-
Medical journals
- Career
Inhibition of 12/15 lipoxygenase by curcumin and an extract from Curcuma longa L.
Authors: Lýdia Bezáková; Daniela Košťálová; Marek Obložinský; Peter Hoffman; Mária Pekárová; Renáta Kollárová; Ivana Holková; Silvia Mošovská; Ernest Šturdík
Authors‘ workplace: Comenius University, Faculty of Pharmacy
Published in: Čes. slov. Farm., 2014; 63, 26-31
Category: Original Articles
Overview
Curcumin (diferuloylmethane) is an orange-yellow secondary metabolic compound from the rhizome of turmeric (Curcuma longa L.), a spice often found in curry powder. It is one of the major curcuminoids of turmeric. For centuries, curcumin has been used in some medicinal preparations or as a food colouring agent. A variety of enzymes that are closely associated with inflammation and cancer were found to be modulated by curcumin. This paper summarized the results of the inhibitory effect of curcumin and a Curcuma longa L. ethanolic extract on lipoxygenase from the rat lung cytosolic fraction. The positional specificity determination of arachidonic acid dioxygenation by RP - and SP-HPLC methods showed that in a purified enzyme preparation from the rat lung cytosol the specific form of lipoxygenase (LOX) is present exhibiting 12/15-LOX dual specificity (with predominant 15-LOX activity). The inhibitory activity of curcumin and Curcuma longa extract on LOX from cytosolic fraction of rat lung was expressed in the percentage of inhibition and as IC50. Lineweaver-Burk plot analysis has indicated that curcumin is the competitive inhibitor of 12/15 LOX from the rat lung cytosolic fraction.
Keywords:
Curcuma longa L., curcumin, turmeric extract, linoleic acid, lipoxygenase inhibitionIntroduction
Turmeric (the common name of Curcuma longa L.) is an Indian spice, food preservative and colouring agent, which has been traditionally used for the treatment of various ailments. Curcumin, a hydrophobic polyphenol, is the principal active compound of turmeric. The major curcuminoids isolated from turmeric are curcumin, demethoxycurcumin and bisdemetohoxycurcumin. Commercially available preparations of “curcumin” contain predominantly 70% of curcumin, 17% of demethoxycurcumin and 3% of bisdemethoxycurcumin2) (Fig. 1). Curcumin is a highly pleiotropic molecule with anti-inflammatory, anti-oxidant, chemopreventive activities in vitro and in animal models3, 4). The mechanisms of these effects are diverse and appear to involve the regulation of various molecular targets, including transcription factors (such as nuclear factor kapa B, a protein that influences the genetic code to produce inflammatory cytokines), such as the tumour necrosis factor, interleukin 1 and interleukin 6, mitogen-activated protein kinases and other enzymes, such as cyclooxygenase 2 and 5-lipoxygenase, phospholipase A2 (PLA2) and inducible nitric oxide synthase (iNOS)1, 4). Several studies have provided interesting insights into the multiple mechanism by which curcumin may mediate chemotherapy and chemopreventive effects on cancer, including colorectal cancer trough the modulation of multiple molecular targets such as surface adhesion proteins, survival pathways and cytokines5–7).
Fig. 1. Structure of curcumin, demethoxycurcumin and bisdemethoxycurcumin 
The anti-inflammatory properties of curcumin were shown to inhibit the 5-lipoxygenase activity in rat peritoneal neutrophils as well as the 12-lipoxygenase activity and the cyclooxygenase activity in human plateles8). The arachidonic acid (AA) pathway constituents are one of the main mechanisms for the formation of mediators of inflammation, as well as controlling homeostatic function. AA is the major precursor of several classes of signal molecules and alteration of its metabolism is involved in human carcinogenesis by interfering with signalling events9).
Lipoxygenases (LOXs) belong to the multigenes family of dioxygenases with a content of non-heme iron in the active site of the enzyme. LOX catalyze dioxygenation of polyunsaturated fatty acids with a cis-1,4-pentadiene configuration to their corresponding hydroperoxide derivatives. In animal organisms LOX is the key enzyme in the biosynthesis of leukotrienes playing an important role in the pathophysiology of several inflammatory diseases because the products of lipoxygenase catalyze oxygenation as hydroxyeicosatetraenoic (HETE) or hydroperoxyeicosatetraenoic acid (HPETE)10). Lipoxygenases catalyze the oxidation of arachidonic acid to form therapeutically important signalling molecules including leukotrienes and lipoxins. The involvement of lipoxygenases in many inflammatory diseases reactions (rheumatoid arthritis and psoriasis), cancer, atherosclerosis, asthma, diabetes mellitus and Alzheimer’s disease has prompted the search for inhibitors for these enzymes11, 12).
