-
Medical journals
- Career
Production of flavonoids and isoflavonoids in jasmonic acid-induced red clover suspension cultures
Authors: Marie Kašparová; Tomáš Siatka
Authors‘ workplace: Faculty of Pharmacy in Hradec Králové ; Charles University in Prague
Published in: Čes. slov. Farm., 2014; 63, 17-21
Category: Original Articles
Overview
Effect of exogenously applied jasmonic acid (JA) in combination with calcium and verapamil (a calcium channels blocker) on the production of flavonoids and isoflavonoids in suspension cultures of Trifolium pratense L. was investigated. The culture was cultivated in Gamborg medium with an addition of 2 mg.l-1 of 2,4-dichlorophenoxyacetic acid and 2 mg.l-1 of 6-benzylaminopurine, at the temperature of 25 °C, 16-hr light/8-hr dark period. The best effect of jasmonic acid on the production of flavonoids and isoflavonoids was manifested after a 24-hour application of the 50 μmol.l-1 concentration. The maximum production of JA-induced suspension culture was observed when cells were treated with a high level of calcium (10 mmol.l-1). The addition of all concentrations of verapamil to JA-induced suspension culture decreased production of flavonoids and isoflavonoids.
Keywords:
flavonoids, isoflavonoids, jasmonic acid, elicitation, Trifolium pratense suspension cultureIntroduction
Red clover (Trifolium pratense L., Fabaceae) is a rich source of isoflavonoids with multiple potential protective functions, plant secondary metabolites belonging to the group of phenylpropanoids. Red clover contains isoflavones, which have a high affinity to estrogen receptor α (ERα), estrogen receptor β (ERβ), progesterone receptor and androgen receptor. The higher affinity to ERβ compared to ERα has been used as an explanation why red clover extracts function as food additives to treat menopausal disorders and may reduce risk of breast cancer1). The main isoflavones present in red clover are formononetin and biochanin A, of which formononetin is more abundantly found in roots2). Other isoflavones found in roots include daidzein, genistein, pratensein, pseudobaptigenin, calycosin, methylorobol, irilin B, afrormosin and irilone3). Isoflavonoids are synthesised mainly constitutively in intact plant and in suspension culture of Trifolium pratense L., but their concentrations may be influenced by biotic or abiotic stresses (elicitors) such as ozone, UV light, pathogens, heavy metals, phytohormones4–8). The elicitation method can be an important strategy towards improved production of plant secondary metabolites. Jasmonic acid, plant hormonal regulator, is an integral part of a general signal transduction system regulating inducible defense genes in plants. Moreover, exogenously applied jasmonic acid and methyl jasmonate induce de novo transcription of the gene of the key enzyme of the phenylpropanoid pathway, phenylalanine ammonia lyase. Jasmonic acid and the jasmonates are, therefore, key signal compounds in the elicitation process leading to de novo transcription and translation and, ultimately, to the biosynthesis of secondary metabolites in plant cell cultures. Calcium functions as a versatile messenger in mediating responses to hormones, biotic/abiotic stress signals and a variety of developmental cues in plants9, 10).
This experiment investigated the influence of jasmonic acid and the effect of jasmonic acid in combination with calcium and verapamil (a calcium channels blocker) on the production of flavonoids and isoflavonoids in Trifolium pratense suspension cultures.
Experimental part
Instruments
A 200S analytical scales made by Sartorius, Göttingen; a PS 20A autoclave, by Chirana, Brno; a HS 31A hot-air sterilizer by Chirana, Brno; a laminar flow workbench by Fatran LF, Žilina; a roller by Vývojové Dílny AV ČR, Praha; a 2010 shaker by Unimax, Heidolph; a CE 1010 spectrophotometer by Cecil Instruments, Cambridge; a liquid chromatograph (PU-2089 pump, MD-2015 detector, AS-2055 automatic sample injector) by Jasco, Tokyo.
Trifolium pratense L. suspension culture
The suspension culture (variety Sprint) was derived from callus culture mechanically by shaking in the Gamborg liquid nutrient medium11) supplemented with 2 mg.l-1 of 2,4-dichlorophenoxyacetic acid and 2 mg.l-1 of 6-benzylaminopurine. The culture was cultivated on a roller at the temperature of 25 °C, and a 16-hr light/8-hr dark photoperiod. The experiments used 4-year-old suspension cultures. The subcultivation interval lasted 14 days12).
