-
Medical journals
- Career
Changes in Paraoxonase 1 activity and concentration of conjugated dienes in connection with number of metabolic syndrome components
Authors: B. Staňková; L. Vávrová; J. Rychlíková; A. Žák
Authors‘ workplace: th Department of Internal Medicine, 1st Faculty of Medicine, Charles University and General University Hospital Prague 4
Published in: Klin. Biochem. Metab., 24, 2016, No. 2, p. 88-93
Overview
Objective:
Paraoxonase 1 is an antioxidant enzyme with a variety of physiological roles – one of them is the inhibition of LDL (low-density lipoprotein) lipid peroxidation and inactivation of LDL-derived oxidized phospholipids. The aim of this study was to investigate the PON1 activity and levels of conjugated dienes in precipitated LDL (CD/LDL) in subjects, who fulfill different numbers of metabolic syndrome (MetS) criteria.Design:
Cross-sectional studySettings:
4th Department of Internal Medicine, 1st Faculty of Medicine, Charles University and General University Hospital PragueMaterial and methods:
The population under study consisted of 354 Caucasian subjects (188 females/166 males) divided into 6 groups according to the number of presented components of metabolic syndrome. All groups were age matched. The activity of paraoxonase 1 and concentration of conjugated dienes in precipitated LDL (CD/LDL) were both assessed spectrophotometrically.Results:
The activity of PON1 was significantly decreased in subjects with all 5 components of MetS in comparison with those with 0 to 3 components of MetS (p < 0.05). The concentrations of CD/LDL were increased in subjects with 4 or 5 components of MetS compared to subjects with 0-3 components of MetS (p < 0.001).Conclusion:
As was shown in this study, the levels of PON1 are extensively affected by the concentration of HDL-C and ApoA1. PON1 activity is depressed and CD/LDL levels are increased mainly in subjects who fulfill all five criteria of MetS.Keywords:
Paraoxonase 1, conjugated dienes, metabolic syndrome components.Introduction
Paraoxonase 1 (PON1; EC 3.1.8.1.) is synthesized in the liver and secreted into the blood, where associates with HDL (high-density lipoprotein) particles [1]. In ancho-ring of PON1 to HDL through Apo-A1, the hydrophobic N terminus of PON1 is thought to be involved [2].
A variety of physiological roles have been proposed for PONs. Serum PON1 catalyzes the hydrolysis and thereby the inactivation of oxons like paraoxon, is also able to hydrolyze the nerve agents sarin and soman. In addition, PON1 hydrolyzes arylesters and different aromatic and aliphatic lactones as well as cyclic carbonates [3-6]. It was hypothesizes that the physiological substrates could be some derivatives of fatty acid oxidation process such as 5-hydroxy - 6E, 8Z, 11Z, 14Z -eicosatetraenoic acid (5-HETE) lactone that resides in HDL or some lactones which are consumed as food ingredients or drug metabolites. Furthermore was shown that homocysteine thiolactone is naturally occurring substrate of PON1 [7].
In vitro assays demonstrated that PON1 can inhibit LDL (low-density lipoprotein) lipid peroxidation and inactivate LDL-derived oxidized phospholipids. This could potentially reduce the serum content of the oxidized lipids involved in the initiation of atherosclerosis [8,9]. Furthermore was shown, that PON1 have also peroxidase-like activity [10,11] – it is capable to hydrolyze hydrogen peroxide, reduce lipoprotein pe-roxides (by 19 %) and cholesteryl linoleate hydrope-roxides (by 90 %).
One of the most sensitive indicators of lipid peroxidation is supposed to be the concentration of conjugated dienes in precipitated LDL (CD/LDL) [12].
The aim of this study was to investigate the PON1 activity and levels of CD/LDL in subjects, who fulfil different numbers of metabolic syndrome (MetS) criteria. The main components of MetS are accumulation of intraabdominal fat, impaired metabolism of glucose, atherogenic dyslipidemia (low HDL cholesterol, hypertriacylglyceridemia) and arterial hypertension.
Materials and methods
Settings and subjects
This cross-sectional study was carried out at the 4th Department of Internal Medicine of the General University Hospital in Prague from January 2012 to July 2015. The study protocol was approved by the institutional review board and the Ethics Committee of the General University Hospital in Prague. Informed consent was obtained from all participants.
The population under study consisted of 354 Caucasian subjects (188 females/166 males) divided into 6 groups according to the number of presented components of metabolic syndrome. All groups were age matched.
For the definition of metabolic syndrome components the criteria of the International Diabetes Federation [13] were used: central obesity (waist circumference ≥ 94 cm for men and ≥ 80 cm for women), raised TG level (≥ 1.7 mmol/l), reduced HDL-C (< 1.03 mmol/l in males and < 1.29 mmol/l in females), or specific treatment for these abnormalities, raised blood pressure (BP): systolic BP > 130 (16.25kPa) or diastolic BP ≥ 85 mmHg (10.625kPa), or treatment of previously diagnosed hypertension, raised fasting plasma glucose (≥ 5.6 mmol/l), or previously diagnosed type 2 of diabetes mellitus.