Lipoxygenases are categorized as 5‑LOX [EC 1.13.11.34], 8‑LOX [EC 1.13.11.40], 12‑LOX [EC 1.13.11.31] and 15‑LOX [EC 1.13.11.33]. LOXs are regio - and stereo-selective and thus the resulting eicosanoids differ not only in the position of oxidation and the location of the remaining double bonds but also in chirality, which is a tremendously crucial factor in their biological activity. A single mutation glycine-alanine can change not only the chirality S-R but also the site of oxidation. The mutation of other residues in the vicinity of the iron site can have a similar effect on regio - and stereo-specificity of catalysis12). These differences imply great difficulties in planning pharmacological interventions with the intention to change the functions regulated by LOXs.
5‑LOX is the most explored enzyme according to the ability to synthesize leukotrienes, formerly known as the slow reacting substances of anaphylaxis, and their involvement in inflammatory and allergic diseases13, 14). However, some LOXs were found to have a dual positional specificity and produce 12 ‑ and 15‑hydroperoxides of arachidonic acid. These are called 12/15‑LOX. The 12/15‑LOX enzyme has been linked to the development of the metabolic syndrome including atherosclerosis, obesity, diabetes, liver injury in non-alcoholic fatty liver disease15–18) and in the pathogenesis of another inflammatory diseases including arthritis19) and lung injury20).
The aim of the present study was to prepare a partially purified LOX enzyme from the rat lung cytosol fraction and use this enzyme preparation as a model enzyme for testing potential LOX inhibitors: standard curcumin and an extract from powdered turmeric rhizomes (Curcuma longa L.). The kinetic parameters of KM and Vmax and the type of inhibition were specified with respect to curcumin. For the determination of the positional specific forms of LOX from the rat lung cytosolic fraction, the LOX reaction products were separated on a non-polar system (RP-HPLC) and identified on a polar adsorbent (SP-HPLC).
Experimental part
Standard curcumin (purity > 95 %) was purchased from Sigma (St. Louis, Missouri, USA).
The powdered turmeric rhizomes (Curcuma longa L.) were purchased from the local market of Select Horeca, Slovak Republic.
All chemicals and solvents used were of analytical grade.
Extraction of curcuminoids
Ground dry turmeric rhizomes (500 g) were defatted with hexane (1500 ml) before being extracted with an ethanol-water 95 : 5 mixture (3000 ml). Curcuminoids where then crystallized by adding hexane (200 ml) to the hydroalcoholic solution and the resulting mixture was allowed to stand for 24 hours. A microcrystalline orange solid was filtered and dried at 60°C under vacuum. The determination of the amount of curcuminoids was performed with a Shimadzu UV-1700 spectrophotometer in the visible range at 420 nm.
Purification and determination of lipoxygenase activity
The cytosolic fraction from the rat lungs (Wistar rat, male 180 g), as the source of LOX, was isolated according to the procedure reported by Kulkarni21). Briefly, rat lung homogenate was centrifuged at 1000 × g for 5 min. The pellet obtained contained unbroken cells and the debris was discarded. The resulting supernatant was centrifuged at 10 000 ⋅ g for 15 min to obtain the mitochondrial fraction. The postmitochondrial supernatant was further centrifuged at 100 000 ⋅ g for 60 min to obtain microsomes and cytosol. This fraction was further purified by ammonium sulfate precipitation (60 %), chromatography on Sephadex G-100 (Pharmacia, Sweden), and on Phenyl-Sepharose CL-4B (Pharmacia, Sweden). The purified enzyme was used for LOX activity determination. The protein content in the enzyme preparation was estimated by the method of Bradford22). The enzyme with specific activity (SA) of 3053,15 μkat.mg-1 was used in all measurements. Linoleic acid (99 %, Sigma, USA) was used as the substrate prepared in solubilized state as described23) in the concentration of 0.2143 ⋅ 10-5 – 0.7143 ⋅ 10-5 mol.l-1. The assay of LOX was monitored as an increase in the absorbance at 234 nm, which reflects the formation of hydroperoxylinoleic acid. For the LOX activity assay, a UV/VIS Spectrometer Perkin-Elmer Lambda 35 (USA) was used. The reaction medium contained a 50 mM Tris-HCl buffer (pH 7.0), 5 μl of the enzyme protein and solubilized linoleic acid. The final concentration of the curcumin tested was in the range of 1.06–5.32 ⋅ 10-5 mol.l-1. Inhibitory effects of the compounds tested were expressed in the percentage of inhibition and as IC50.
