-
Medical journals
- Career
Assessment of Prospective Memory – a Validity Study of Memory for Intentions Screening Test
Authors: O. Bezdicek 1; S. A. Raskin 2; M. Altgassen 3,4; E. Ruzicka 1
Authors‘ workplace: Department of Neurology and Centre of Clinical Neuroscience, Charles University, 1st Medical Faculty and General University Hospital in Prague, Czech Republic 1; Trinity College, Department of Psychology and Neuroscience Program, Hartford, CT, USA 2; Donders Institute for Brain, Cognition and Behaviour, Centre for Cognition, Radboud University Nijmegen, Nijmegen, The Netherlands 3; Department of Psychology, Technische Universitaet Dresden, Dresden, Germany 4
Published in: Cesk Slov Neurol N 2014; 77/110(4): 435-443
Category: Original Paper
Overview
Aim:
The goal of the present study was to validate the Czech version of the Memory for Intentions (Screening) Test (MIST, 2010). We included standardized testing material, translation of administration and scoring, and assessment of normative data for the MIST in the Czech population.Introduction:
Prospective memory (PM), i.e., the ability to remember and implement intentions after a delay, is essential as a subsystem of episodic memory for the maintenance of independence and execution of activities of daily living. PM assessment thus plays an important role in the diagnosis of episodic memory disorders. However, there are currently no standardized and validated PM tools in Czech language.Methods:
The Czech version of MIST was administered to 30 healthy persons.Results:
The MIST Summary score correlated at a medium level with a range of neuropsychological measures including memory retention, mental flexibility, and resistance to interference (all rho = 0.37 – 0.42; all p < 0.05). The reliability of MIST in terms of internal consistency was insufficient when analyzing the eight individual MIST trials (α = 0.50), as was split ‑ half reliability (split ‑ half reliability = 0.56). In contrast, there was a high degree of reliability between six subscales classified by type (delay, cue and mode of response; α = 0.88, split ‑ half = 0.95).Conclusion:
The reliability and validity of the Czech version of MIST is comparable to the original English version. The study opens access to standardized PM assessment in clinical populations in the Czech Republic.Key words:
Memory for Intentions (Screening) Test – prospective memory – declarative memory – validity – reliability – episodic memoryIntroduction
Prospective memory (PM) refers to the ability to carry out intentions after a delay; for instance, to phone someone at a particular time in the future. PM is a subsystem of declarative, and in particular, episodic memory [1 – 4]. It is theorized to be comprised of a number of cognitive processes, in particular intention formation, intention retention, intention initiation and intention execution (Fig. 1) [5 – 7]. The interval between the formation and execution of an intention may last minutes, hours or days. On functional imaging (fMRI), PM tasks have been shown to primarily activate the prefrontal cortex (Brodmann area 10) and the medial temporal cortex (hippocampal formation) [8,9]. The successful performance of PM tasks requires the ability to initiate and plan an action, inhibit ongoing activities and identify a cue for the task to be carried out in the future (Fig. 1) [10,11].
Fig. 1. Process model of prospective memory. 
The model was adapted according to Kliegel and Knight et al [6,7]. The text in boxes describes individual mental processes (components) while working on a prospective memory task sequence. The dashed-line boxes indicate their bilateral correlation with the environment and, simultaneously, their dissimilarity to the process of retention. The (thin- -line) terms at the top and on the left indicate moderating variables, such as may influence prospective memory processes. The notations below the arrows on the left suggest the basic mental functions within which the particular prospective memory processes are evolving (e.g., intention formation is part of planning as the fundamental process of executive function; intention storage as one of the processes of retrospective memory dependent on the hippocampal formation). This process model is the groundwork for our understanding of the construct of prospective memory and its role in the system of mental function. Early studies on PM explored common and distinctive characteristics with respect to retrospective memory [12]. Contemporary research has placed greater emphasis on the critical role of PM in the preservation of functional independence, including instrumental activities of daily living (IADL) such as adhering to medication regimes, managing finances, grocery shopping, housekeeping and employment [13]. Considering that a patient without intact PM cannot be relied upon to take their medication at the right time and in the right quantity, the construct of PM and its assessment may have great clinical utility. Thus, the evaluation of PM may yield knowledge of immediate value in clinical practice.
Although the integration of PM testing into comprehensive memory testing would be beneficial, PM testing tools are rarely reviewed and their use in clinical practice remains limited [14]. A survey by Rabin et al (2005) determined that the Rivermead Behavioral Memory Test was the only validated tool among memory tests in use to include a specific index for PM [14,15]. As stated by Woods et al [16], this surprising fact may be due to the small number of tools for PM measurement that have been standardized and have an analysis of psychometric characteristics and normative data. However, two standardized tools for PM measurement have recently been published: the CAMbridge PROspective Memory Test (CAMPROMT; [17]), and the Memory for Intentions (Screening) Test (MIST; [18]). Currently, there are no practical, standardized tools for PM measurement available for use in the Czech Republic, only unvalidated experimental techniques [19]. Moreover, there are no comprehensive descriptions of PM in the Czech literature [20].