Exclusion criteria for all groups were the following: current antioxidant therapy kidney disease (creatinine > 150 µmol/l), clinically manifest proteinuria (urinary protein > 500 mg/l), and liver cirrhosis, malignancies, chronic immunosuppressive and anti-inflammatory therapy, as well as chemotherapy. Further criteria for exclusion were: contraception, acute and chronic pancreatitis; heart insufficiency (NYHAIII/IV), unstable angina pectoris, stage within 1 year after acute myocardial infarction, respectively coronaro-aorto bypass grafting, or percutaneous coronary intervention, and stroke.
Blood samples
Blood samples were collected after a 12 hour overnight fast, puncturing a peripheral vein. All samples were marked with unique anonymized identification numbers, merging data only after assays had been completed. All parameters were assessed in serum. Serum was prepared following coagulation in vacutainer tubes; by centrifugation at 3500 rpm at 4 °C for 10 min. Samples were stored at -80 °C until the assay.
Methods
The arylesterase activity of PON1 was measured according to the method of Eckerson et al. (1983) [14] using phenylacetate as a substrate. The rate of phenol generation was monitored at 270 nm. Blank was run for each sample. Arylesterase activity of PON1 was calculated using the molar extinction coefficient of the produced phenol, 1310 M-1cm-1 and expressed as U/ml serum (U = μmol/min), as described earlier [15].
The concentration of CD in precipitated LDL was determined by the modified spectrophotometric method of Wieland at 234 nm [16,17]. All routine clinical tests were measured in Institute for Clinical Biochemistry and Laboratory Diagnostics of General University Ho-spital in Prague.
The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as HOMA-IR = [fasting serum glucose (mmol/l) * fasting serum insulin (µU/ml)]/22.5 [18].
Statistical analysis
Data are expressed as mean and standard deviation or median (25th-75th percentile) for data different from normal distribution. Normality of the distribution was tested by the Shapiro-Wilks W test. Comparisons between the groups were carried out by one-way ANOVA with Newman-Keuls post test. Kruskal-Wallis ANOVA was used for non-parametric comparisons. Spearman correlation coefficients were used for correlation analyses. All analyses were performed using version 12.0 of StatSoft Statistica software (2013, CZ). The p value < 0.05 was considered statistically significant.
Results
Table 1 summarizes basic clinical and biochemical data of the studied groups. As shown in Table 1 with increasing number of presented components of me-tabolic syndrome, the increase in levels of BMI, waist circumference, triacylglycerols, glucose, insulin and HOMA-IR and decrease in HDL-C and ApoA1 levels were observed.
1. Basic characteristic of studied groups 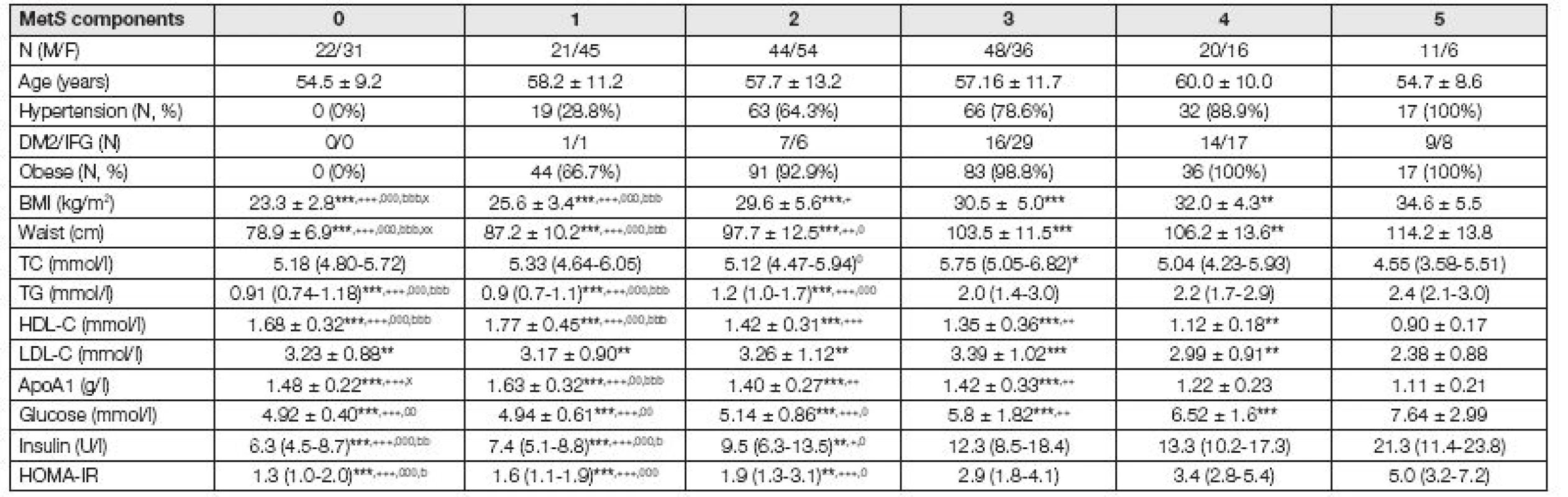
MetS: Metabolic syndrome, DM2: diabetes mellitus type 2, IFG: increased fasting glucose; BMI: body mass index, TC: total cholesterol, TG: triacylglycerols, HDL-C high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol; Data presented as mean ± standard deviation (S.D.) for parametric and median (IQR) for non-parametric variables; * 5 vs. 4,3,2,1,0 MetS-components; + 4 vs. 3,2,1,0 MetS-components; 0 3 vs. 2,1,0 MetS-components; b 2 vs. 1,0 MetS-components; x 1 vs. 0 MetS-components; * p < 0.05, ** p < 0.01, *** p < 0.001 The activity of PON1 was significantly decreased in subjects, who fulfill all 5 components of MetS in comparison with those, who fulfil 0 to 3 components of MetS. The concentrations of conjugated dienes in precipitated LDL were increased in subjects with 4 or 5 components of MetS compared to subjects with 0-3 components of MetS (Fig. 1).