HPLC analysis of positional specific forms of LOX
HPLC analysis was performed on a Hewlett‑Packard 1050 (Holbron, Germany) HPLC system equipped with MWD, autosampler and quaternary pump. 10 μl of purified proteins were incubated with the substrate (10 μl, 5% methanol solution of AA v/v) in a total volume of 1ml TRIS-HCl buffer (0.1 mol.l-1, pH 7.2) for 20 min at ambient temperature. The reaction was stopped by adding 10 mg of NaBH4 and 100 μl of concentrated HCl. HETEs were isolated by diethyl ether (2 ⋅ 1 ml; Sigma‑Aldrich, St. Luis, USA) and evaporated to dryness in the nitrogen stream. After drying, the sample was reconstituted in 100 μl of methanol and kept at –20°C under a nitrogen atmosphere until analysis.
HETEs were separated on a Nucleosil 120-5 C18 100A column (Watrex, Prague) using a gradient separation at the mobile phase prepared from acidified methanol (A); 0.5 ml of glacial acetic acid in 500 ml of methanol) and deionized water (B). The products were collected between 21.5–23.5 min (100% A + 0% B, flow rate 0.4 ml.min-1) and detected at the wavelength of 235 nm.
The eluate was evaporated under a nitrogen stream and reconstituted in n‑hexane. The compounds were separated on a Zorbax Rx‑Sil 150 ⋅ 2.1 mm (Agilent Technologies, Holbron, Germany) by using isocratic elution at the mobile phase prepared of n‑hexane and acidified with 2‑propanol (0.5 ml of glacial acetic acid in 500 ml of 2‑propanol, 98/2 (v/v)) and a flow rate of 0.15 ml.min-1 with UV detection at 235 nm (optimization by Hoffman)24).
Results and discussion
The content of total curcuminoids in turmeric extract estimated by UV spectrophotometry was 93.6 % by measuring the absorbance at 420 nm in ethanol.
It is known that the structures of curcumin (also designated diferuloylmethane) and its derivatives demethoxycurcumin and bisdemethoxycurcumin (Fig. 1) exhibit many pharmacological activities. The hydroxyl and phenol groups in the molecule of curcuminoid are also essential for the inhibition of leukotriene and prostaglandin synthesis and anti-inflammatory action is associated with the beta-dicarbonylic system, which has the conjugated double bonds (dienes)1, 25). Several research studies provided an interesting insight into the multiple mechanisms by which curcumin and curcuminoids may mediate a number of molecular targets justifying its chemopreventive effects on cancer, including colorectal cancer, anti-inflammatory and antioxidative activities. Curcumin has the ability to inhibit carcinogenic promotion through the modulation of multiple molecular targets, such as transcription factor, enzymes, cell cycle proteins, cell surface adhesion proteins, survival pathways and cytokines26–30).
The inhibitory effect of curcumin on LOX activity from the rat lung cytosolic fraction is expressed in the percentage of inhibition and in the value of IC50 in Graph 1 and Table 1. The final concentrations of curcumin in incubation mixtures were 1.06; 1.77; 3.55 and 5.32 ⋅ 10-5 mol.l-1 of curcumin. The highest inhibition of LOX from the rat lung cytosolic fraction by curcumin expressed in the percentage of inhibition was 91.82% and IC50 = 6.13 ⋅ 10-6 mol.l-1 by using a 5.32. 10-5 mol.l-1 of curcumin in incubation mixture. The turmeric extract in the concentrations of 1.07; 1.78; 3.57; 5 ⋅ 35 mg.ml-1 on LOX activity from the rat lung cytosolic fraction showed the highest inhibitory effect of 76.06% (Graph 2). These results showed a lower inhibitory effect of Curcuma longa extract on LOX activity and is possible that in this extract mainly phenolic curcumin and curcuminoids may be responsible for inhibitory activity.