MIST was developed and introduced by Sarah Raskin and Carol Buckheit in 2004. A few years later, in 2010, an English version was standardized and validated on an American population [18]. Several studies have described the psychometric characteristics of MIST, such as reliability (internal consistency, inter ‑ rater and test ‑ retest reliability) and validity (content and convergent validity) [16,18,21,22]. These studies have shown that PM correlates with executive function, verbal working memory and retrospective memory. Regarding the relationship between executive function or working memory and PM, Schitzspahn et al (2013) reported that inhibition and shifting appeared to be essential aspects of cognitive control involved in PM performance in young and old adults, whereas working memory was not revealed to be a significant predictor of PM performance [13]. Various studies have documented discriminatory validity in clinical cohorts. For example, MIST has revealed evidence of impaired PM in patients with Alzheimer’s disease, Parkinson’s disease, and brain injury [23 – 29]. Carey et al (2008) demonstrated the ecological validity of MIST in predicting IADL deficits in HIV ‑ positive subjects [23], and MIST has been shown to predict medication adherence in individuals with schizophrenia [24]. An overview of all studies concerning MIST validity in clinical cohorts is available in the Raskin et al study [18].
MIST has proven to be a robust tool for the measurement of the PM construct in a number of studies [17,18,22, 24 – 26,28,29], however, it is still unavailable to Czech psychologists. Hence, the primary objective of the present study was to develop a version of MIST for use in the Czech population. Specifically, we endeavored to convert the test to a Czech version (translation and back ‑ translation), standardize test materials, validate the basic psychometric characteristics (demographic factors, inner consistency and split ‑ half reliability) on sample of the Czech population and describe its construct validity by correlation with established gold ‑ standard tests.
Patients and methods
Participants were recruited through flyers and advertisements from the general community and afterwards using snowball sampling. We then obtained a brief medical history of each subject via telephone (OB). Subjects meeting the inclusion criteria were then tested (OB). A cohort of 30 healthy subjects were included in the study and met the following criteria for enrollment: interviews excluded all participants with a history of head trauma with loss of consciousness, cerebrovascular accident, abuse of alcohol or other psychoactive substances, and individuals with a history of neurological or psychiatric disease (e. g., epilepsy, multiple sclerosis, schizophrenia or ongoing delirium). We additionally excluded persons currently undergoing radio ‑ or chemotherapy or with serious internal diseases, myocardial infarction, diabetes mellitus, etc.) or with sensory deficits. Participants meeting the above criteria were then tested for cognitive efficiency, manifestations of depression and activities of daily living (ADL). In order to prevent the inclusion of subjects at risk of developing neurodegenerative disease, limits for enrollment were set on the Mini‑Mental State Examination (MMSE) [30] at a value of ≥ 26 points, the Beck Depression Inventory (BDI ‑ II) [31] at a score of < 13, and the Functional Activities Questionnaire (FAQ) [32] at ≤ 4 points. They underwent a comprehensive neuropsychological assessment as part of a research project supported by Charles University Grant Agency. Demographic characteristics of the cohort and their basic functional characteristics are presented in Tab. 1. With all conditions met, we administered a comprehensive neuropsychological battery including MIST (Tab. 2). The test battery was divided into several cognitive domains according to the classification of mental function. The study was approved by the local ethics committee and all participants provided signed, informed consent. All the tests were administered under standard neuropsychological laboratory conditions and were conducted by single, trained psychologist (OB). The author of the Czech translation (OB) had the permission of the license owner (Psychological Assessment Resources) to use the test in the Czech population and to translate the original to Czech. Back ‑ translation was done by a translation agency, and comparison of the original and back ‑ translation by the original author (SR) and the author of the translated version (OB) was performed.
