Determination of the colorants in vitamin E by HPLC with photodiode array detection
Authors:
Monika Šuleková; Alexander Hudák
Authors‘ workplace:
The University of Veterinary Medicine and Pharmacy, Košice, Slovak Republic
Published in:
Čes. slov. Farm., 2015; 64, 279-282
Category:
44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Besides active ingredients, most pharmaceutical products contain also a variety of additives. Among them, colorants are included as well. Colorants are added to drugs and nutritional supplements for commercial, psychological and practical reasons1). They are substances of natural origin, or are prepared synthetically. Natural dyes are often unstable and easily subject to degradation during the process of manufacture of medicinal products. Therefore, the natural dyes are often replaced by synthetic dyes, which have a higher stability and a low price. Some of them, when eaten in large amounts, pose a potential health risk. Recently, it has also been reported that artificial dyes may be responsible for children’s Attention Deficit Hyperactivity Disorder (ADHD), immune system problems, and certain allergic reactions2). In Europe, the use of colorants in food and pharmaceutical products is regulated by the Food Colour Directive N° 94/36/EC and N° 95/45/EC3, 4). Due to the regulatory restrictions regarding the use of food dyes, it seems important that the control of medicinal products encompasses not only qualitative and quantitative studies of active ingredients, but also that of inactive ingredients including colorants5). Vitamin E is a substance with many positive effects. Therefore, it is often a popular pharmaceutical product. On the market, it comes in the form of coloured capsules made of gelatine. The present work is aimed to determine the presence and amount of colour used in capsules of vitamin E colorization from a variety of manufacturers. The analysis was carried out by means of HPLC with a DAD detector.
Experimental methods
Chemicals and solutions
Gradient-grade methanol for HPLC (Sigma-Aldrich, France) and Acetonitrile for LC-MS (Merck, Czech Republic) was used for analysis. Solid dye standard of Ponceau 4R (Cochineal Red, E124) was obtained from the Institute for Engineering of Polymer Materials and Dyes, Department of Dyes and Organic Products, in Zgierz (Poland), and the standard of carmine (Cochineal, E120) was obtained from Progast (Bratislava). Analytical grade sodium acetate trihydrate was supplied by Lach-Ner (Czech Republic). Ultra-pure water (Purelab Flex with purification pack: LC208) was used for preparation of all solutions. Working solution was prepared by dissolving the appropriate amounts of powder of each colour in water to obtain a concentration of 0.5 mg ml-1. Calibration standards of dye were prepared by dilution of aliquots of the stock working solutions. The concentration range for the standard solution was 1–10 μg ml–1 of Ponceau 4R and 2,5–15 μg ml–1 of carmine. All solutions were stored in dark flasks at 5 °C.
Sample preparation
All the tested samples of vitamin E were obtained from the local pharmacy from a variety of manufacturers: Zentiva (Czech Republic), Noventis (Czech Republic), Dr. Max (Great Britain), Generica (Slovakia) and Jamieson (Canada). The gelatine capsules of vitamin E were accurately weighed and dissolved in water by heat. The sample solutions were placed in an ultrasonic bath (Sonorex Digitec, Bandelin, Germany) for 15 min for the complete extraction of the colorant, followed by centrifugation (B. Braun Sigma 2 K15, Germany) at 19000 rpm for 10 min. These solutions were filtered through a folder paper filter and the filtrate was collected in a volumetric flask of 10 ml. All solutions were injected after filtering. One ml of the supernatant liquid was then transferred into an HPLC auto-sampler vial for injection of 20 μL onto the column.
Instrumentation and HPLC analysis
The samples of vitamin E and colorants were analysed using high-performance liquid chromatography (HPLC). The HPLC system Dionex UltiMate 3000 RS (Thermo Fisher Scientific, Germany) consisted of a quaternary pump, a degasser, an automated injector, a column oven and a diode array detector. The DAD detector was set to collect signals within the range of 190–800 nm. Separation was performed on a chromatographic column (250 mm ×⋅ 4.6 mm) Polaris C18-A with a particle size of 5 μm (Varian, USA). The injection volume was 20 μL. During the chromatographic separation the mobile phase was kept isocratic, at a flow rate of 1.2 ml min–1. The mobile phase system consisted of A (CH3CN) and B (CH3COONa : CH3OH = 85 : 15, v/v) in the ratio of 20 : 80, v/v. The column was kept at 40 °C in the column oven. Analyses were performed with the Chromeleon Chromatography Data System, Version 7,2 (Thermo Fisher Scientific, Germany) for collecting and processing data. Each analysis was performed in three replicates.