Elicitation
During the elicitation process, on the 21st day of cultivation12), the suspension culture (volume 25 ml) received 1.0 ml of a jasmonic acid solution (in the concentrations of 5 μmol.l-1, 50 μmol.l-1 and 500 μmol.l-1 dissolved in 96% ethanol), calcium chloride (in the concentrations of 0.1 mmol.l-1, 1 mmol.l-1 and 10 mmol.l-1) and verapamil (in the concentrations of 1 μmol.l-1, 10 μmol.l-1 a 100 μmol.l-1 )13–16). The control cultures received 1.0 ml of distilled water. The elicitor application durations were 6, 24, 48, and 168 hours8, 16). Inspection cultures were collected after 6 and 168 hours since their production does not change notably in such short time intervals.
Determining the flavonoids and isoflavonoids
The elicited and the inspection samples underwent a photometric determination of flavonoids in accordance with the Czech Pharmacopoeia 200917) and a determination of isoflavonoids via the HPLC method18). The HPLC conditions were as follows: a RP-18 Lichrospher column (250 × 4 mm, particle size 5 μm) with a precolumn made of the same material; elution: linear gradient of a mobile phase A (methanol) in phase B (water containing 0.15% (v/v) of phosphoric acid) 30–80% (v/v) from 0 to 9 minutes was followed by an isocratic elution with a mixture of 80% (v/v) of phase A in phase B from 9 to 15 minutes; the flow rate was 1.1 ml.min-1; the detection was carried out at the 260 nm wavelength. The contents of the monitored substances were quantified by using the mathematical method of normalization and by comparing with the calibration curve drawn by the external standard of the same substance. The obtained results were statistically evaluated by the t-test at p = 0.05.
Results and discussion
The study investigated the effect of exogenously applied jasmonic acid (JA) in combination with calcium and verapamil on the production of flavonoids and isoflavonoids in Trifolium pratense suspension cultures. The cultures were treated with jasmonic acid (5, 50 and 500 μmol.l-1). The results show that the best effect of jasmonic acid on the production of flavonoids and isoflavonoids was manifested after a 24-hour application of the 50 μmol.l-1 concentration. The maximum content of flavonoids was determined at 2.95 mg.g-1 DW and the production was stimulated by 72% in comparison with the control (Table 1). The maximum increase in the content of isoflavonoids was as follows: genistin by 579% (0.95 mg.g-1 DW), daidzein by 150% (0.50 mg.g-1 DW) and genistein by 127% (0.50 mg.g-1 DW) (Table 2).
1. The effects of jasmonic acid, calcium and verapamil on flavonoids production in Trifolium pratense L. suspension cultures 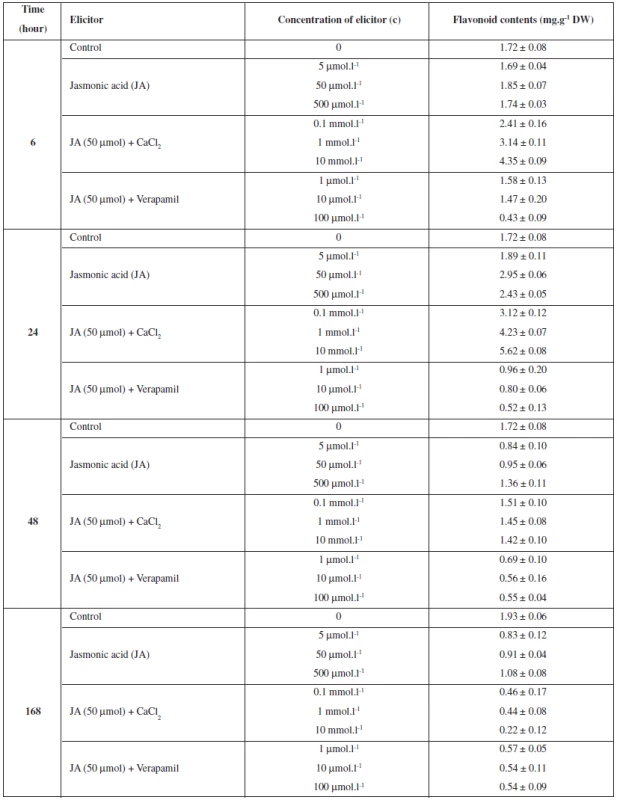
Data represent the mean value ± SD (n = 3). 2. The effects of jasmonic acid, calcium and verapamil on isoflavonoids production in Trifolium pratense L. suspension cultures 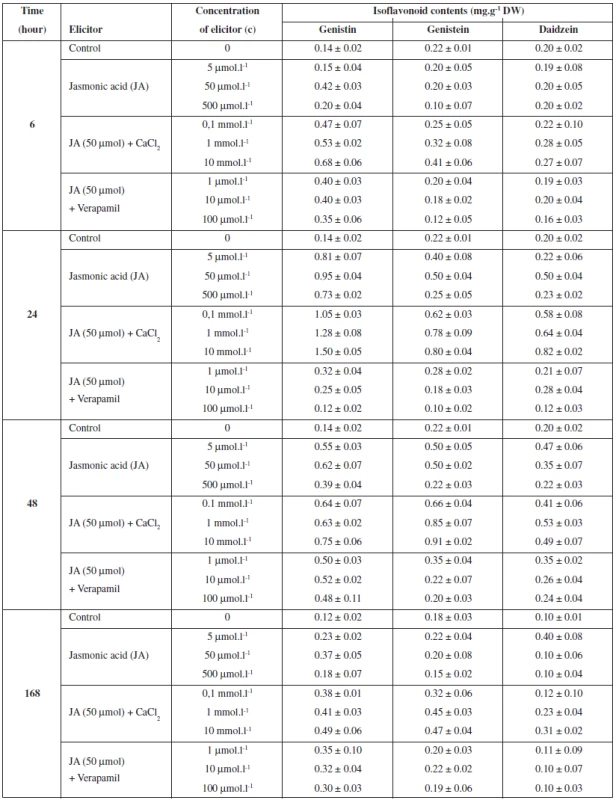
