-
Medical journals
- Career
Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
Authors: Svetlana Dokupilová; Lucia Veizerová; Jaroslav Galba; Peter Mikuš
Authors‘ workplace: Faculty of Pharmacy, Comenius University, Bratislava, Slovak Republic
Published in: Čes. slov. Farm., 2015; 64, 283
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Flavonoids are water soluble polyphenolic molecules which belong to a group of plant metabolites thought to provide health benefits through cell signalling pathways and antioxidant effects. Flavonoids are the most abundant and widely studied, and have enjoyed greater attention among grape researchers1).
Experimental methods
Grape leaves of various grape varieties obtained from the Research Institute of Viticulture and Enology in Slovakia were analysed. Samples were collected in September 2013. Leaves were dried and frozen (–20 °C) until treated prior to analysis. Samples were homogenized and 2.5 g of each sample was extracted with a 40 ml mixture of methanol/water 80 : 20 (v/v) for 120min and 2 × 20 ml dichloromethene was used for sample clean-up. 2.5 ml of the water-methanolic part was dried and dissolved in the mobile phase. Analysis was performed on an LC Agilent 1200 Infinity System (Agilent Technologies, USA) with an Agilent 1260 Infinity Diode Array Detector and an Agilent 6520 Accurate-Mass Quadrupole Time-of-Flight. Separation column was Kromasil C18 (4.0 × 150 mm, 3.5 μm). Mobile phase consisted of acetonitrile and formate buffer (ammonium formate of the concentration 10 mmol/l and pH adjusted to 3.1 by formic acid). Gradient elution 10–95% of acetonitrile in 62 min was used.
MS Detection conditions were: negative mode (ESI–), capillary voltage: 3.5 kV, drying gas temperature: 300 °C, drying gas flow rate: 10 l/min, nebulizer pressure: 40 psi, fragmentor voltage: 140 V, collision energy: 20 eV.
Results and discussion
Identification of the separated compounds was carried out using a UV, MS and MS/MS spectra. 7 quercetin and 4 kaempferol glycoside flavonoids (quercetin-3-O-rutinoside, quercetin-glucuronide, quercetin-3-O-galactoside, quercetin-3-O-glucoside, quercetin-pentoside 1, kaempferol-3-O-rutinoside, kaempferol-hexoside 1, quercetin - -pentoside 2, quercetin-3-O-rhamnoside, kaempferol-hexoside 2, kaempferol-deoxyhexoside) were identified. A DAD detector and external calibration curves of quercetin and kaempferol (in the range 5–100 μg/ml) were used for quantification. 9 of the 13 identified compounds could be quantified (Table 1).