Graph 1. Inhibition of LOX activity by curcumin expressed in percentage of inhibition (the values are means ± SD from triplicate experiments, n = 3, p < 0.01) 
1. Kinetic parameters of curcumin by inhibition of LOX activity 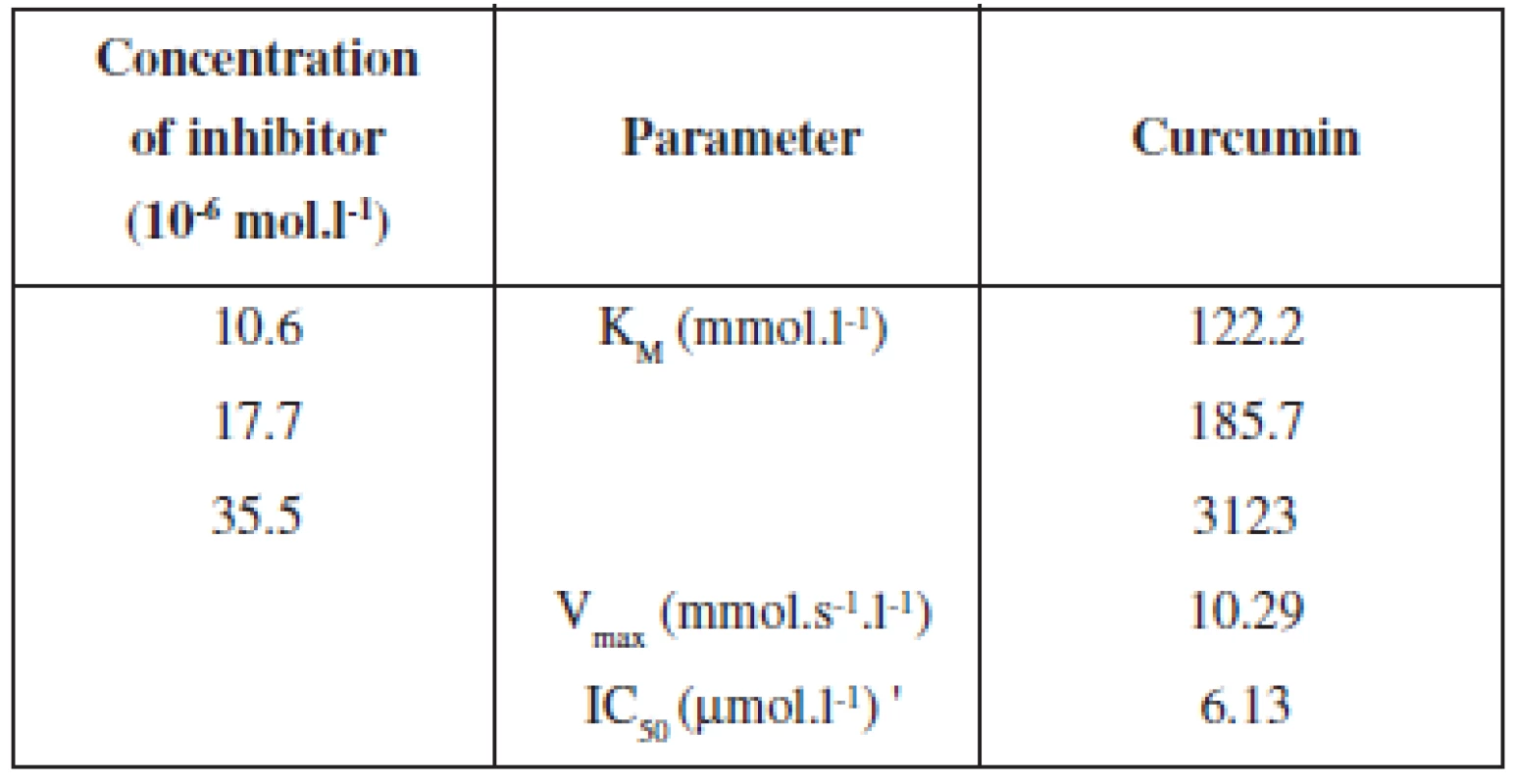
Graph 2. Inhibition of LOX activity by extract of Curcuma longa L. expressed in percentage of inhibition (the values are means ± SD from triplicate experiments, n = 3, p < 0.01) 
When the effects of linoleic acid on LOX inhibition by curcumin were determined, the rate of dioxygenation became greater, with an increase in substrate concentration. The Lineweaver-Burk plots analysis (Graph 3, Table 1) at fixed curcumin concentration showed that Michaelis-Menten kinetic constant (KM) increased without changing Vmax, suggesting that curcumin is the competitive inhibitor of LOX from the rat lung cytosol fraction with KM of 122.2; 185.7; 312.3 mmol.l-1 and Vmax 10.29 mmol.s-1 by the presence of curcumin for linoleic acid and by using of 12/15 LOX from the rat lung cytosol fraction. The competitive type of inhibition of curcumin on LOX activity was also determined by using linoleic acid bound to the phosphatidylcholine micelles and by using soybean lipoxygenase LOX-131). On the other hand, by using X-ray diffraction and mass spectrometry of the lipoxygenase-curcumin complex (soybean LOX-L3) Skrzypczak-Jankun and colleages32) have found an electron mass located near the soybean L3 catalytic site and hypothesize the noncompetitive type of inhibition.