1. Descriptive characteristics of subjects (n = 30). 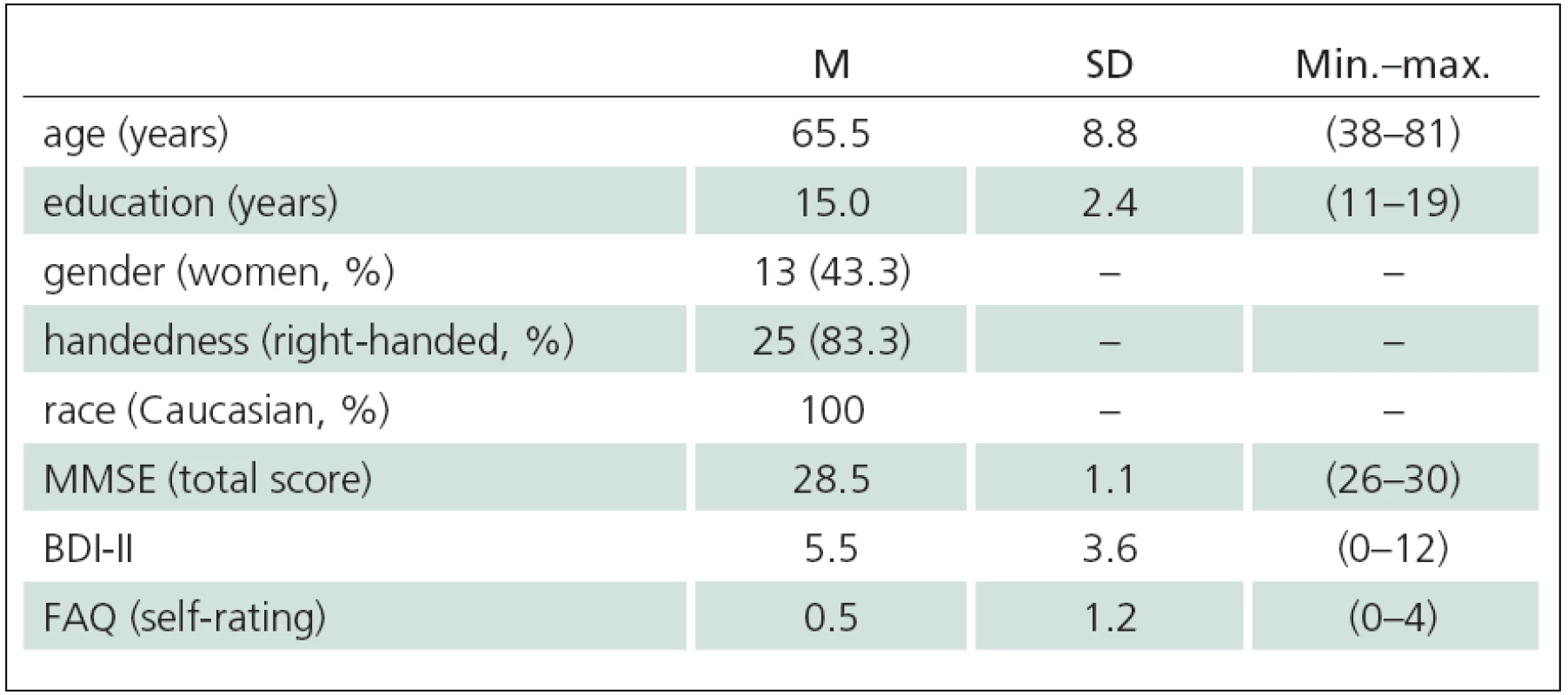
M – arithmetic mean, SD – standard deviation, Min.–max. – range of values, MMSE – Mini-Mental State Examination [28]; BDI-II – (Beck Depression Inventory – Second Edition) [31], FAQ (self-rating) – Functional Activities Questionnaire [32]. 2. Performance characteristics of participants on neuropsychological battery and correlation with the MIST Summary score. 
The data represent the arithmetic mean of the given test, its standard deviation ± SD, range (Min.–max.) and median (Md) relative to non-parametric analysis (Spearman rank order correlation coefficient; rho). For orientation, we present the score range or units of measurement for each scale: Czech National Adult Reading test (CART; 0– 50 points), Mini-Mental State Examination (MMSE; 0– 30 points), Trail Making Test (TMT– A and B, time in seconds; Digit and Spatial forward (0– 16 points), Digit span backward (0– 14), Spatial span back ward (0– 16 points), Letter- Number Sequencing (0– 21 points), Tower of London task; TOL (0– 108 points), Verbal and semantic fluency (N + K + P and animals + clothing + shopping = total word count); Wisconsin Card Sorting Test (WCST-64; 0– 64 errors), Victoria Stroop Test (VST; time in seconds), Wechsler Adult Intelligence Scale (WAIS– R Similarities; 0– 28 points), Rey Auditory Verbal Learning Test (RAVLT; sum of words in trials 1– 5, i.e., 0– 75 words), RAVLT – delayed recall (0– 15 words), Wechsler Memory Scale (WMS– III Family pictures IR; immediate recall 0– 64 points), WMS- III (Family pictures DR; delayed recall 0– 64 points), Memory Capacity Test (MCT; both lists 0– 32 points), Benton Judgment of Line Orientation Test (BJOL; 0– 30 points); Beck Depression Inventory (BDI- II, 0– 64 points), Functional Activities Questionnaire (FAQ, 0– 30 points). * – α < 0.05. MIST test construction
MIST contains a set of eight PM trials. The test was constructed to satisfy the accepted criteria of a PM task [3,6,33]:
- a) intentions are supposed to be realized after a delay,
- b) there is a separate ongoing activity (attention distraction task),
- c) the test provides a limited period of time after which the intention has to be retrieved from memory and implemented.
The duration of the test was approximately 30 minutes, during which the subject was administered an ongoing, attention ‑ distraction task (crossword puzzle). All of the MIST tasks were divided in a balanced way between:
- delay interval (either 2 or 15 minutes),
- cue type (either time‑based, e. g., “tell me in 15 minutes time that we should have a break”, or event‑based, e. g., “when I pass the red pen to you sign your real name on the paper”),
- mode of response (verbal, such as in the first example, or activity‑related such as in the second example), which may then be used in choosing a strategy for rehabilitation [22].
The Retrieval index was constructed according to Carey et al [23], (Tab. 3). A 24 - hour delayed recall followed completion of the eight items in MIST to determine how many hours the subject had slept the day of testing (a task designed to simulate everyday life). The ongoing activity was a Word Search Form (range 0 – 40 words). Following completion of the eight recall tasks, recognition items consisting of three multiple choice items and were scored (right answer out of three). All tasks were related to everyday activities so as to have as much ecological validity as possible [22,34]. MIST also allows for error analysis regarding the cause of PM failure [35], which was recorded with the aid of a qualitative scoring system designed to differentiate six types of errors:
- failure to remember that there was an intention (to realize the intention),
- loss of content error (substitution of the target activity by an alternative activity at the correct time, or failure to recall the content at all, e. g., remembering at the correct time that “something” should have been done),
- loss of time error (executing the correct intention at the wrong time),
- task substitution (e. g., mistaking a verbal response for an activity‑based response or vice ‑ versa),
- place losing omission error (completing only part of the task or repeating the previous one),
- random error (errors that did not fit into any of the previous categories).