Result and discussion
Ponceau 4R and carmine are used extensively as colour additives in drugs. For the purpose of the analysis in question, five preparations of vitamin E, produced by various manufacturers, were purchased from pharmacies. Three companies offer gelatine red coloured capsules, while two of the listed companies sell colourless gelatine capsules. The manufacturers Dr. Max and Jamieson do not state the use of any colorants. In the package leaflet for the user of the vitamin E (Zentiva), synthetic dye Ponceau 4R was mentioned among additives. A large number of analytical methods for Ponceau 4R have been proposed, such as thin layer chromatography (TLC)6, 7), polarography8), spectrophotometry9), ion chromatography10) and high-performance liquid chromatography (HPLC)11–14). Other manufacturers (Generica, Noventis) used to colorize the aforementioned capsules of vitamin E using the natural dye carmine. Carmine is usually produced by means of precipitation of carminic acid in aluminium hydroxide in the form of aluminium or of that of aluminium-calcium salt. Carminic acid is obtained from dried bodies of the female insect Dactylopius coccus or Cocus cacti L., which lives on cactus plants. Several analytical methods have been applied in order to determine the presence of carmine, including adsorptive stripping voltammetry15), spectrophotometry16, 17) and HPLC18). The manufacturers who do use the dyes do neither provide the data related to the amount of dye per capsule, nor per unit. For the sake of confirmation of the presence of declared colours in the samples, UV/VIS spectra of solutions of all samples were measured, as well as standard solutions of Ponceau 4R (7 μg ml–1) and carmine (10 μg ml–1). The spectra of the standards, in comparison with the spectrum of the sample of vitamin E, are shown in Figure 1 and 2. The identity of the UV/VIS spectra of the standards Ponceau 4R, or carmine with the spectrum of samples confirmed the presence of the dye declared by the manufacturer. The calibration solutions of Ponceau 4R (1–10 μg ml–1) and carmine (2,5–15 μg ml–1) were prepared by means of diluting the working solution in order to establish the quantity of the dye that is accounted for per capsule. The calibration solutions and solutions of samples were analysed by HPLC method by using a DAD detector, at 510 nm for Ponceau 4R and at 520 nm for carmine. The characteristics of the calibration plot are given in Table 1.
