-
Medical journals
- Career
Synthesis of triclosan derivatives and their antimycobacterial effect
Authors: Rudolf Vosátka; Martin Krátký; Jarmila Vinšová
Authors‘ workplace: Department of Inorganic and Organic Chemistry, Faculty of Pharmacy Charles University, Hradec Králové, Czech Republic
Published in: Čes. slov. Farm., 2015; 64, 302
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Tuberculosis (TB) represents one of the leading causes of morbidity and mortality worldwide. Development of new potential drugs is essential because of the existence of latent TB and development of drug-resistant TB forms (multidrug-resistant TB, extensively drug-resistant TB and recently reported totally drug-resistant TB)1, 2). Triclosan (irgasan) is a broad spectrum antibacterial agent used in household products. Triclosan has been shown to inhibit InhA, an essential enoyl acyl carrier protein which leads to the lysis of Mycobacterium tuberculosis3). Esterification of triclosan to form its prodrugs can produce compounds with improved properties – enhanced bioavailability or absorption, higher activity and/or lower toxicity.
Experimental methods
We used two synthetic procedures to obtain these esters. The first pathway consists in the reaction of triclosan (1 eq.) with various acyl chlorides (1.3 eq.) in presence of triethylamine (1.5 eq.). The second approach of the preparation triclosan esters is the Steglich esterification. Common yields were around 70 %. Synthesized derivatives were evaluated for their in vitro antimycobacterial activity against Mycobacterium tuberculosis H37Rv, M. avium and two strains of M. kansasii.
Results and discussion
It was prepared 28 triclosan esters based on various aliphatic, cycloaliphatic, aromatic and heteroaromatic acids. 5-Chloro-2-(2,4-dichlorophenoxy)phenyl 4-bromobenzoate (TRC-B-4Br) showed the best in vitro activity with minimum inhibitory concentrations (MIC) 16 μmol/L against Mycobacterium tuberculosis H37Rv. Against next strains had the best activity 5-chloro-2-(2,4--dichlorophenoxy)phenyl isonicotinate (TRC-ISO). The MIC of TRC-ISO was similar for M. kansasii 6509/96 and better for M. avium and M. kansasii 235/80 with comparison of INH (Table 1).
1. The most active derivatives 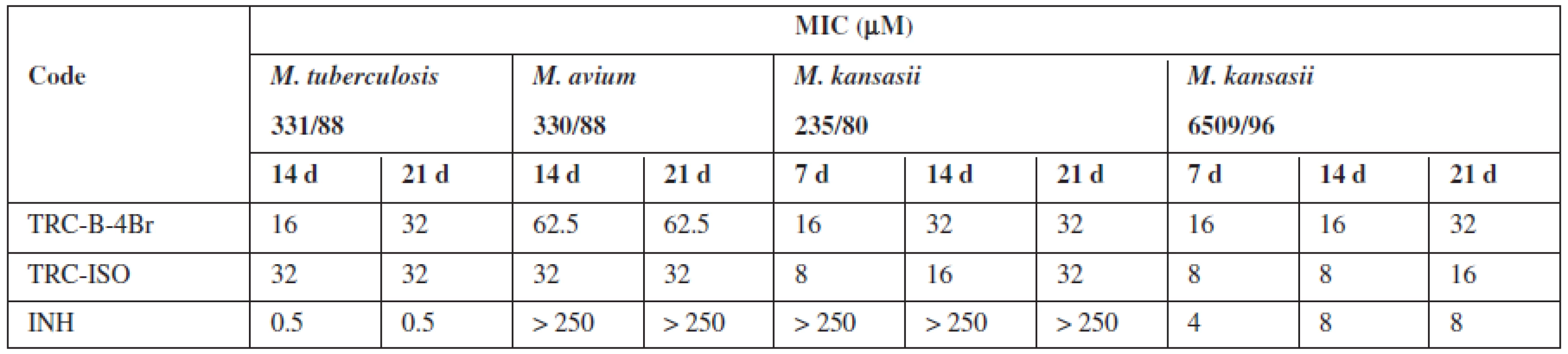
Conclusions
The in vitro evaluation of 28 triclosan-based esters showed promising antimycobacterial activity. The further research of the most active analogues will continue, particularly with regard to cytotoxicity.
Grant dedications: IGA NT 13346 (2012).
Conflicts of interest: none.
Mgr. Rudolf Vosátka
Department of Inorganic and Organic Chemistry
Faculty of Pharmacy Charles University
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: vosatkar@faf.cuni.cz
Sources
1. World Health Organization. Global tuberculosis report 2014. http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1 (30. 6. 2015).
2. Krátký M., Vinšová, J. Pokroky ve vývoji antituberkulotik působících na multilékově rezistentní kmeny. Chem. Listy 2010; 104, 998–1005
3. Stec J., Vilchéz C., et al. Biological evaluation of potent triclosan-derived inhibitors of the enoyl-acyl carrier protein reductase InhA in drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis. ChemMedChem. 2014; 9, 2528–2537.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2015 Issue 6-
All articles in this issue
- Antibacterial activity of natural compounds – essential oils
- Cholinergic system of the heart
- Body surface area and body weight of Czech adult cancer population
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Antibacterial activity of natural compounds – essential oils
- Body surface area and body weight of Czech adult cancer population
- Cholinergic system of the heart
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

