-
Medical journals
- Career
Flow Cytometry in Monoclonal Gammopathies
Authors: L. Kovářová 1,2; T. Varmužová 1,2; P. Zarbochová 3; R. Suská 1; Muthu Raja K. R. 2,4; J. Štossová 2; M. Penka 4; R. Hájek 1,2,5
Authors‘ workplace: Laboratory of Experimental Hematology and Cell Immunotherapy, Department of Clinical Hematology, University Hospital Brno, Czech Republic 1; Babak Myeloma Group, Department of Pathological Physiology, Faculty of Medicine, Masaryk University, Brno, Czech Republic 2; Institute of Experimental Biology, Faculty of Science, Masaryk University, Brno, Czech Republic 3; Department of Clinical Hematology, University Hospital Brno, Czech Republic 4; Department of Internal Medicine – Hematooncology, University Hospital Brno, Czech Republic 5
Published in: Klin Onkol 2011; 24(Supplementum 1): 24-29
Overview
The technological development of flow cytometry (FC) together with new findings reveal the need for immunophenotyping in research of monoclonal gammopathy (MG) because of its diagnostic, prognostic and predictive significance. The aim of the European Myeloma Network (EMN) is to standardize this analytical method and implement it into routine clinical examination. Since the overall significance and application of FC are still analysed, standardisation could help obtain more clinical relevant information in terms of MG pathophysiology.
Key words:
multiple myeloma – monoclonal gammopathy – flow cytometry – plasma cell
This work was supported by research grants of The Ministry of Education, Youth and Sports: LC06027, MSM0021622434; research projects of IGA of The Ministry of Health: NT12425, NS10406, NS10408 and grants of GACR GAP304/10/1395, GP301/09/P457.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.Background
Multiparametric FC is applied in the diagnostics of many hematologic malignancies; however, in MG research, it was performed rather complementarily. One of the reasons why immunophenotyping is still not done as part of routine diagnostics is the fact that it underestimates the number of plasma cells (PCs), when compared to routine morphological evaluation. However, the sensitivity of flow cytometry is similar to the light microscopy – results obtained using both approaches correlate and % of PC provided by FC is also an independent prognostic factor affecting the overall survival of patients [1]. FC provides detailed analysis of leukocyte subpopulations and is able to discriminate PC subtypes, even if they are present in very low numbers, what is useful in differential diagnostics and in the identification of high-risk asymptomatic MGs [2,3]. Multiparametric flow cytometry is also used to confirm stringent complete remission (sCR) as defined by The International Myeloma Working Group [4]. Determination of PC immunophenotype has prognostic significance and can help find new therapeutic targets as well [3,5]. Development of flow cytometry, including powerful instruments with possibility to analyse many fluorochromes, availability of new dyes and antibodies, together with accessible specific software for complex phenotype analysis, require reviewing current settings in MG analyses. This review is focused on methodology and using FC in clinical and research laboratories.
Standardization of Flow Cytometry
FC is able to analyse many parameters of a large number of cells, and it is necessary to be consistent in providing analyses and reporting results. Incorporation of FC into routine analysis should be associated with standardization and validation of this method [6]. Two flow cytometric workshops were organized by the EMN for standardization of immunophenotyping in MGs; these workshops were followed by publication summarizing the findings and recommendations for PC analysis [7]. The third workshop was focused on the possibility of monitoring minimal residual disease (MRD), and technological advances in the field of FC were discussed as well. Higher sensitivity and more possibilities with multicolour flow cytometry will lead to defining of new recommendations in the future which are going to be associated with the development of a uniform protocol for the analysis of biological material using the Euroflow group settings. The Czech Myeloma Group began the standardization process in 2009; currently, there are more than 12 laboratories in the Czech Republic working on the unification of flow cytometry analysis in correlation with European standards [8,9].