Graph 3. Kinetic types of LOX inhibition by curcumin 
Graph 4. Chromatogram of standards 8-, 12-HETE (2.5 μg cm<sup>-3</sup>), 5- and 15-HETE 
Studies on the inhibition of LOXs have shown that inhibitors can act through a number of mechanisms. One of the examples is a reduction of the catalytically active ferric enzyme to its inactive ferrous form through the formation of free radical metabolites33) or by preventing the formation of the activated Fe(III) form of LOX. Inhibition of lipoxygenase reaction seems to be derived from inactivation of the active site of the enzyme or scavenging of the free radical at the active site. Therefore, most antioxidants are inhibitors of LOXs. The use of curcumin as the inhibitor of lipoxygenase31) estimated that curcumin inhibits LOX by binding with an active site iron.
HPLC analysis revealed a production of 12 - and 15 - -HETE in all samples of purified preparation of lipoxygenase (Graph 5). (HPLC analysis of 5-, 8-, 12 - and 15-HETE (Graph 4). According to our previous studies we assumed the presence of 12/15‑LOX in rat lung cytosol. 12/15‑LOX enzymes, the non-heme iron‑containing dioxygenases, insert molecular oxygen into arachidonic acid resulting in the formation of 12(S)‑HETE and 15(S)‑HETE33). According to the product spectrum it is classified as 15‑LOX‑1 in humans and rabbits whereas pigs, rats and mice express leukocyte‑type 12‑LOX34). These enzymes are highly related in their primary structures and are thought to be homologues of each other35). They can be found in a variety of cells, including the lung, heart, atherosclerotic vessel, liver, gut, spleen, lymph node, kidney, adipose tissue and macrophages36). Huo and colleages37) suggested that 12/15‑LOX exists in mice liver tissue mainly as macrophage LOX. Alternatively, 12/15‑LOX in this tissue may be regulated by 12/15‑LOX‑expressing macrophages.
Acknowledgments
We would like to acknowledge the support of the Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic for the Structural Funds of the EU (Project ITMS 26240220040) for a partial financial support of this project.
This work was also supported in part by the Agency of the Ministry of Education, Science and Sport – VEGA 1/0885/13 and by the Comenius University Grant for Young Researchers UK/234/2013.
Conflicts of interests: none.
Received 14 November 2013
Accepted 16 December 2013
doc. RNDr. Lýdia Bezáková, CSc. (∗) • M. Obložinský • P. Hoffman • M. Pekárová • R. Kollárová • I. Holková
Comenius University, Faculty of Pharmacy
Department of Cell and Molecular Biology of Drugs
Kalinčiakova 8, 832 32 Bratislava, Slovak Republic
e - mail: bezakova@fpharm.uniba.sk
D. Košťálová • S. Mošovská • E. Šturdík
Slovak University of Technology
Faculty of Chemical and Food Technology
Institute of Biochemistry, Nutrition and Health Protection
Radlinského 9, 812 37 Bratislava, Slovak Republic
Sources
1. Jayaprakasha G. K., Jagan L., Rao M., Sakariah K. K. Chemistry and biological activities of C. longa. Trends in Food Science Technology 2005; 16, 533–548.
2. Gupta S. C., Prasad S., Kim J. H., Patchva S., Webb L., Priyadarsini I. K., Aggarwal B. B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011; 28, 1937–1953.
3. Ak T., Gúlcin I. Antioxidant and radical scavenging properties of curcumin. Chemico-Biological Interaction 2008; 174, 27–37.