3. Performance characteristics on the Memory for Intentions (Screening) Test (MIST) correlated with demographic data (n = 30) 
M – arithmetic mean, Min.– max. – range of values, MIST – Memory for Intentions (Screening) Test), rho – Spearman rank order correlation coefficient, SD – standard deviation, Retrieval index – number of correct responses in recognition – number of correct responses in free recall (time-related cue + event-related cue/ 2), i.e., higher scores – worse performance, * – α < 0.05, ‡ – α < 0.01. Each of the tasks was related to the six subscales of MIST (time of delay, type of cue, mode of response, each was rated from 0 to 8 points), each containing four particular tasks for PM. Their sum was represented by a summary PM score (ranging from 0 to 48 points). Recognition was a forced ‑ choice task between three alternatives (total 0 – 8 points). The 24 – hour task (range 0 – 2) consisted in sending a text message to the administrator within a precisely defined interval (9 – 10 a. m.), with the subject allowed to use any strategy to initiate intent retrieval (e. g., diary) without being explicitly instructed to do so during testing.
A comprehensive neuropsychological battery was administered and consisted of tests for reading (National Adult Reading Test in the Czech version, CART [36]) as well as general cognitive performance (MMSE [30]; sustained attention: Trail Making Test, part A (TMT ‑ A [37]), Digit span from WAIS ‑ III [38], Spatial span from WMS ‑ III [39]; Working memory: Digit span backwards from WAIS ‑ III [38], Spatial span backwards from WMS ‑ III [39], Letter ‑ Number Sequencing from WMS ‑ III [39]; executive function: Tower of London (TOL [40], verbal fluency (letters N + K + P [37]), Trail Making Test, part B (TMT ‑ B [37]), Wisconsin Card Sorting Test for the number of perseverative responses (WCST‑64 [41]), Victoria Stroop Test, interference condition (VST [42]); speech: Similarities from WAIS ‑ R [43], semantic fluency (animals + clothing + shopping [37]); memory: Rey Auditory Verbal Learning Test (RAVLT [37]), Family Pictures from WMS ‑ III [39] and Buschke’s Memory Capacity Test (MCT [44]); visuospatial abilities: Benton Judgement of Line Orientation Test (BJOL [45]); manifestations of depression: Beck Depression Inventory (BDI ‑ II [31]); activities of daily living (FAQ [32], see Tab. 2).
To assess normality, we examined Q ‑ Q plots and performed the Kolmogorov ‑ Smirnov test. Given that deviation from normality was detected, we used the Spearman rank correlation coefficient (rho) to evaluate correlation between MIST and the demographic variables age and years of education, as well as other cognitive measures. In accordance with convention, the strength of correlation was rated as low (rho = 0.10 – 0.29), medium (rho = 0.30 – 0.49) or high (rho = 0.50 – 1) [46]. For demographic variables we used the nonparametric Mann‑Whitney U test. To analyze reliability, the Cronbach α was determined in the case of internal consistency and the Spearman ‑ Brown formula in the case of split ‑ half reliability. The magnitude of effect in nonparametric tests (r) was estimated according to Cohen’s effect sizes [47] as small (> 0.1), medium (> 0.3), or large (> 0.5). The level of statistical significance was set at α = 0.05. All presented analyses were performed using IBM SPSS Statistics software (Version 22.0, SPSS Inc., Chicago, IL, USA).
Results
Descriptive characteristics of the cohort are presented in Tab. 1. Descriptive cha-racteristics of MIST performance are presented in Tab. 3. Deviation from normality was detected in the distributions of all MIST variables with the exception of Word Search Form (p = 0.36). To evaluate correlation between demographic variables and MIST performance, we therefore applied the nonparametric Spearman correlation coefficient (rho), the results of which are shown in Tab. 3. With regard to several significant correlations between MIST indices and gender (Tab. 3), we looked for any gender‑related performance differences. The Mann‑Whitney U test revealed significant gender differences for the MIST Summary score (men: Md = 38.8, n = 17; women: Md = 42.5, n = 13; U = 62, z = – 2.1, p = 0.038, r = 0.38). Additionally, positive correlation was detected between MIST and demographic variables with respect to age (recognition rho = 0.52) and education (time cue and recognition rho = 0.42 – 0.43).
Analysis of inter ‑ item consistency of the eight individual MIST trials revealed Cronbach α = 0.50. Internal consistency of six of the MIST subscales classified according to response modality (delay, 2 versus 15 minutes; response type, verbal versus action; time‑based versus event‑based cue) resulted in Cronbach α = 0.88. The split ‑ half reliability of eight MIST trials adjusted according to the Spearman ‑ Brown formula = 0.56; that of six subscales = 0.95.
Regarding construct validity, Tab. 2 shows the correlation between MIST and the items in the comprehensive neuropsychological battery. MIST correlated most highly with RAVLT delayed recall, then with TMT ‑ B, TOL, Spatial span forward, CART and MCT. The effect sizes of correlations were determined as medium ‑ large and the differences between particular correlation coefficients were small (rho = 0.37 – 0.42).
Correlations between all the MIST indices are presented in Tab. 4. The MIST Summary score correlated with other MIST indices (rho = 0.58 – 0.97), with the exception of the 24 hour delay item, recognition task and interfering task (Word Search Form). Overall, the error scores were highly correlated with the Summary score, although in some error subtypes no correlation was detected (task substitution, random error), in one case due to zero variability in all subjects (PLO).
