-
Medical journals
- Career
Stable gold nanoparticles – synthesis, bioconjugation and application
Authors: Paweł Nalepa 1; Anna Mrozek-Wilczkiewicz 2; Jarosław Polański 1
Authors‘ workplace: Institute of Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia Katowice, Poland 1; Silesian Center for Education and Interdisciplinary Research, Chorzów, Poland 2
Published in: Čes. slov. Farm., 2015; 64, 269-272
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Gold nanoparticles (GNPs) are good biocompatible materials due to their special physical and chemical properties1). There is more and more research where GNPs are used in the medical imaging and in the cancer therapy. Nanotechniques, the usage and production of nanomaterials, have been developed in our team for many years2–4). Furthermore, we synthesise various compounds which have anticancer activity and can be developed into novel anticancer treatment drugs5, 6). Therefore, it is not surprising that we decided to combine these two paths of our studies.
Indeed, there are many examples described in the literature of chemically7, 9) and biologically8, 10) functionalized GNPs; however, these conjugates are very often instable. Although GNPs are rather stable within the period of many months, modified nanoparticles – conjugates GNPs to drug, dye or antibody are often instable within a long period, particularly in the presence of high salts and proteins, which is the essence of the human body8, 11).
There exist some reports where glycerol is used as an efficient reducing agent12–14). Glycerol is an environmentally friendly and completely biocompatible reagent. Thus we decided to use glycerol and to synthesise GNPs with various concentration of cross-linked polyvinylpyrrolidone (PVP) as the stabilizing agent. We also wanted to determine the size, shape, dispersity and stability of the obtained GNPs. Our aim was to obtain stable GNPs with diameters smaller than 60 nm because these GNPs have the most appropriate physical and biological properties1). The next step will be coupling nanoparticles with our potential drugs. These bioconjugates will be examined in the in vivo biological studies.
Experimental methods
Preparation of gold nanoparticles
Gold nanoparticles were obtained using wet bottom-up approach. All glassware was cleaned in aqua regia, rinsed with water and dried prior to the use. All solution was prepared with ultrapure water. A mixture (10 ml) containing glycerol : H2O solution (1 : 4 v/v) and various amounts of cross-linked (i.e. crospovidine) PVP (0%, 0.015%, 0.03%, 0.05% and 0.1% w/v) were prepared. Then, 20 μl of tetrachloroauric acid (HAuCl4) was added (final concentration: 0.5 mM). After 2-hour pre-incubation, NaOH was added (1 mM) and the reaction was left to react overnight (16 hours). The mixture was kept in the room temperature and mixed during the whole process of preparation.
Characterization of gold nanoparticles
The transmission electron microscopy (TEM) images of the resultant GNPs were obtained on a JEOL 2000 FX microscope and for GNP-conjugates they will be also obtained on a high resolution (HRTEM) JEM3010 microscope (both equipped with a EDS detector). The UV-Vis absorption spectra of the GNPs were obtained by using a UV-Vis V-530 spectrophotometer (Jasco). The size distribution profiles (by DLS) of GNPs were received by a Zetasizer Nano ZS (Malvern) (light source – 633 nm).
Preparation of nanoparticles-drug conjugates
Gold nanoparticles-drug conjugates will be obtained by mixing GNPs (dissolved in a water:glycerol solution) and the drug solution in a proper ratio at room temperature. Conjugation time was previously fixed as 1 hour. Thiosemicarbazones will be the most common group amount of used drugs. They will be both obtained in our group of novel compounds and the others, investigated in clinical or preclinical trials such as Dp44mT or triapine. Because of their lack of solubility in water, thiosemicarbazones will be dissolved in DMSO.
Biological tests in vivo
For GNP-drug conjugates the biological tests will be conducted. In this study the bare GNP and the drug alone will be used as the reference. The conducting of GNP-drug combination studies will provide a possibility to observe a potential synergistic effect. Biological studies of obtained nanoparticles and theirs conjugates will be carried out by using cell cultures and the MTT assay.