Identification and Phenotype of PCs – Recommendation for Basic Analysis
Most PCs are available in the bone marrow (BM), but there are also circulating plasma cells/plasmablasts in peripheral blood (PB). The identification of PCs is based on expression of two markers – CD38 and CD138. CD38 is not a specific marker, but its bright expression could help to discriminate PCs from other leukocytes in PB and/or BM. It is known that expression of CD138 is influenced by age of the sample and can be reduced over time by shedding of CD138 into plasma; however, this is the specific marker of PCs. Only very few BM samples show absent or low expression of CD138, although its expression in PB is lower or missing when compared to BM [3,10,11]. Acquisition of sufficient amount of CD38+CD138+ PCs (minimum of 100 neoplastic PCs) in the whole BM is crucial, so the first portion of bone marrow aspirate is essential for analysis to avoid hemodilution of sample with PB [3,7]. The major advantage of FC when compared to other methods is the possibility to discriminate between normal polyclonal (N-PCs) and abnormal clonal (A-PCs) PCs. Although clonal cells could express CD38 and CD138 with lower intensity than normal PC and CD45 is mostly not expressed on these aberrant PCs, precise discrimination is possible only when other markers are analysed [12]. Many studies confirmed that clonal PCs have different phenotype characterized by underexpression and/or lack of CD19, CD27, CD38 and CD45; on the other hand, these clonal PCs should overexpress CD20, CD28, CD33, CD56 and CD117; benefit of other markers, such as CD81 and CD200 is still discussed [5,13]. Minimum of 4 markers is recommended for basic PC analysis so that expression of CD38/CD138/CD19//CD56 should be analyzed in every MG case to identify CD38+CD138+PCs and to discriminate N-PCs (CD19+CD56–) and A-PCs (CD19+CD56+, CD19–CD56+, CD19–CD56–) [7,14].
Routine Setting in MG Analysis
Basic clinical applications of FC are a) differential diagnostics of multiple myeloma (MM) and other plasma cell-related disorders [15–20]; b) determination of the risk of progression of monoclonal gammopathy of undetermined significance (MGUS) or asymptomatic MM to symptomatic form [2,9,17]; and c) the detection of MRD after treatment [4,21–23]. For these applications, particular discrimination, enumeration and characterization of myelomatous PCs in context of whole PC population are essential [3,7,24,25]. Every clinical laboratory should be able to perform basic analysis using 4 markers (CD38/CD138//CD19/CD56) by one-laser flow cytometer (Fig. 1). However, there could be some uncertainty about PC clonality, so using not only the essential markers CD19 and CD56, but also CD20, CD27, CD28, CD45 and CD117 is recommended for aberrant PC detection [7]. As CD27 is expressed by both normal and clonal PCs, higher expression of CD27 is specific for N-PCs, while lower intensity and/or lack of expression CD27 is typical for A-PC [26]. Unfortunately, there is no clear evidence which subpopulations are really polyclonal and/or clonal in selected cases, so cytoplasmic expression of kappa and lambda immunoglobulin light chains should be used for verification of PC normality using minimum of 6-colours (Fig. 2).
Fig. 1. Detection of normal and aberrant PCs. Diff erent subpopulations of CD38+CD138+PCs according to CD19 and CD56 expression are visualised in MGUS case (A), newly diagnosed MM case (B), and MM case after treatment (C). Percentage of CD19+CD56- (blue dots) and CD19-CD56+ (purple dots) are showed. Analyses made by fl ow cytometr FACSCantoII using acquisition software Diva 6.0 (Becton Dickinson) and analysis software Infi - nicyt (Cytognos). 
Fig. 2. Analysis of CD19+ cells in bone marrow. CD19+ B cells (blue dots) and/or PCs (red dots) were identifi ed according to expression of CD19, CD38 and CD138 in whole bone marrow (A). Phenotype profi le match normal PCs (CD19+CD45+CD56-D27+CD117-CD20-), but slightly higher number of PC (1.5% of leukocytes) was the reason for clonality analysis. Predominance of clonal clambda+ PCs in comparison with normal B cells is evident (F). Analyses made by fl ow cytometr FACSCantoII using acquisition software Diva 6.0 (Becton Dickinson) and analysis software Infi nicyt (Cytognos). 
Advanced PC Analysis in MGs
Polychromatic assays performed by two - and/or three-laser flow cytometer (6 - and/or 8-colour analyses) should provide complex information about PC immunophenotype, what could be useful not only in clinical, but also in research approach. The significance of some surface and/or cytoplasmic markers is known, while others are still analysed for their prognostic/predictive significance or for use in the detection of MRD and/or as a potential therapeutic target etc. (Tab. 1) [5,7,27–31].