4. Correlation (rho) between MIST Summary score, subscales and error scores (n = 30). 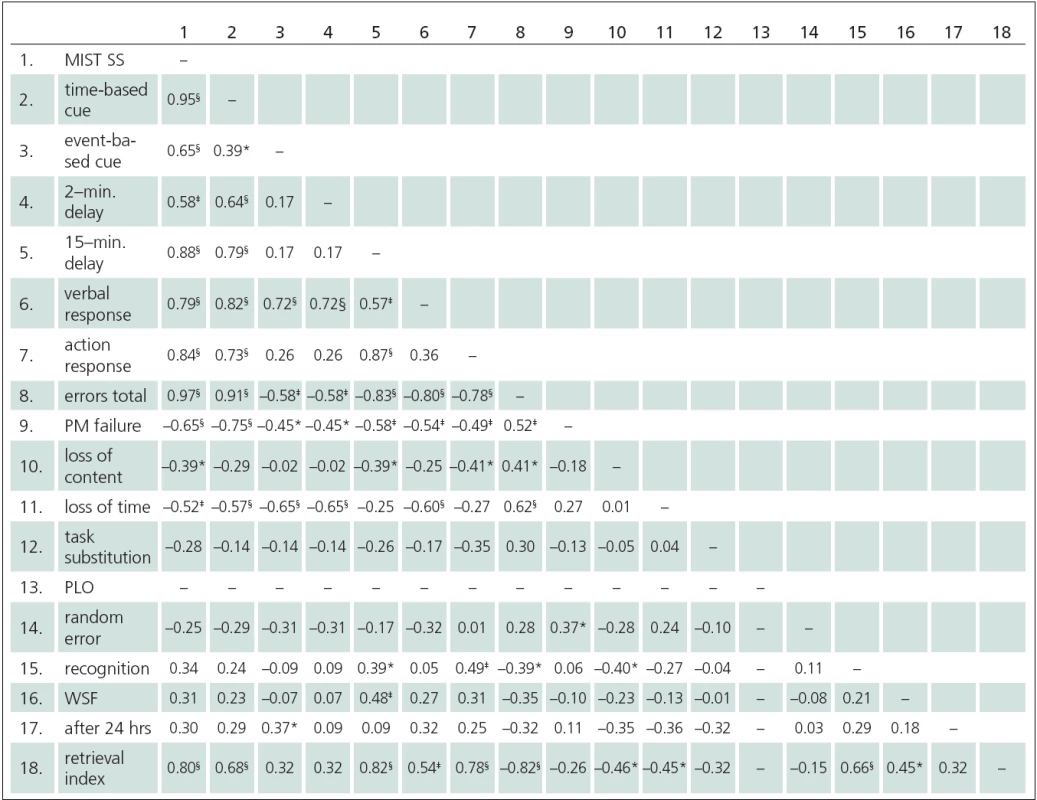
MIST – Memory for Intention (Screening) Test score, MIST SS – MIST Summary Score, 2- min. delay – delay after 2 minutes, 15- min. delay – delay after 15 minutes, PLO – Place Losing Omission, WSF – Word Search form, after 24 hrs – intention retrieval after 24 hours, rho – Spearman rank correlation coefficient, * – α < 0.05, ‡ – α < 0.01, § – α < 0.001. Our study may serve as a source of normative PM performance in the Czech version of MIST. As the above correlation analyses suggest, the MIST Summary score is independent of age and education, though it is dependent on gender (as men and women differed from one another significantly). The distribution of that score cannot be regarded as meeting the demands for normality (see normality analysis). Hence, it is impossible to construct a regression equation for analyzing the effect of gender on MIST performance. For tentative estimation of MIST performance in healthy persons (age range 38 – 81 and years of education 11 – 19) we recommend using Md (median) values (women Md = 45.0, men Md = 39.0), or descriptive characteristics separately for women (M (mean) = 42.5 ± 6.2 SD) and men (M = 38.8 ± 5.7 SD). The average duration of MIST administration was 24.2 ± 1.7 minutes, which is comparable to the original [22].
Discussion
In Czech neuropsychology and neurology, PM represents a new and unexploredtopic [19,20]. The purpose of the present study was to introduce the concept of PM to a broader Czech neuroscience audience, and to make available the first measurement tool for PM assessment (MIST). PM assessment in clinical populations and different age groups requires data on PM performance in healthy populations on MIST [10]. Therefore created a standardized Czech version (supervised by the original author of MIST) with back translation of the administration, scoring and original‑based record sheet [18]), validating the translated version of MIST on healthy Czech subjects.
We found performance on the MIST Summary score (which indicates general PM performance) to depend on certain demographic characteristics (Tab. 3). Unlike previous studies [16,18], neither age nor education correlated with the Summary score on MIST. That correlation was not detected may be due to the relatively homogeneous demographic characteristics of the sample under study and the small sample size. Given a larger sample and a greater range of ages, our results may have been more in agreement with those report previously in US studies [16,18,48], which found a significant relationship between age and gender. Regarding the influence of education, our results in adults with a higher level of education are surprisingly in agreement with a normative study published previously, where no differences were found between groups of college ‑ educated persons (13 – 15 years of education) and those with a longer record of education ≥ 16 years [18]. Differences between groups of men and women were significant in the MIST Summary score, with a medium effect size. This finding concurs with previous studies [16,18]. As a result, we also present separate descriptive statistics for men and women for normative values on the MIST Summary score. However, considering group size in individual genders, our results should be interpreted cautiously.
