan Da Xue Xue Bao Yi Xue Ban 2019; 50(5), 684–687 (in Chinese).
14. Zohreh S. H., Azita A., Leila J., Farzad S. Effect of quercetin on oxidative stress and liver function in beta-thalassemia major patients receiving desferrioxamine: A double-blind randomized clinical trial. J. Res. Med. Sci. 2019; 24, Article 91. https://www.jmsjournal.net
15. Haleagrahara N., Hodgson K., Miranda-Hernandez S., Hughes S., Kulur A. B., Ketheesan N. Flavonoid quercetin-methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology 2018; 26(5), 1219–1232.
16. Eman E. B., Kamal M. M. The possible protective effect of quercetin on methotrexate induced hepatotoxicity in albino rats: biochemical , histological and immunohistochemical study. Egyptian Journal of Histology 2019; 43(1), 220–229.
17. Vietrova K. V. Experimental evidance of the expediency of correction of the toxic effects of anticancer drugs by derivatives of glucosamine and their combination with quercetin. Kharkiv: Ph.D. Thesis 2015 (in Ukrainian).
18. Guide for the care and use of laboratory animals, 8th ed. Washington: National Academies Press 2011.
19. Sharp P., Villano J. S. The laboratory rat, 2nd ed. Boca Raton: CRC Press 2013.
20. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union 2010; L276, 33–79.
21. Zupanets I. A. Experimental rationale for the use of glucosamine and its derivatives in medicine. M.D. Thesis, Kupavna, 1993 (in Russian).
22. Vardi N., Parlakpinar H., Cetin A., et al. Protective effect of β-carotene on methotrexate-induced oxidative liver damage. Toxicologic. Pathology 2010; 38, 592–597.
23. Flecknell P. A. Laboratory animal anesthesia, 4th ed. Oxford: Academic Press 2015.
24. Stefanov O. V. Preclinical studies of drugs. Kiev: Avicenna 2001 (in Ukrainian).
25. Devasagayam T. P. A., Boloor K. K., Ramasarma T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian Journal of Biochemistry & Biophysics 2003; 40, 300–308.
26. Rebrova O. Yu. Statistical analysis of medical data. Application of the STATISTIKA software package, 3rd ed. Moscow: Media Sphere 2006 (in Russian).
27. Alexander V. A. D., Namani S., Subramani P., Sengodan B., Radhakrishnan A. Ameliorative Effect of Quercetin on Methotrexate Induced Toxicity in Sprague-Dawley Rats: A Histopathological Study. Indian J. Pharm. Educ. 2016; 50(3), S200–S208.
28. Erdogan E., Ilgaz Y., Gurgor P. N., Oztas Y., Topal T., Oztas E. Rutin ameliorates methotrexate induced hepatic injury in rats. Acta Cir. Bras. 2015; 30(11), 778–784.
29. Bhakti A. M., Thankamani M. Protective effect of Morinda citrifolia L. (fruit extract) on methotrexate-induced toxicities – hematological and biochemical studies. Cogent Biology 2016; 2, Article 1207879. https://www.cogentoa.com
30. Kumari S. Methotrexate Induced Hepatotoxicity and its Management. Int. J. Sci. Res. 2016; 5(9), 1477–1481.
31. Cao Y., Shi H., Sun Z., Wu J., Xia Y., Wang Y., Wu Y., Li X., Chen W., Wang A., Lu Y. Protective Effects of Magnesium Glycyrrhizinate on Methotrexate-Induced Hepatotoxicity and Intestinal Toxicity May Be by Reducing COX-2. Front. Pharmacol. 2019; 10, Article 119. https://www.frontiersin.org
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 5-6-
All articles in this issue
- Automated preparation of radiopharmaceuticals as a tool of radiation protection optimisation of staff
- Determination of thiamine and pyridoxine in food supplements and beverages by the simple capillary zone electrophoresis in combination with UV detection
- K životnímu jubileu prof. RNDr. Jozefa Csölleiho, CSc.
- Focus on perchlozone, an anti-tuberculosis drug from the Russian Federation
- Development and validation of a HPLC method for quantifica-tion of degradation impurities of salbutamol sulfate with following long-term stability studies in multicomponent cough syrup
- The hepatoprotective effect of the combination of glucosamine derivatives with quercetin against methotrexate-induced liver toxicity
- Meropenem serum concentrations in intensive care patients: a retrospective analysis
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Meropenem serum concentrations in intensive care patients: a retrospective analysis
- K životnímu jubileu prof. RNDr. Jozefa Csölleiho, CSc.
- Automated preparation of radiopharmaceuticals as a tool of radiation protection optimisation of staff
- Determination of thiamine and pyridoxine in food supplements and beverages by the simple capillary zone electrophoresis in combination with UV detection
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