4. Basnet P., Skalko-Basnet N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011; 16, 4567–4598.
5. Shehzad A., Khan S., Shehzad D., Lee Y. S. Curcumin therapeutic promises and bioavailability in colorectal cancer. Drug Today 2010; 47(7), 523–532.
6. Caroll R. E., Bena R. V., Turgeon D. K., Varee S., et al. Phase II clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011; 4(3), 354–364.
7. Shureiqi I., Baron J. A. Curcumin Chemoprevention: The long road to clinical translation. Cancer Prev. Res. 2011; 4(3), 296–298.
8. Ammon H. P., Safayhi H., Mack T., Sabieraj J. Mechanism of anti-inflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993; 38(2–3): 113–119.
9. Kohll K., All J., Ansari J., Raheman Z. Curcumin: A natural antiinflammatory agent. Indian J. Pharmacol. 2005; 37(3), 141–147.
10. Jankun L., Aleem A. M., Malgorzewicz S., Szkudlarek M., Zavodszky M. I., Devitt D. L., Feig M., Selman S. H., Skrzypczak-Jankun E. Synthetic curcuminoids modulate the arachidonic acid metabolism of human platelet 12-lipoxygenase and reduce sprout formation of human endothelial cells. Mol. Cancer Ther. 2006; 5(5), 1371–1382.
11. Wu J., Xiang Xia H. H., Ping Tu S., Fan D. M., Lin M. Ch., Kung H. F., Lam S. K., Wong B. Ch. 15-lipoxygenase-1 mediates cyclooxygenase-2 inhibitor-induced apoptosis in gastric cancer. Carcinogenesis. 2003; 24(2), 243–247.
12. Segraves S., Walther M , Ivanov I., et al. Sequence determinants for the reaction specificity of murine (12R)-lipoxygenase: targeted substrate modification and site-directed mutagenesis. J. Biol. Chem. 2005; 280, 36633–36641.
13. Werz O., Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Therapeut. 2006; 112 : 701–718.
14. Montuschi P., Sala A., Dahlén S. E., Folco G. Pharmacological modulation of the leukotriene pathway in allergic airway disease. Drug Discov. Today 2007; 12, 404–412.
15. Wen Y., Gu J., Vandenhoff G. E., Liu X, Nadler J. L. The role of 12/15‑lipoxygenase in the expression of MCP-1 in mouse macrophages. Am. J. Physiol. Heart Circ. Physiol. 2008; 294, H1933–H1938.
16. Chakrabarti S. K, Cole B. K, Wen Y., Keller S. R., Nadler J. L. 12/15‑lipoxygenase products induce inflammation and impair insulin signaling in 3T3‑L1 adipocytes. Obesity 2009; 17, 1657–1663.
17. Yuan H., Lanting L., Xu Z. G., Li S. L., Swiderski P., Putta S., Jonnalagadda M., Kato M., Natarajan R. Effects of cholesterol-tagged small interfering RNAs targeting 12/15‑lipoxygenase on parameters of diabetic nephropathy in a mouse model of type 1 diabetes. Am. J. Physiol‑Renal 2008; 295, F605–F617.
18. Martínez-Clemente M., Ferré N., Titos E., Horrilo R., González-Périz A., Morán-Salvador E., López-Vicario C., Miquel R., Arroyo V., Funk C. D., Claria J. Disruption of the 12/15‑lipoxygenase gene (Alox15) protects hyperlipidemic mice from nonalcoholic fatty liver disease. Hepatology 2010; 52, 1980–1991.
19. Krönke G., Katzenbeisser J., Uderhardt S., Zaiss M. M., Scholtysek C., Schabbauer G., Zarbock A., Koenders M. I., Axmann R., Baencler H. W, van den Berg W., Voll R. E., Kuhn H., Joosten L. A., Schett G. 12/15‑lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 2009; 183, 3383–3389.
20. Rossaint J., Nadler J. L., Ley K., Zarbock A. Eliminating or blocking 12/15-lipoxygenase reduces neutrophil recruitment in mouse models of acute lung injury. Crit. Care 2012; 16, R166.
21. Kulkarni A. P., Cai Y., Richards I. S. Rat pulmonary lipoxygenase: dioxygenase activity and role in xenobiotic metabolism. Int. J. Biochem. 1992; 24, 255–261.
22. Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976; 72, 248–254.
23. Kemal C., Louis-Flamberg P., Krupinski-Olsen R., Shorter A. R. Reductive inactivation of soybean lipoxygenase 1 by catechols: a possible mechanism for regulation of lipoxygenase activity . Biochemistry. 1987; 26, 7064–7068.