1. Possible combinations of surface and intracellular antigens for PC identifi cation and detailed phenotype analysis in bone marrow and/or peripheral blood. 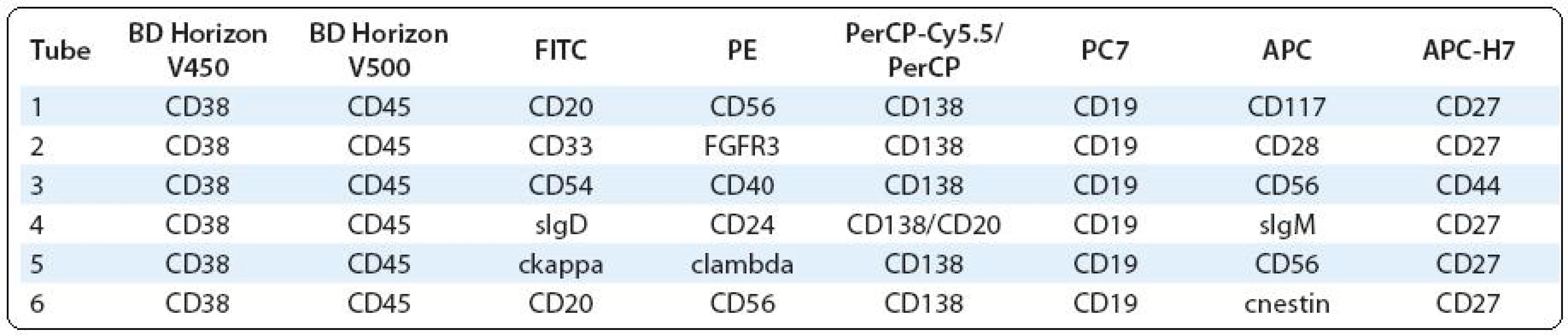
Using polychromatic 8-colour FC saves sample volume – with same volume for one tube (60–120 μl per tube depending on cellularity and PCs content), twice as many markers could be analysed than before with 4-colour FC. Polychromatic FC is important when low volume of bone marrow is available (problem with aspiration of bone marrow etc.) or other low volume liquid sample (cerebrospinal fluid etc.) has to be analysed, so using 8 markers in one tube allows both detection of leukocyte subpopulations and PC characterisation. In addition to continual acquisition of a large number of cells (a huge listmode), two-step acquisition process could be used. First, several thousand cells are analysed, and then, using a gate, a defined population of PCs is acquired for further analyses [3,17]. Co-expression of different markers may be found by merge of different tubes using so-called backbone markers (markers which are present in all analysed tubes) through special software. This approach increases number of PCs as well; for example, from 3 tubes which individually contain 50 PCs there is possibility to obtain one file with 150 PCs using data merging. Thus, polychromatic assays are helpful in determination of the complexity of PC phenotype in routine and also in research analyses.
Assessment of PCs clonality seems to be very important for differential diagnostics in unusual MG samples, for exclusion of non-MG samples and especially for sCR confirmation in MRD analysis. However, detection of clonal cells based only on CD19/CD56 expression is mostly insufficient in these cases (Fig. 3). On the other hand, in typical MM cases, majority of PCs are clonal in the bone marrow, so analysis of PC clonality is not required. Minimum of 6 markers should be used, CD38 and CD138 for PC identification, CD19 and CD56 for detection of different PC subpopulation and cytoplasmic kappa and lambda light chains for detection of real clonal PCs [32]. Other markers useful in clonality analysis are CD45 and CD27, which could better identify polyclonal PCs, thus 8-colour FC is more accurate.
Fig. 3. Assessment of PC clonality. Diff erent subpopulations of CD38+CD138+ PCs according to CD19, CD56 and CD27 expression are visualised in AL amyloidosis sample (A, B). Majority of clonal clambda+ PCs is presented in CD19-CD56- region and these clonal PCs also lack CD27. Remaining regions seems to be polyclonal with high expression of CD27. Analyses made by fl ow cytometr FACSCantoII using acquisition software Diva 6.0 (Becton Dickinson) and analysis software Infi nicyt (Cytognos). 
There is association between the phenotypic profile and cytogenetic abnormalities [5], although the role of FC is only informative. However, FC analysis of FGFR3 expression is an available method for the detection and management of new therapeutic approaches for t(4;14) positive MM with poor prognosis [33]. Recently, a study was also published on progression from MGUS to SMM and eventually to MM, involving a clonal expansion of genetically abnormal PC [34].