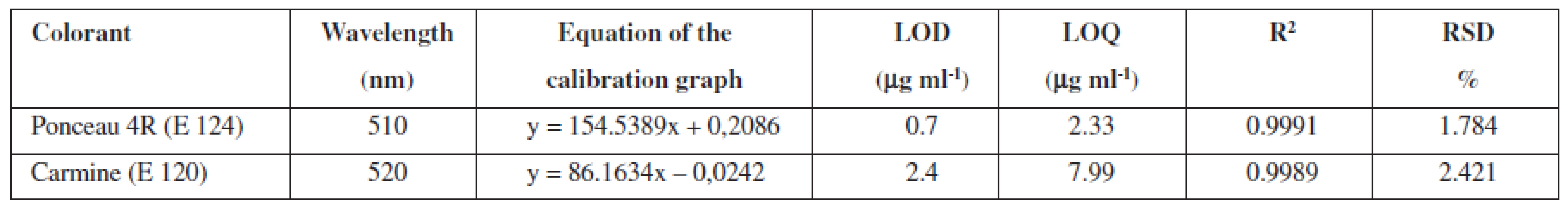
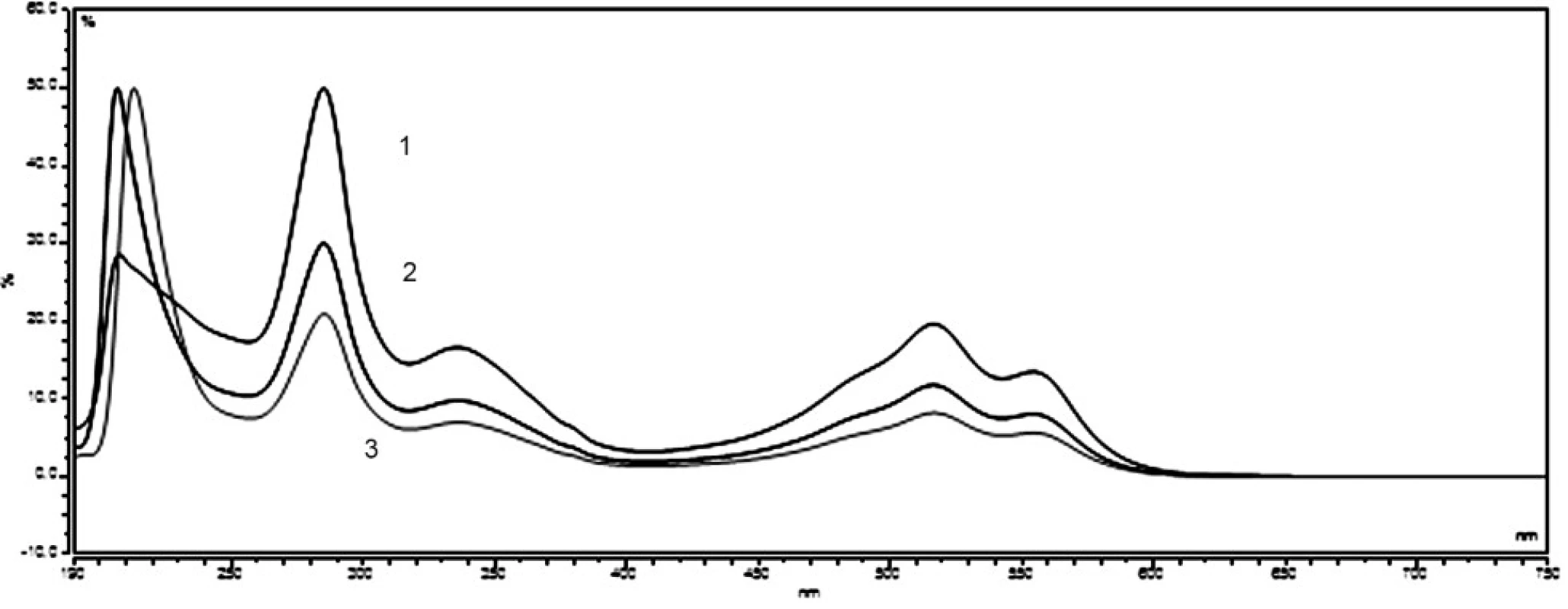
In furtherance of the analysis, the exact number of three capsules of vitamin E, which were individually weighed and then dissolved in ultra-pure hot water, was employed every time. Warmed to room temperature, the sample solution was transferred to a volumetric flask and the volume was adjusted to 10 ml with ultra-pure water. Each sample was measured three times. Table 2 demonstrates the results of the analysis. In the products of companies that stated no use of dye addition, HPLC analysis did not detected the presence of any dye.
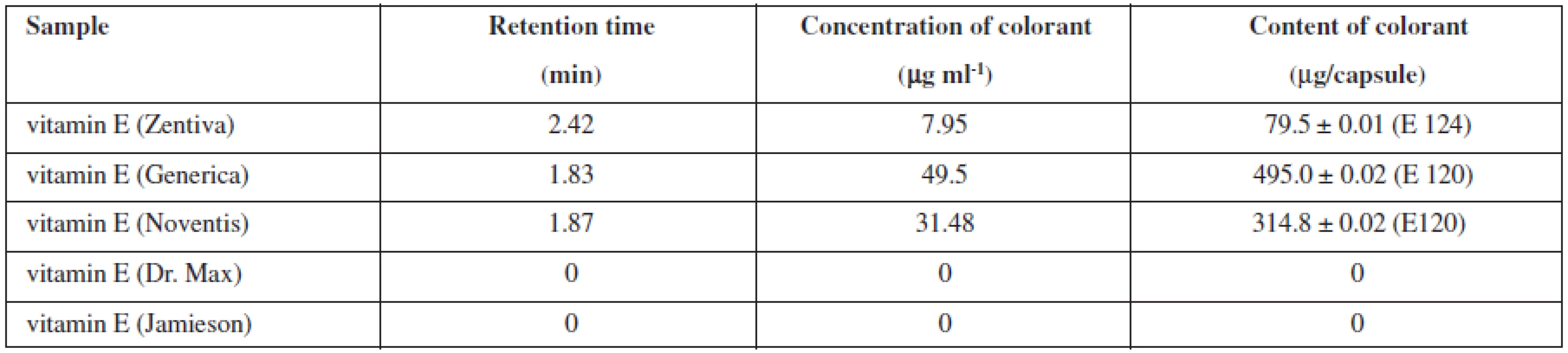
Manufacturers do not always inform us about the nature of the dyes used; what is more, they rarely establish the dye content as attributed to the weight unit of a particular drug. The expert medical circles have been increasingly talking about the relation between the use of additives in drugs and some health problems, such as allergy, immune deficiency syndrome and ADHD19, 20). The Acceptable Daily Intake (ADI) of Ponceau 4R is 0.7 mg/kg bw/day and of carmine is 5 mg/kg, based on body weight21, 22).
Conclusions
The presence of the synthetic dye Ponceau 4R in the capsule of vitamin E was declared by one manufacturer. The two products state the use of natural dye carmine. In two cases, no colorant is used. When using a dye, manufacturers do not establish the amount of the dye as attributed to the capsule or to the weight unit. So far, many studies have pointed to the negative impact of various synthetic, but also natural colorants on human health. A trend of tightening the limits for the use of mainly synthetic dyes in food and pharmaceutical products can be observed nowadays. Based on the results of the study of the impact of additive substances on human health, it is very well-founded and vital to monitor the amount of such substances, especially in drugs falling into the non-prescription category, and in various products for children.