24. Hoffman P., Rauová D., Bezáková L., Obložinský M., Mikuš P. HPLC method for determination of lipoxygenase positional specific products. J. Pharm. Biomed. Anal. 2013; 84, 53–58.
25. Aggarwal B., Harikumar K. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. & Cell Biol. 2009; 41, 40–59.
26. Lev-Ari S., Strier L., Kazanov D., Shapiro L. M., Sobol H. D., Pinchuk I., Marian B., Lichtenberg D., Arber N. Celecoxib and curcumin synergistically inhibit the growth of colerectal cancer cells. Clin. Cancer Res. 2005; 11, 6738.
27. Basnet P., Skalko-Basnet N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011; 16, 4567–4598.
28. Caroll R. E., Bena R. V., Turgeon D. K., Varee S., et al. Phase II clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011; 4(3), 354–364.
29. Gupta S. C., Prasad S., Kim J. H., Patchva S., Webb L., Priyadarsini I. K., Aggarwal B. B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011; 28, 1937–1953.
30. Shureiqi I., Baron J.A. Curcumin Chemoprevention: The long road to clinical translation. Cancer Prev. Res. 2011; 4(3), 296–298.
31. Began G., Sudharshan E., Appu Rao A. G. Inhibition of lipoxygenase 1 by Phospatidylcholine micelles-bound curcumin. Lipids. 1998; 33(12), 1223–1228.
32. Skrzypczak-Jankun E., McCabe N. P., Selman S. H., Jankun J. Curcumin inhibits lipoxygenase by binding to its central cavity: theoretical and X-ray. evidence. Int°. J. Mol. Med. 2000; 6, 521–526.
33. Hajek A. R., Lindley A. R., Favoreto S., Carter R., Schleime R. P., Kuperman, D. A. 12/15-lipoxygenase deficiency protects mice from allergic airways inflammation and increases secretory IgA levels. J. Allergy Clin. Immunol. 2008; 122, 633–639.
34. Zarbock A., Distasi M. R., Smith E., Sanders J. M., Kronke G., Hardy B. L., Vietinghoff S. Improved survival and reduced vascular permeability by eliminating or blocking 12/15 - lipoxygenase in mouse models of acute lung injury (ALI). J. Immunol. 2009; 183, 4715–4722.
35. Kawakami Y., Hosokawa T., Morinaka T., Irino S., Hirano S., Kobayashi H., Yoshioka A., Suzuki-Yamamoto T., Yokoro M., Kimoto M., Tsuji H., Yamashita H., Doi S., Yutani C., Kato R., Itabe H., Kanada T., Hada T., Takahashi Y. Antiatherogenic effect of guava leaf extracts inhibiting leukocyte-type 12-lipoxygenase activity. Food Chem. 2012; 131, 1069–1075.
36. Rong S., Cao Q., Liu M., Seo J., Lia L., Boudyguina E., Gebre R. K. Macrophage 12/13 lipoxygenase expression increases plasma and hepatic lipid levels and extracerebrates atherosclerosis. J. Lipid Res. 2012; doi: 10.1194/jlr. MO22723.
37. Huo Y., Zhao L., Hyman M. C., Shashkin P., Harry B. L., Burcin T., Forlow S. B., Stark M. A., Smidth D. F., Clarke S. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004; 110, 2024–2031.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2014 Issue 1-
All articles in this issue
- Separation of amino acid enantiomers by high performance liquid chromatography
-
Where does the development of new antituberculotics go?
Part 2 – In vitro evaluation -
Study of local anaesthetics: Part 204*
Determination of critical micelle concentrations of selected derivatives of pyrrolidino-m-alkoxyphenylcarbamic acid using pyrene as a probe - Identifying and solving drug-related problems in terms of the community pharmacist
- Globalization and its impact on pharmacy services in the Slovak Republic
- Production of flavonoids and isoflavonoids in jasmonic acid-induced red clover suspension cultures
- Inhibition of 12/15 lipoxygenase by curcumin and an extract from Curcuma longa L.
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Separation of amino acid enantiomers by high performance liquid chromatography
- Identifying and solving drug-related problems in terms of the community pharmacist
- Globalization and its impact on pharmacy services in the Slovak Republic
- Inhibition of 12/15 lipoxygenase by curcumin and an extract from Curcuma longa L.
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