Specific part of research analyses is BM microenvironment, as its interactions could play a key role in the proliferation, survival and drug resistance of clonal PCs [35,36]. Lack, decreased and/or increased expression of adhesive markers (CD44, CD54 etc.) and chemokine receptors may result in migration of PCs to PB or other tissues; expression of other markers supports survival and proliferation of PCs, so adhesive system of MGs is an attractive potential therapeutic target [37,38].
As PCs differentiate from B cells, analysis of B-cell subsets can help to better understand pathophysiology of PC-related disorders, as well as putative MM initiating cells can circulate through PB and/or BM as less and/or more mature forms of B cells [39]. MM initiating cells hypothesis could explain incurability of MM, since they are relatively resistant to anticancer agents and have potential to self-renewal; so looking for them is still a challenge for research. [40]. Recently, a study was published on the expression of nestin in mature PCs. Nestin is a characteristic marker of multipotent proliferative precursors, what could be important for identification of MM initiating cells, but clinical consequence of nestin expression is still unknown [41]. Detailed analysis of CD19+ subpopulations (immature, transitional, naïve, activated, memory, isotype switched, plasmablast, plasma cell etc.) helps in diagnostics of unusual cases.
Methodological Pitfalls in Multiple Myeloma Research
Identification of PCs should be done by both CD38 and CD138, while CD45 has informative character with no clear prognostic significance. Separation of mononuclear cells is not recommended for reasons of cell loss; on the other hand, there is no limitation in terms of erythrocyte lysis. PCs are very heterogeneous in their size, and they could be highly autofluorescent, so negative control for staining is a method of choice [42]. Discrimination of PC doublets by comparison of signal pulse height and width or area is necessary as PCs are sticky, especially after fixation for cytoplasmic analyses [43]. The very important process in FC is choosing fluorochromes conjugated with monoclonal antibodies, because of their significantly different staining index [44]. For example, as CD38 is expressed by PCs with high intensity, so using bright phycoerythrine (PE) conjugate for this marker is not recommended, since fluorescence can be out of scale or the sample can be uncompensable etc. Acquisition of sufficient PC number is a prerequisite for successful evaluation in low-infiltrated samples. This is important mostly in MRD analysis, where a minimum of 3,000 acquired PCs is needed [4]. Although analysis of CD19 and CD56 on CD38+CD138+ PCs could discriminate majority of normal and abnormal PCs, in some cases more detailed analysis using other markers and/or analysis of cytoplasmic expression of kappa and lambda light chains is necessary. Thus, minimalistic 4-colour PC analysis should be replaced by polychromatic FC in uncertain cases, especially in other PC dyscrasias (MGUS, primary amyloidosis, Waldenström macroglobulinemia etc.), if possible. Clonality assessment should be done also in every case with low PC infiltration and unclear phenotype as increasing sensitivity of methods for monoclonal protein detection could lead to suspicion of MG even in normal cases. Lymphomas with plasmacytic differentiation, where PCs have mostly CD19+ phenotype were described; however, they are clonal in lymphoplasmocytic lymphoma and polyclonal in marginal zone lymphoma [45,46]. Verification of clonality is then important for differential diagnostics as some polyclonal PCs should not express CD19, and CD56 could be expressed by polyclonal PC subpopulation as well.
Conclusion
Flow cytometry is a sophisticated method which allows detection and further analysis of plasma cells, especially in polychromatic setting. Its clinical significance, in particular stringent complete response assessment, is unquestionable and this method should be used in both routine analyses of monoclonal gammopathies as well as in research.
Mgr. Lucie Kovářová, Ph.D.
Department of Clinical Hematology
University Hospital Brno
Jihlavská 20
625 00 Brno
Czech Republic
e-mail: lkovarova@fnbrno.cz
Sources
1. Paiva B, Vidriales MB, Pérez JJ et al. Multiparameter flow cytometry quantification of bone marrow plasma cells at diagnosis provides more prognostic information than morphological assessment in myeloma patients. Haematologica 2009; 94(11): 1599–1602.
2. Pérez-Persona E, Mateo G, García-Sanz R et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol 2010; 148(1): 110–114.
3. Paiva B, Almeida J, Pérez-Andrés M et al. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom 2010; 78(4): 239–252.
4. Rajkumar SV, Harousseau JL, Durie B et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011; 117(18): 4691–4695.
5. Mateo G, Montalbán MA, Vidriales MB et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol 2008; 26(16): 2737–2744.