Two groups of cell line culture will be used. Firstly, the group of cancer cells consisting of HCT116 (human colon adenocarcinoma cells), CT26-CEA (murine colon cancer cell line with stable expression of carcinoembryonic antigen) and T41 (murine breast cancer cell line); secondly, the group of normal cells such as human fibroblasts (NHDF), hCMEC/D3 (human model of the blood brain barier) and RAW 264.7 (murine macrophages from blood). The comparison between these two groups of cells will make it possible to determine not only cytotoxicity but also therapeutic index of the examined conjugates.
All cell lines will be cultured in a proper medium (supplemented according to the requirements of each cell line) under standard conditions at 37 °C in a humidified atmosphere at 5% CO2 and will be subcultured every 3–4 days as required. After a 24 - or 48-h incubation with the drug, GNPs or their conjugate (in different concentrations), the MTT assay will be conducted.
Results and discussion
The gold nanoparticles were obtained as described above. During GNPs preparation changes in the colour of the mixtures from light yellow to light red or even pinkish violet were observed. Metal nanoparticles (especially Ag, Au and Cu) exhibit a phenomena known as surface plasmon resonance. The volume (size) and shape of a nanoparticle determines, via interactions with light, the observed colour of the GNPs. A change of size induces a shift of the peak maximum location and a change of shape (e.g. spherical nanocrystal into nanorode) causes the formation of new band at a longer wavelength. Figure 1 shows an exemplary series of changes in the UV-Vis spectrum during the reaction for the sample with 0.03% of PVP concentration.
1. Change in UV-Vis spectrum for 0.03% PVP concentration 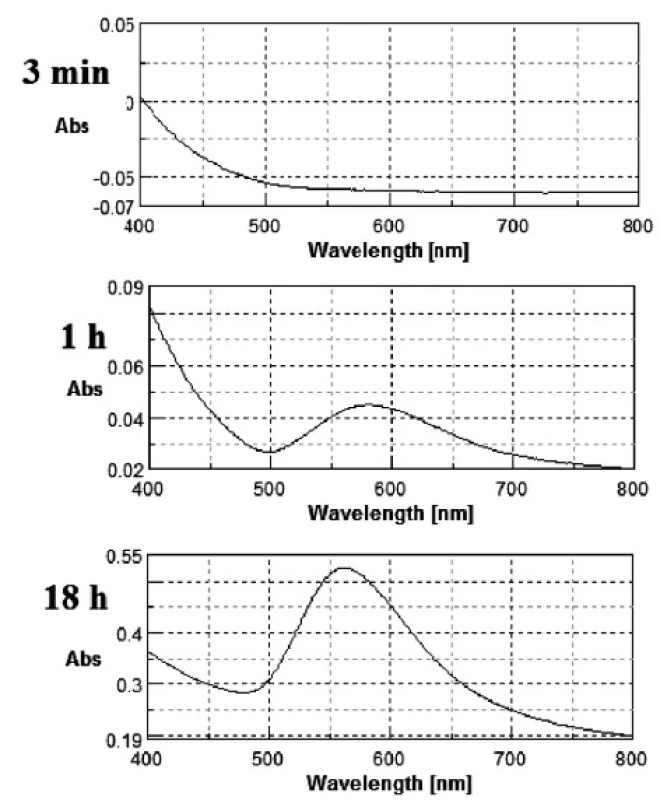
There were five versions of the experiment, where different amounts of the stabilizing agent (PVP) were added. For each version of the experiment, size distribution profiles by dynamic light scattering (DLS) phenomena and TEM images were obtained. The data from these two techniques are shown in Table 1 and in Figure 2.