Data represent the mean value ± SD (n = 3). To study the role of calcium on flavonoids and isoflavonoids production, JA-induced (50 μmol.l-1) suspension cultures of Trifolium pratense were treated with CaCl2 (0.1, 1 and 10 mmol.l-1) and verapamil (1, 10, and 100 μmol.l-1). The highest flavonoids and isoflavonoids production was observed when cells were treated with a high level of calcium (10 mmol.l-1). The maximum content of flavonoids (5.62 mg.g-1 DW) was induced by a 24-hour application of calcium and the production was increased by 227% in comparison with the control (Table 1). The production of isoflavonoids genistin (1.50 mg.g-1 DW) and daidzein (0.82 mg.g-1 DW) was best stimulated after a 24-hour application, the increase being 970% and 310%, respectively. The maximum content of genistein (0.91%) was found after a 48-hour application and the production was stimulated by 314% in comparison with the control (Table 2). An addition of all concentrations of verapamil to JA-induced (50 μmol.l-1) suspension cultures decreased the production of flavonoids and isoflavonoids in comparison with the control culture and the JA-induced culture (Tables 1 and 2).
The process of elicitation activates various Ca2+ – and calmodulin-dependent protein kinases by increasing the level of free Ca2+ in the cytoplasm and triggers the cellular responses, which may include alterations in gene expression. Calcium ions seem to play a role in the control of the production of secondary metabolites but in a different manner depending on the species. It has a stimulatory as well as an inhibitory role in the production of the secondary metabolites in plants. The importance of jasmonic acid, calcium ions, and verapamil in the process of biotic elicitation can be documented in other examples13–15, 19–23).
This study was financially supported by the grant of Charles University in Prague SVV 267 004.
Conflicts of interest: none.
Received 6 November 2013
Accepted 19 December 2013
PharmDr. Marie Kašparová, Ph.D. (∗) • T. Siatka
Charles University in Prague
Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy
Heyrovského 1203, 500 05 Hradec Králové
e-mail: kasparova@faf.cuni.cz
Sources
1. Beck V., Rohr U., Jungbauer A. Phytoestrogens derived from red clover: An alternative to estrogen replacement therapy? J. Steroid Biochem. Molecul. Biolog. 2005; 94, 499–518.
2. Saviranta N. M., Anttonen M. J., Wright A., Karjalainen R. O. Red clover (Trifolium pratense L.) isoflavones: determination of concentrations by plant stage, flower colour, plant part and cultivar. J. Sci. Food Agric. 2008; 88, 125–132.
3. Wu Q., Wang M., Simon J. E. Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J. Chromatogr. A 2003; 1016, 195–209.
4. Saviranta N. M., Julkunen-Tiitto R., Oksanen E., Karjalainen R. O. Red clover (Trifolium pratense L.) isoflavones: root phenolic compounds affected by biotic and abiotic stress factors. J. Sci. Food Agric. 2010; 90, 418–423.