Reliability is the rate up to which scores are devoid of errors of measurement. In the present study, we tested the internal consistency of MIST, using Cronbach’s α and also split ‑ half reliability. We showed that the inner consistency of the eight MIST trials was insufficiently low (α = 0.50 – 0.56), which is consistent with the α values in the normative study [18]. However, comparing the internal consistency of six subscales (delay of 2 versus 15 minutes, type of verbal versus action response, time‑based versus event‑based cue; each score ranging from 0 to 8 points), we obtained high internal consistency and split ‑ half coefficients (0.88 – 0.95) comparable to the original normative study [18].
Validity generally means that the test measures the construct (presently, the construct of PM, compare with Fig. 1) that it is supposed to measure [49]. MIST content validity is shown in Fig. 1. The PM process model shows the basic processes that any test designed to measure PM should adhere to. Fig. 1 shows MIST as a test covering the basic PM processes (intention formation, intention retention, intention initiation, and intention execution relative to different lengths of delay (2 and 15 minutes, 24 hours), cue modality (time ‑ and event‑based) and the type of response (verbal and action)). Correlation between the MIST Summary score and all basic MIST subscales may been seen in Tab. 4. The only exception, the subscale of delay after 2 minutes (medium effect size), may be the result of the ceiling effect (see Tab. 3), in agreement with previous studies [16,18]. The construct validity of MIST has been proven in previous studies and surveys [16,18,22,28,50]. In the present study, we show the construct validity of MIST in connection with whether some of the MIST indices correlate with other well established measures of psychic efficiency and effectively delimit the construct of PM as measured by MIST in healthy individuals: the MIST Summary score is at the medium level of association with RAVLT delayed recall. This appears to represent a retrospective component of PM (see Fig. 1). Also with regard to TMT ‑ B, a test generally viewed as an indicator of mental flexibility, divided attention and set shifting [51]. In our opinion, this is a component associated with intention execution, an act requiring considerable mental flexibility, and monitoring (executive processes; see Fig. 1). As follows from functional imaging studies, tasks for PM activate the rostral prefrontal cortex [8,52]. The MIST Summary score correlates to a slightly lower degree with the total score of TOL, which is a task regarded as a paradigm of executive functioning, implicit learning and planning in particular [39], thus lending support to previous findings. It also correlates significantly with the Spatial Span forward, a task significantly activating visual focused attention. This is also one of the aspects necessary for effective performance of MIST consisting of visual monitoring and awareness of time while simultaneously solving a parallel task (Word Search Form). Correlation with the Czech National Adult Reading Test (CART), i.e., a test of premorbid intelligence and semantic memory, is another interesting feature. Since the construct of intelligence is a general factor with significant impact on most of the tasks for mental efficiency [53], this correlation is not surprising. The last and lowest medium ‑ level correlation is that between MCT and the MIST Summary score attributable to the relationship with memory processes in MCT (a verbal memory test based on an interference paradigm), where resistance to interference is probably one of the basic mechanisms essential for successful performance on MIST (with the particular tasks interfering with one another at the time of execution and in a sequence of event‑based cues). Critically speaking, in all of the above mentioned coefficients of correlation (Tab. 2) their coefficient of determination (as a rate of the joint variability of both variables) was r2 = 0.14 – 0.18, which is about one tenth to one fifth of their joint variance. Hence, it cannot be argued that any of the mentioned variables would share a higher proportion of joint variance with MIST.
The present validation study has some limitations. The size of the cohort and its members’ average education and age (a relatively homogeneous group), both clearly restrict any generalization of the results to the Czech population as a whole, especially in individuals with a lower level of education or age. The results of reliability analyses may have differed considerably if we also had use of data from clinical groups (e. g., from Parkinson’s disease with dementia). This would certainly lead to greater variability in the scores obtained and in the number of errors [54].
The main objective of the present study was to make a standardized tool for PM measurement available and to adapt the MIST Czech version to clinical practice. MIST enables analysis of the basic components and processes of PM (intention formation and retention (at different lengths of delay), intention retrieval and execution, recognition, time ‑ and event‑based PM differentiation and error analysis). PM is a significant component of our mental life, and its measurement may prove to be of major clinical consequence for the diagnosis and treatment of patients with memory disorders. We hope this tool will open the door in the Czech Republic to further research on PM; a construct close to our everyday functioning and potentially important and sensitive in neurodegenerative diseases causing memory disorders.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Mgr. Ondrej Bezdicek
Department of Neurology and Centre of Clinical Neuroscience
Charles University, 1st Medical Faculty and General University Hospital in Prague
Katerinska 30
128 21 Prague 2
e-mail: ondrej.bezdicek@gmail.com
Accepted for review: 17. 10. 2013
Accepted for print: 23. 1. 2014
Přílohy
1. Instrukce Testu paměti pro záměry (MIST)
2. Test na prospektivní paměť (MIST) a jeho administrace
Sources
1. Kožený J. Theory, data and validity of construct measurement. Ceskoslov psychol 1998; 42(6): 481–502.
2. Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem 2004; 82(3): 171–177.