1. Content of glycoside flavonoids in grape leaves: 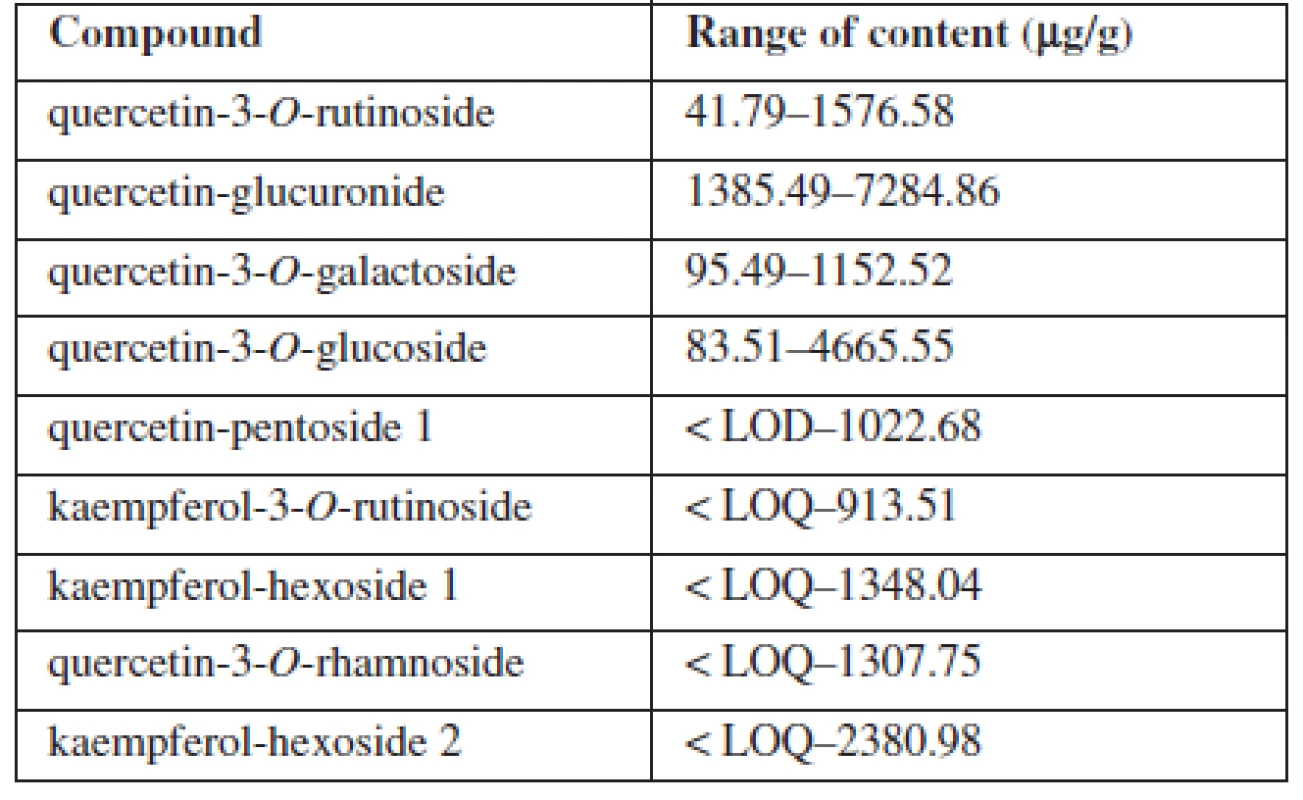
It was found that the highest contained flavonoid was quercetin-glucuronide, the content of which ranged from 1385 to 7284 μg/g. The amount of other components varied from 41.79 μg/g to the maximum value of 4665 μg/g. The method validation confirmed sufficient linearity, precision, accuracy and separation efficiency. Low limits of detection and limit of quantification (LOD = 1.12 μg/g, LOQ = 3.73 μg/g for quercetin and LOD = 2.35 μg/g, LOQ = 7.84 μg/g for kaempferol) were detected.
Conclusions
It was confirmed that the proposed method is suitable for a comprehensive analysis of complex plant matrices, such as grape leaves. The results of research will contribute to the creation of profiles of pharmaceutically active substances contained in the leaves of nearly 30 Slovak vine varieties that could be used in the future to prepare nutraceuticals.
Research was supported by grant APVV-0550-11.
Conflicts of interest: none.
RNDr. Svetlana Dokupilová, PhD.
Department of Pharmaceutical Analysis and Nuclear Pharmacy
Faculty of Pharmacy, Comenius University
Odbojárov 10, 832 32 Bratislava, Slovak Republic
e-mail: dokupilova@fpharm.uniba.sk
Sources
1. Liu C., et al. Resveratrols in Vitis berry skins and leaves: Their extraction and analysis by HPLC. Food Chem. 2013; 136, 643–649.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2015 Issue 6-
All articles in this issue
- Antibacterial activity of natural compounds – essential oils
- Cholinergic system of the heart
- Body surface area and body weight of Czech adult cancer population
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Antibacterial activity of natural compounds – essential oils
- Body surface area and body weight of Czech adult cancer population
- Cholinergic system of the heart
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