1. Summary of GNPs size at different PVP (cross-linked) concentrations 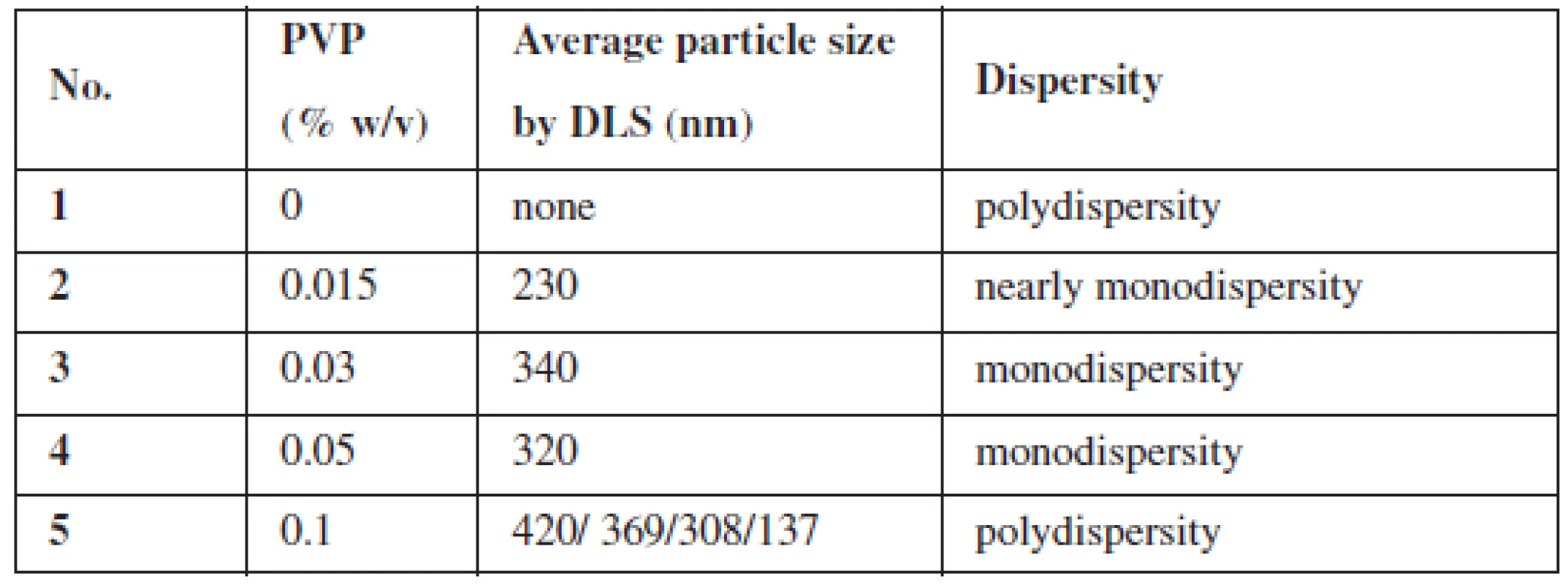
2. Dispersity of the obtained GNPs with different PVP concentration (obtained with DLS technique) 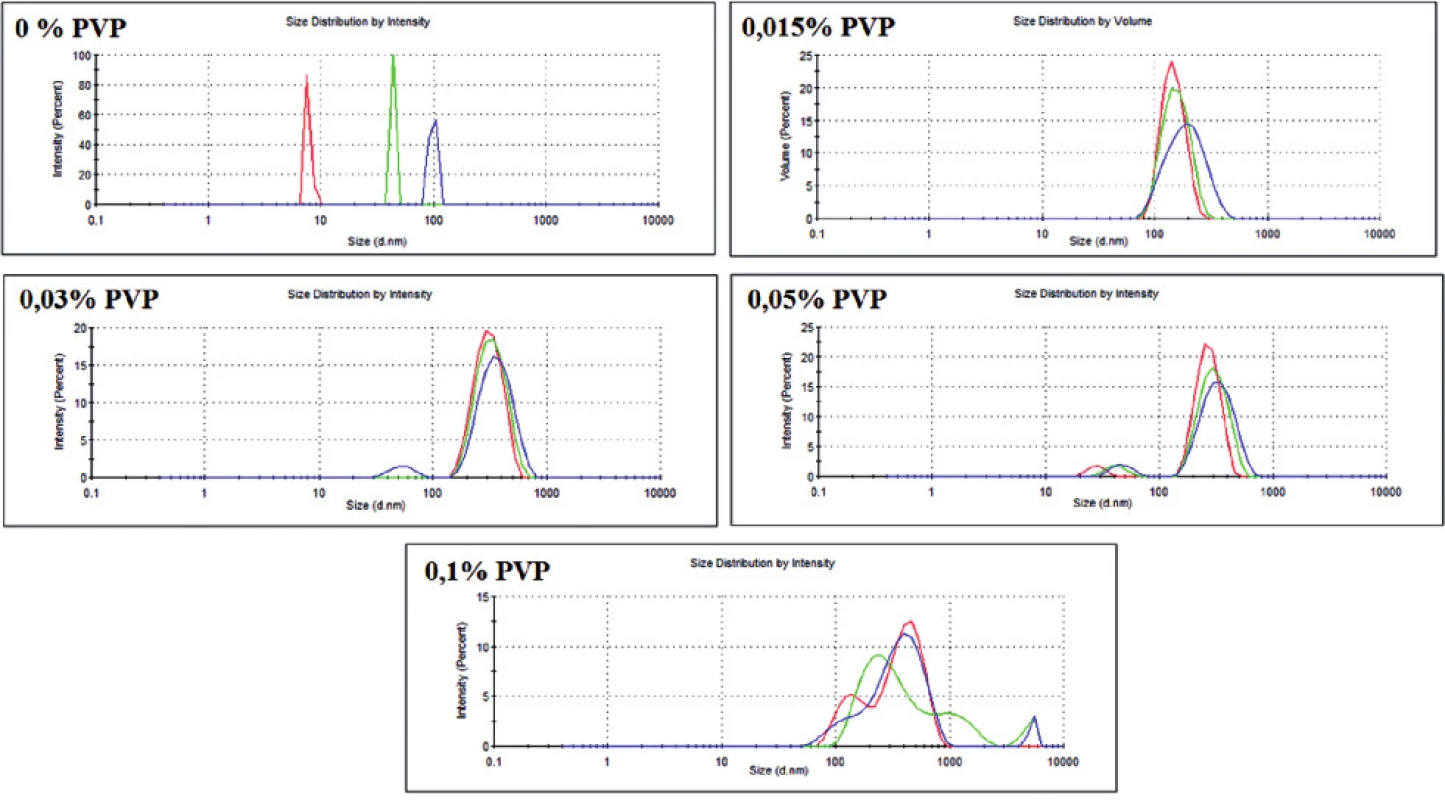
As it can be observed, PVP was found to be a very sufficient stabilizer of GNPs. Even with a small concentration of PVP (0.015%), gold nanoparticles were created. However, PVP was proved to be indispensable for obtaining nanoparticles in a similar size (i.e. monodisperse) (Fig. 2). Moreover, without the stabilizing agent, the reduction of gold proceeded with very low efficiency, which could be seen in the UV-Vis spectrum (data not shown). Otherwise, PVP with the highest concentration (0.1%) seems to be inappropriate for the preparation of monodisperse nanoparticles (Fig. 2). It is probably because of the incorrect ratio between the polymer and gold ions – the arising nanoparticle is randomly included into the PVP structure, which stops further growth of GNP. This hypothesis seems to be confirmed by TEM images (not shown). Therefore, for the next GNPs preparation experiments three middle concentrations of PVP (0.015–0.05%) will be chosen.
Although we managed to obtain monodisperse gold nanoparticles, the size of all obtained nanoparticles occurred to be too big for further medical application and investigation, where GNPs smaller than 60 nm are required. Thus, changes in gold nanoparticles preparation are necessary. The first option to be tested is to use the straight PVP polymer (povidone) instead of a cross-linked one (crospovidone). Other biocompatible polymers such as polyethylenimine (PEI) or poly-L-lysine (PLL) should also be investigated.
Conclusions
In this research, stable gold nanoparticles were synthesised using polyvinylpyrrolidone (PVP) as the stabilizing agent. Then, using many different techniques, size, shape and dispersity of nanoparticles were investigated. The presented results suggest that the most appropriate concentration of cross-linked PVP for GNPs preparation lies within 0.01–0.05% range. The obtained data showed in which way these investigated physical properties depend on the concentration of polymers. However, further examination is necessary because of the fact that we did not manage to obtain gold nanoparticles in the required size.
In further studies, stable gold nanoparticles of an appropriate size will be conjugated with several approved or potential anticancer drugs. The chosen promising conjugates will be examined in a basic anticancer in vivo study with different cell lines.
These researches seem to be interesting and helpful in the design and improvement of medical application for nanoparticles.
The experiments were conducted under National Research and Development Center (NCBiR) Grant ORGANOMET no: PBS2/A5/40/2014 (J.P.).
The financial support of the National Center for Science NCN grants 2014/13/D/NZ7/00322 (A.M.W.) is also greatly appreciated.