3. McDaniel MA, Einstein GO. Prospective memory: an overview and synthesis of an emerging field. Los Angeles: Sage Publications 2007.
4. Kliegel M, McDaniel MA, Einstein GO (eds). Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. Mahwah (NJ): Erlbaum 2008.
5. Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol 2002; 53 : 1–25.
6. Kliegel M, Altgassen M, Hering A, Rose NS. A process-model based approach to prospective memory impairment in Parkinson’s disease. Neuropsychologia 2011; 49(8): 2166–2177. doi: 10.1016/j.neuropsychologia.2011.01.024.
7. Knight RG. Prospective memory in aging and neurodegenerative disease. In: Tröster, AI (ed). Memory in neurodegenerative disease: Biological, clinical and cognitive perspectives. New York, NY: Cambridge University Press 1998 : 172–183.
8. Simons JS, Schölvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia 2006; 44(8): 1388–1397.
9. Martin T, McDaniel MA, Guynn MJ, Houck JM, Woodruff CC, Bish JP et al. Brain regions and their dynamics in prospective memory retrieval: a MEG study. Int J Psychophysiol 2007; 64(3): 247–258.
10. Cockburn J. Assessment and treatment of prospective memory deficits. In: Brandimonte M, Einstein G, McDaniel M (eds). Prospective memory: theory and applications. Mahwah, NJ: Lawrence Erlbaum Associates 1996 : 327–350.
11. Hicks JL, Marsh RL, Russell EJ. The properties of retention intervals and their effect on retaining prospective memories. J Exp Psychol Learn Mem Cogn 2000; 26(5): 1160–1169.
12. Einstein GO, McDaniel MA. Normal aging and prospective memory. J Exp Psychol Learn Mem Cogn 1990; 16(4): 717–726.
13. Schnitzspahn KM, Stahl C, Zeintl M, Kaller CP, Kliegel M. The role of shifting, updating, and inhibition in prospective memory performance in young and older adults. Dev Psychol 2013; 49(8): 1544–1553. doi: 10.1037/a0030579.
14. Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Arch Clin Neuropsychol 2005; 20(1): 33–65.
15. Wilson BA, Cockburn J, Baddeley AD. The Rivermead Behavioral Memory. Test manual. 2nd ed. Bury St. Edmunds (UK): Thames Valley Test Company 1991.
16. Woods SP, Moran LM, Dawson MS, Carey CL, Grant I. Psychometric characteristics of the memory for intentions screening test. Clin Neuropsychol 2008; 22(5): 864–878. doi: 10.1080/13854040701595999.
17. Groot YC, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. J Int Neuropsychol Soc 2002; 8(5): 645–654.
18. Raskin S, Buckheit C, Sherrod Ch. Memory for Intentions test. Professional Manual. Lutz: Psychological Assessment Resources 2010.
19. Szente V. The role of executive functions in prospective memory. A diploma thesis. Brno: Masaryk University 2012.
20. Klenerová V, Hynie S. Is there any correlation between stress, memory and strong emotions such as apprehension and fear? Ceskoslov fyziol 2007; 56(3): 97–103.
21. Raskin S. Memory for intentions screening test [abstract]. J Intern Neuropsychol Soc 2004; 10 (Suppl 1): 110.
22. Raskin SA. Memory for intentions screening test: psychometric properties and clinical evidence. Brain Imp 2009; 10(1): 23–33.
23. Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I. Prospective memory in HIV-1 infection. J Clin Exp Neuropsychol 2006; 28(4): 536–548.
24. Woods SP, Twamley EW, Dawson MS, Narvaez JM, Jeste DV. Deficits in cue detection and intention retrieval underlie prospective memory impairment in schizophrenia. Schizophr Res 2007; 90(1–3): 344–350.
25. Pearce R, Raskin S. An examination of age, prospective memory, and memory errors: How are the elderly forgetting to remember future actions? (Unpublished honors thesis). Trinity College, Hartford, CT 2000.
26. Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams R et al. A differential deficit in time versus event-based prospective memory in Parkinson’s disease. Neuropsychology 2011; 25(2): 201–209. doi: 10.1037/a0020999.
27. Raskin SA, Buckheit CA, Waxman A. Effect of type of cue, type of response, time delay and two different ongoing tasks on prospective memory functioning after acquired brain injury. Neuropsychol Rehabil 2012; 22(1): 40–64. doi: 10.1080/09602011.2011.632908.
28. Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I. HIV Neurobehavioral Research Center Group. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology 2008; 22(1): 110–117. doi: 10.1037/0894-4105.22.1.110.
29. Raskin SA, Maye J, Rogers A, Correll D, Zamroziewicz M, Kurtz M. Prospective Memory in Schizophrenia: Relationship to Medication Management Skills, Neurocognition, and Symptoms in Individuals With Schizophrenia. Neuropsychology Nov 4. [Epub ahead of print].
30. Folstein MF, Folstein SE, Fanjiang G. Mini-Mental State Examination. Clinical Guide. Lutz, FL: Psychological assessment Resources 2001.
31. Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2nd ed. San Antonio, TX: Pearson 1996.
32. Bezdíček O, Lukavský J, Preiss M. Validizační studie české verze dotazníku FAQ. Cesk Slov Neurol N 2011; 74/107(1): 36–42.