6. Johnsen HE, Bøgsted M, Klausen TW et al. Nordic Myeloma Study (NMSG). Myeloma Stem Cell Network (MSCNET). Multiparametric flow cytometry profiling of neoplastic plasma cells in multiple myeloma. Cytometry B Clin Cytom 2010; 78(5): 338–347.
7. Rawstron AC, Orfao A, Beksac M et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica 2008; 93(3): 431–438.
8. Kovarova L, Buresova I, Suska R et al. Comparison of plasma cells phenotype in MGUS and MM. Brno: 4th Myeloma and 2nd Immunotherapy workshop 2009.
9. Kovarova L, Varmuzova T, Zarbochova P et al. Is Flow Cytometry Able to Distinguish Monoclonal Gammopathies and/or Multiple Myeloma Subtypes with Good Prognosis? Brno: 5th Myeloma workshop 2010.
10. Yang Y, Yaccoby S, Liu W et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood 2002; 100(2): 610–617.
11. Caraux A, Klein B, Paiva B et al. Myeloma Stem Cell Network. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138 - and CD138+ plasma cells. Haematologica 2010; 95(6): 1016–1020.
12. Bataille R, Jégo G, Robillard N et al. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica 2006; 91(9): 1234–1240.
13. Cannizzo E, Bellio E, Sohani AR et al. Multiparameter immunophenotyping by flow cytometry in multiple myeloma: The diagnostic utility of defining ranges of normal antigenic expression in comparison to histology. Cytometry B Clin Cytom 2010; 78(4): 231–238.
14. Kovarova L, Hajek R. Flow cytometric analysis of plasma cells in multiple myeloma. Klin Onkol 2008; 21 (Suppl 1): 249–252.
15. Ocqueteau M, Orfao A, Almeida J et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. Am J Pathol 1998; 152(6): 1655–1665.
16. Sezer O, Heider U, Zavrski I et al. Differentiation of monoclonal gammopathy of undetermined significance and multiple myeloma using flow cytometric characteristics of plasma cells. Haematologica 2001; 86(8): 837–843.
17. Pérez-Persona E, Vidriales MB, Mateo G et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007; 110(7): 2586–2592.
18. Paiva B, Vidriales MB, Mateo G et al. The persistence of immunophenotypically normal residual bone marrow plasma cells at diagnosis identifies a good prognostic subgroup of symptomatic multiple myeloma patients. Blood 2009; 114(20): 4369–4372.
19. Kovarova L, Buresova I, Buchler T et al. Phenotype of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance. Neoplasma 2009; 56(6): 526–532.
20. Kovarova L, Buresova I, Suska R et al. Flow cytometric discrimination between neoplastic clonal and physiological polyclonal plasma cells. Klin Onkol 2008; 21 (Suppl 1): 254–257.
21. Rawstron AC, Davies FE, DasGupta R et al. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood 2002; 100(9): 3095–3100.
22. Sarasquete ME, García-Sanz R, González D et al. Minimal residual disease monitoring in multiple myeloma: a comparison between allelic-specific oligonucleotide real-time quantitative polymerase chain reaction and flow cytometry. Haematologica 2005; 90(10): 1365–1372.
23. Paiva B, Vidriales MB, Cerveró J et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood 2008; 112(10): 4017–4023.
24. Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol 2010; 149(3): 334–351.
25. Yuan CM, Stetler-Stevenson M. Role of flow cytometry of peripheral blood and bone marrow aspirates in early myeloma. Semin Hematol 2011; 48(1): 32–38.
26. Kovarova L, Buresova I, Raja KR et al. Expression of CD27 on plasma cells in multiple myeloma. Haematologica 2009; 94 (Suppl 2): 381.
27. Bataille R, Pellat-Deceunynck C, Robillard N et al. CD117 (c-kit) is aberrantly expressed in a subset of MGUS and multiple myeloma with unexpectedly good prognosis. Leuk Res 2008; 32(3): 379–382.
28. Guikema JE, Hovenga S, Vellenga E et al. CD27 is heterogeneously expressed in multiple myeloma: low CD27 expression in patients with high-risk disease. Br J Haematol 2003; 121(1): 36–43.
29. Plowright EE, Li Z, Bergsagel PL et al. Ectopic expression of fibroblast growth factor receptor 3 promotes myeloma cell proliferation and prevents apoptosis. Blood 2000; 95(3): 992–928.
30. Grand EK, Chase AJ, Heath C et al. Targeting FGFR3 in multiple myeloma: inhibition of t(4;14)-positive cells by SU5402 and PD173074. Leukemia 2004; 18(5): 962–966.