5. Swinny E. E., Ryan K. G. Red clover Trifolium pratense L. phytoestrogens: UV-B radiation increases isoflavone yield, and postharvest drying methods change the glucoside conjugate profiles. J. Agric. Food Chem. 2005; 53, 8273–8278.
6. Sivesind E., Seguin P. Effects of foliar application of elicitors on red clover isoflavone content. J. Agron. Crop Sci. 2006; 192, 50–54.
7. Goyal S., Ramawat K. G. Increased isoflavonoids accumulation in cell suspension cultures of Pueraria tuberosa by elicitors. Ind. J. Biotechnol. 2008; 7, 378–382.
8. Tůmová L., Zápalková L. The effect of jasmonic acid on the production of flavonoids by the culture Ononis arvensis L. in vitro. Čes. slov. Farm. 2002; 51, 96–98.
9. Zhao J.-L., Davis L. C., Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005; 23, 283–333.
10. Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. USA 1992; 89, 2389–2393.
11. Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968; 50, 151–158.
12. Kašparová M., Siatka T., Spilková J., Dušek J. Explant culture of Trifolium pratense L. Čes. slov. Farm. 2006; 55, 44–47.
13. Tebayashi S., Ishihara A., Tsuda M., Iwamura H. Induction of clovamide by jasmonic acid in red clover. Phytochemistry 2000; 54, 387–392.
14. De D., De B. Elicitation of diosgenin production in Trigonella foenum-graecum L. seedlings by heavy metals and signaling molecules. Acta Physiol. Plant. 2011; 33, 1585–1590.
15. Lee-Parsons C. W. T., Ertürk S. Ajmalicine production in methyl jasmonate-induced Catharanthus roseus cell cultures depends on Ca2+ level. Plant Cell Rep. 2005; 24, 677–682.
16. Kašparová M., Siatka T., Dušek J. Vliv kyseliny jasmínové na produkci anthracenových derivátů v kultuře Rheum palmatum L. in vitro. Čes. slov. Farm. 2003; 52, 148–151.
17. Czech Pharmacopoeia 2009. 1. vyd. Praha: Grada Publishing 2009; 1801–1802.
18. De Rijke E., Joshi H. C., Sanderse H. R., Ariese F., Brinkman U. A. Th., Gooijer C. Natively fluorescent isoflavones exhibiting anomalous Stokes’ shifts. Anal. Chim. Acta 2002; 468, 3–11.
19. Sudha G., Ravishankar G. A. Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tiss. Org. Cult. 2002; 71, 181–212.
20. Sánchez-Sampedro M. A., Fernández-Tárrago J., Corchete P. Elicitation of silymarin in cell cultures of Silybum marianum: Effect of subculture and repeated addition of methyl jasmonate. Biotechnol. Lett. 2009; 31, 1633–1637.
21. Balažová A., Blanáriková V., Bilka F., Bilková A., Sepová H. K. The effect of three different elicitors on sanguinarine production in suspension cultures of a low-morphine variety of the opium poppy (Papaver somniferum L.). Čes. slov. Farm. 2011; 60, 237–240.
22. Krzyzanowska J., Czubacka A., Pecio L., Przybyc M., Doroszewska T., Stochmal A., Oleszek W. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha × piperita cell suspension cultures. Plant Cell Tiss. Org. Cult. 2012; 108, 73–81.
23. Shukor M. F. A., Ismail I., Zainal Z., Noor N. M. Development of a Polygonum minus cell suspension culture system and analysis of secondary metabolites enhanced by elicitation. Acta Physiol. Plant. 2013; 35, 1675–1689.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2014 Issue 1-
All articles in this issue
- Separation of amino acid enantiomers by high performance liquid chromatography
-
Where does the development of new antituberculotics go?
Part 2 – In vitro evaluation -
Study of local anaesthetics: Part 204*
Determination of critical micelle concentrations of selected derivatives of pyrrolidino-m-alkoxyphenylcarbamic acid using pyrene as a probe - Identifying and solving drug-related problems in terms of the community pharmacist
- Globalization and its impact on pharmacy services in the Slovak Republic
- Production of flavonoids and isoflavonoids in jasmonic acid-induced red clover suspension cultures
- Inhibition of 12/15 lipoxygenase by curcumin and an extract from Curcuma longa L.
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Separation of amino acid enantiomers by high performance liquid chromatography
- Identifying and solving drug-related problems in terms of the community pharmacist
- Globalization and its impact on pharmacy services in the Slovak Republic
- Inhibition of 12/15 lipoxygenase by curcumin and an extract from Curcuma longa L.
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