33. Ellis J. Prospective memory or the realization of delayed intentions: A conceptual framework for research. In: Brandimonte M, Einstein G, McDaniel M (eds). Prospective memory: Theory and applications. Mahwah, NJ: Lawrence Erlbaum Associates 1996 : 1–22.
34. Philips L, Henry J, Martin M. Adult aging and prospective memory: the importance of ecological validity. In: Kliegel M, McDaniel MA, Einstein GO (eds). Prospective memory: cognitive, neuroscience, developmental, and applied perspectives. Mahwah: Erlbaum 2008 : 161–186.
35. Cockburn J, Smith PT. Anxiety and errors of prospective memory among elderly people. Br J Psychol 1994; 85(2): 273–282.
36. Krámská L, Preiss M. Evaluation of premorbid status – word reading test. Psychiatrie 2007; 11(1): 4–7.
37. Preiss M, Rodriguez M, Kawaciuková R, Laing H. Neuropsychological batters of the Psychiatric Centre Prague. Clinical examination of basic cognitive functions. 2nd ed. Praha: Psychiatrické centrum Praha 2007.
38. Wechsler D. Wechsler adult intelligence scale. Manual. Prague: Hogrefe Testcentrum 2010.
39. Wechsler D. Wechsler memory scale. A technical manual. Bratislava: Psychodiagnostika 1999.
40. Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 1982; 298(1089): 199–209.
41. Kongs SK, Thompson LL, Iverson GL, Heaton, RK. Wisconsin Card Sorting Test-64 card computerized version. Odessa, FL: Psychological Assessment Resources 2000.
42. Troyer AK, Leach L, Strauss E. Aging and response inhibition: Normative data for the Victoria Stroop Test. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2006; 13(1): 20–35.
43. Wechsler D. Wechsler adult inteligence test. Bratislava: Psychodiagnostické a didaktické testy 1983.
44. Buschke H. Screening for pre-symptomatic AD. Lecture. Charles University in Prague: Prague 2007.
45. Benton AL, Sivan AB, Hamsher K, de Varney NR, Spreen O. Contributions to neuropsychological assessment. 2nd ed. Orlando, Fla: Psychological Assessment Resources 1994.
46. Pallant J. SPSS Survival Manual. New York, NY: McGraw-Hill 2007.
47. Cohen JW. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates 1988.
48. Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychol Aging 2004; 19(1): 27–39.
49. Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychol Bull 1955(4); 52(4): 281–302.
50. Fleming JM, Shum D, Strong J, Lightbody S. Prospective memory rehabilitation for adults with traumatic brain injury: a compensatory training programme. Brain Inj 2005; 19(1): 1–10.
51. Bezdicek O, Motak L, Axelrod BN, Preiss M, Nikolai T, Vyhnalek M et al. Czech version of the Trail Making Test: normative data and clinical utility. Arch Clin Neuropsychol 2012; 27(8): 906–914. doi: 10.1093/arclin/acs084.
52. Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: what have we learnt so far? Neuropsychologia 2011; 49(8): 2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014.
53. Deary IJ. Intelligence. Annu Rev Psychol 2012; 63 : 453–482. doi: 10.1146/annurev-psych-120710-100353.
54. Delis DC, Jacobson M, Bondi MW, Hamilton JM, Salmon DP. The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: lessons from memory assessment. J Int Neuropsychol Soc 2003; 9(6): 936–946.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2014 Issue 4-
All articles in this issue
- Sensory Examination
- Disorders of the Nervous System Arising out of Nutritional Insufficiency after Bariatric Surgery
- Genetic Variability in Attention Deficit Hyperactivity Disorder
- Anaplastic Oligodendrogliomas – the Age of Personalized Medicine Has Arrived?
- The Value of Repeated Non-confirmatory Multiple Sleep Latency Test (MSLT) for the Diagnosis of Narcolepsy
- Differences in Spatial Navigation Impairment in Neurodegenerative Dementias
- Assessing Handwriting in Patients with Parkinson‘s Disease
- AMETYST – Observational Phase IV Study Following the Influence of Intramuscularly Administered Interferon Beta‑1a in Patients with Clinically Isolated Syndrome/ Clinically Definite Multiple Sclerosis
- Hereditary Ulceromutilating Sensory Neuropathy – Clinical, Electrophysiological and Molecular Genetic Study of Three Families
- Limits of Verbal Fluency Tests Use in the Differential Diagnostic of Neurological Diseases
- Validation of the Minimum Question Set for the Diagnosis of the Restless Legs Syndrome in a Population of Czech Pregnant Women
- Findings in Cerebrospinal Fluid among Children with Paretic Involvements
- Long‑term Observation of Cognitive, Emotional and Behavioral Changes in Patient with Multiple Sclerosis – a Case Report
- Spinal Arteriovenous Malformations – Two Case Reports
- Total Avulsion of the Eye Globe Combined with an Injury of the Chiasm – a Case Report
- Assessment of Prospective Memory – a Validity Study of Memory for Intentions Screening Test
- The Significance Of (CA)n Tandem Repeat in GABA(A) Beta‑3 Subunit Gene in Tinnitus Manifestation
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Sensory Examination
- Genetic Variability in Attention Deficit Hyperactivity Disorder
- Spinal Arteriovenous Malformations – Two Case Reports
- The Value of Repeated Non-confirmatory Multiple Sleep Latency Test (MSLT) for the Diagnosis of Narcolepsy
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