31. Hussein M, Berenson JR, Niesvizky R et al. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica 2010; 95(5): 845–848.
32. Marsee DK, Li B, Dorfman DM. Single tube, six-color flow cytometric analysis is a sensitive and cost-effective technique for assaying clonal plasma cells. Am J Clin Pathol 2010; 133(5): 694–699.
33. Chandesris MO, Soulier J, Labaume S et al. Detection and follow-up of fibroblast growth factor receptor 3 expression on bone marrow and circulating plasma cells by flow cytometry in patients with t(4;14) multiple myeloma. Br J Haematol 2007; 136(4): 609–614.
34. López-Corral L, Gutiérrez NC, Vidriales MB et al. The progression from MGUS to smoldering myeloma and eventually to multiple myeloma involves a clonal expansion of genetically abnormal plasma cells. Clin Cancer Res 2011; 17(7): 1692–1700.
35. Perez-Andres M, Almeida J, Martin-Ayuso M et al. Soluble and membrane levels of molecules involved in the interaction between clonal plasma cells and the immunological microenvironment in multiple myeloma and their association with the characteristics of the disease. Int J Cancer 2009; 124(2): 367–375.
36. Katz BZ. Adhesion molecules. The lifelines of multiple myeloma cells. Semin Cancer Biol 2010; 20(3): 186–195.
37. Iqbal MS, Otsuyama K, Shamsasenjan K et al. Constitutively lower expressions of CD54 on primary myeloma cells and their different localizations in bone marrow. Eur J Haematol 2009; 83(4): 302–312.
38. Vincent T, Mechti N. IL-6 regulates CD44 cell surface expression on human myeloma cells. Leukemia 2004; 18(5): 967–975.
39. Perez-Andres M, Paiva B, Nieto WG et al. Primary Health Care Group of Salamanca for the Study of MBL. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom 2010; 78 (Suppl 1): S47–S60.
40. Agarwal JR, Matsui W. Multiple myeloma: a paradigm for translation of the cancer stem cell hypothesis. Anticancer Agents Med Chem 2010; 10(2): 116–120.
41. Svachova H, Pour L, Sana J et al. Stem cell marker nestin is expressed in plasma cells of multiple myeloma patients. Leuk Res 2011. Epub ahead of print.
42. Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A 2006; 69(9): 1037–1042.
43. Donnenberg AD, Donnenberg VS. Rare-event analysis in flow cytometry. Clin Lab Med 2007; 27(3): 627–652.
44. Maecker HT, Frey T, Nomura LE et al. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A 2004; 62(2): 169–173.
45. Morice WG, Chen D, Kurtin PJ et al. Novel immunophenotypic features of marrow lymphoplasmacytic lymphoma and correlation with Waldenström‘s macroglobulinemia. Mod Pathol 2009; 22(6): 807–816.
46. Meyerson HJ, Bailey J, Miedler J et al. Marginal zone B cell lymphomas with extensive plasmacytic differentiation are neoplasms of precursor plasma cells. Cytometry B Clin Cytom 2011; 80(2): 71–82.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2011 Issue Supplementum 1-
All articles in this issue
- Editorial (CZ)
- Radiotherapeutic methods
- Multiple Myeloma
- Monoclonal Gammopathy of Undeterminated Significance: Introduction and Current Clinical Issues
- Sample Processing and Methodological Pitfalls in Multiple Myeloma Research
- Flow Cytometry in Monoclonal Gammopathies
- Flow Cytometric Phenotyping and Analysis of T Regulatory Cells in Multiple Myeloma Patients
- Genomics in Multiple Myeloma Research
- Polymorphisms Contribution to the Determination of Significant Risk of Specific Toxicities in Multiple Myeloma
- Oligonucleotide-based Array CGH as a Diagnostic Tool in Multiple Myeloma Patients
- Visualization of Numerical Centrosomal Abnormalities by Immunofluorescent Staining
- Impact of Nestin Analysis in Multiple Myeloma
- Editorial (EN)
- List of authors and reviewers
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Multiple Myeloma
- Flow Cytometric Phenotyping and Analysis of T Regulatory Cells in Multiple Myeloma Patients
- Monoclonal Gammopathy of Undeterminated Significance: Introduction and Current Clinical Issues
- Flow Cytometry in Monoclonal Gammopathies
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