Conflicts of interest: none.
Paweł Nalepa MSc.
Institute of Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia
Szkolna 9, 40-006 Katowice, Poland
e-mail: pnalepa@us.edu.pl
Sources
1. Dreaden E.C., Mackey M.A., Huang X., Kangy B., El-Sayed M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011; 40, 3391–3404
2. Kapkowski M., Bartczak P., Korzec M., Sitko R., Szade J., Balin K., Lelątko J., Polański J. SiO2–, Cu–, and Ni-supported Au nanoparticles for selective glycerol oxidation in the liquid phase. Journal of Catalysis 2014; 319, 110–118.
3. Korzec M., Bartczak P., Niemczyk A., Szade J., Kapkowski M., Zenderowska P., Balin K., Lelątko J., Polański J. Bimetallic nano-Pd/PdO/Cu system as a highly effective catalyst for the Sonogashira reaction. Journal of Catalysis 2014; 313, 1–8.
4. Bujak P., Bartczak P., Polański J. Highly efficient room-temperature oxidation of cyclohexene and d-glucose over nanogold Au/SiO2 in water. Journal of Catalysis 2012; 295, 15–21.
5. Mrozek-Wilczkiewicz A., Serda M., Musioł R., Małecki G., Szurko A., Muchowicz A., Gołąb J., Ratuszna A., Polański J. Iron Chelators in Photodynamic Therapy Revisited: Synergistic Effect by Novel Highly Active Thiosemicarbazones. ACS Med. Chem. Lett. 2014; 5, 336–339.
6. Serda M., Kalinowski D. S., Rasko N., Potůčková E., Mrozek-Wilczkiewicz A., Musioł R., Małecki J. G., Sajewicz M., Ratuszna A., Muchowicz A., Gołąb J., Šimůnek T., Richardson D. S., Polański J. Exploring the Anti-Cancer Activity of Novel Thiosemicarbazones Generated through the Combination of Retro-Fragments: Dissection of Critical Structure-Activity Relationships. PLOS One 2014; 9, e110291.
7. Park G., Seo D., Chung I. S., Song H. Poly(ethylene glycol) - and Carboxylate-Functionalized Gold Nanoparticles Using Polymer Linkages: Single-Step Synthesis, High Stability, and Plasmonic Detection of Proteins. Langmuir 2013; 29, 13518–13526.
8. Gao J., Huang X., Liu H., Zan F., Ren J. Colloidal Stability of Gold Nanoparticles Modified with Thiol Compounds: Bioconjugation and Application in Cancer Cell Imaging. Langmuir 2012; 28, 4464–4471.
9. Katz E., Shipway A. N., Willner I. Chemically functionalized metal nanoparticles. In: Liz-Marzán, L. M., Kamat, P. V. (eds.) Nanoscale Materials, Springer US 2003.
10. Sperling R. A., Parak W. J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Phil. Trans. R. Soc. A. Advances in Nanoparticles 2010; 368, 1333–1383.
11. Liu S., Han M. Syntheis, functionalization and Bioconjugation of Monodisperse, Silica-Coated Gold-Nanoparticles: Robust Bioprobes. Adv. Funct. Mater. 2005; 15, 961–967.
12. Nalawande P., Mukherjee T., Kapoor S. Green Syntheis og Gold Nanoparticles Using Glycerol as a Reducing Agent. Advances in Nanoparticles 2013; 2, 78–86.
13. Díaz-Álvarez A. E., Cadierno V. Glycerol: A promising Green Solvent and Reducing Agent for Metal-Catalyzed Transfer Hydrogenation Reactions and Nanoparticles Formation. Appl. Sci. 2013; 3, 55–69.
14. Kouz J., Varma R. S. Speedy fabrication of diameter-controlled Ag nanowires using glycerol under microwave irradiation conditions. Chem. Commun. 2013; 49, 692–694.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2015 Issue 6-
All articles in this issue
- Antibacterial activity of natural compounds – essential oils
- Cholinergic system of the heart
- Body surface area and body weight of Czech adult cancer population
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Antibacterial activity of natural compounds – essential oils
- Body surface area and body weight of Czech adult cancer population
- Cholinergic system of the heart
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