Conflicts of interest: none.
RNDr. Monika Šuleková, PhD.
The University of Veterinary Medicine and Pharmacy in Košice, Department of Chemistry, Biochemistry and Biophysics, Institute of Pharmaceutical Chemistry
Komenského 73, 041 81 Košice, Slovak Republic
e-mail: monika.sulekova@uvlf.sk
Sources
1. Jendruchová M. Prečo sa lieky farbia. Nový čas pre ženy 2012; 28.
http://www.sukl.sk/buxus/lib/print_page/print_page.php
2. Swerlick R. A., Campbell C. F. Medication dyes as a source of drug allergy. Journal of Drugs in Dermatology 2013; 12(1), 99–102.
3. European Parliament and council directive 94/36/EC of 30 June 1994 on colours for use in foodstuffs. No L 237/13.
4. Commission Directive 95/45/EC of 26 July 1995, laying down specific purity criteria concerning colours for use in foodstuffs. No L 226/1.
5. Jaworska M., Szulinska Z., Wilk M., Anuszewska E. Separation of synthetic food colourants in the mixed micellar system, Application to pharmaceutical analysis. Journal of Chromatography A 2005; 1081, 42–47.
6. Ashkenazi P., Yarnitzky Ch., Cais M. Determination of synthetic food colours by means of a novel sample preparations system. Anal. Chim. Acta. 1991; 248, 289–299.
7. Sayar S., Őzdemir Y. First-derivative spectrophotometric determination of ponceau 4R, Sunset Yellow and Tartrazine in confectionery products. Food Chemistry 1998; 61(3), 367–372.
8. Chanlon S., Joly-Pottuz L., Chatelut M., Vittori O., Cretier J. L. Determination of Carmoisine, Allura red and Ponceau 4R in sweets and soft drinks by Differential Pulse Polarography. Journal of Food Composition and Analysis 2005; 18(6), 503–515.
9. Sayar S., Őzdemir Y. Determination of Ponceau 4R and Tartrazine in Various Samples by Derivative Spectrophotometric Methods. Tr. J. Of Chemistry 1997; 2, 182–187.
10. Chen Q. Ch., Mou S. F., Hou X. P., Riviello J. M., Ni Z. M. Determination of eight synthetic food colorants in drinks by high-performance ion chromatography. Journal of Chromatography A 1998; 827(1), 73–81.
11. Vlase L., Muntean D., Cobzac S.C., Filip L. Development and validation of an HPLC-UV method for determination of synthetic food colorants. Re. Roum. Chim. 2014; 59(9), 719–725.
12. Yoshioka N., Ichihashi K. Determination of 40 synthetic foo colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008; 74, 1408–1413.
13. Kirschbaum J., Krause C., Pfalzgraf S., Brückner H. Development and Evaluion of an HPLC-DAD. Method for Determination of Synthetic Food Colorants. Chromatogrphia 2003; 57, 115–119.
14. Minioti K. S., Sakellariou Ch. F., Thomaidis N. S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liuid chromatography coupled with diode-array detector. Analytica Chimica Acta 2007; 583, 103–110.
15. Alghamdi A. H., Alshammery H. M., Abdalla M. A., Alghamdi A. F. Determination of Carmine Food Dye (E120) in Foodstuffs by Stripping Voltammetry. Journal of AOAC International 2009; 92(5) 1454–1459.
16. Arslan Z. K., Aycan S. An Example of the Use of Spectrophotometric Method: Determining the Carmine in Various Food Products. Procedia – Social and Behavioral Sciences 2014; 4622–4625.
17. Gupta H. K. L D. F. Spectrophotometric study of the determination of boron by the carminic acid method. Microchimica Acta 1974; 62(3), 415–428.
18. Lim H. S., Choi J. Ch., Song S. B., Kim M. Quntitative determination of carmine in foods by high-performance liquid chromatography. Food Chemistry. 2014; 158, 521–526.
19. McCann D., Barrett A., Cooper A., Crumpler D., Dalen L., Grimshaw K., Kitchin E., Lok K., Porteous L., Prince E., Sonuga-Barke E., Warner JO., Stevenson J. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007; 370(9598), 1560–1567.
20. http://dx.doi.org/10.1016/S0140-6736(07)61306-3
21. Chung K., Baker Jr., J.R., Baldwin J. L., Chou A. Identification of carmine allergens among carmine allergy patients. Allergy 2001; 56, 73–77.
22. Regulations, Commission Regulation (EU) No 232/2012, 2012.
23. Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants. WHO Technical Report Series, No. 901. Geneva 2001; 10–12.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2015 Issue 6
Most read in this issue
- Antibacterial activity of natural compounds – essential oils
- Body surface area and body weight of Czech adult cancer population
- Cholinergic system of the heart
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