Fig. 1. Activity of Paraoxonase 1 and concentration of Conjugated dienes in precipitated LDL 
MetS: Metabolic syndrome; CD: conjugated dienes, LDL-C: low density lipoprotein cholesterol; * 5 vs. 4, 3, 2, 1, 0 MetS-components; + 4 vs. 3, 2, 1, 0 MetS-components; * p < 0.05, ** p < 0.01, *** p < 0.001 We have also studied, if obesity or hypertension influences the levels of PON1 and CD/LDL; subjects with no component of MetS were compared with subjects with only one component (obesity or hypertension) of MetS. There was no difference between obese and non-obese subjects in activity of PON1 (163.4 ± 42.3 vs. 167.3 ± 39.2 U/ml; p = 0.63) or in concentration of CD/LDL (0.0177 ± 0.008 vs. 0.016 ± 0.004; p = 0.22). And also no difference in PON1 activity (167.2 ± 38.8 vs. 169.2 ± 40.0 U/ml; p = 0.85) or CD/LDL levels (0.0155 ± 0.0045 vs. 0.0162 ± 0.0045; p = 0.55) between subjects with and without hypertension was observed.
Strong correlation between PON1 activity and concentrations of HDL-C (r = 0.331; p < 0.001), ApoA1 (r = 0.334; p < 0.001) and TC (r = 0.324; p < 0.001) was observed. The levels of conjugated dienes correlated with concentrations of TC (r = -0.388; p < 0.001) TG (r = 0.304; p < 0.001) and HDL-C (r = -0.303; p < 0.001).
Studied subjects were also divided into 3 groups according to the levels of HDL-C and ApoA1. As shown in Fig. 2, the activity of PON1 was the lowest in subjects with depressed levels of both HDL-C and ApoA1 (H+A) and the highest in subjects with normal levels of both HDL-C and ApoA1 (N).
Fig. 2. Activity of Paraoxonase 1 according to the concentration of HDL-C and ApoA1 
A+H: subjects with decreased levels of both HLC-C and ApoA1, (n = 21); A/H: subjects with decreased levels of only HLC-C or ApoA1, (n = 44); N: subjects with normal levels of both HDL-C and ApoA1 (n = 289); * N vs. A+H or A/H, * p < 0.05, *** p < 0.001 Discussion and Conclusion
The arylesterase activity of PON1 and levels of CD/LDL were measured in subjects with different numbers of presented components of MetS. Decreased acti-vity of PON1 was found in subjects with 5 presented components of MetS compared to subjects with fewer components of MetS. The levels of CD/LDL were increased in subjects with 5 and 4 presented components of MetS in comparison with subjects with fewer components of MetS.
In our previous study we have investigated the activities of PON1 in subjects with metabolic syndrome compared with healthy controls [19] and found decreased PON1 activity and increased concentration of conjugated dienes in subjects with MetS compared to healthy controls. Also in other studies [20-23] decreased PON1 activity in MetS patients was observed. However in studies of Tabur et al. (2010) [24], Yilmaz et al. (2010) [25] and Lagos et al. (2009) [26] equivalent levels of PON1 in MetS patients and in CON were found. As was shown in this study PON1 activity is depressed mainly in subjects who fulfill all five criteria of MetS, in subjects with severe form of MetS. These findings could help to explain the inconsistent results in PON1 activity in MetS subjects.
Strong positive correlation between PON1 activity and HDL and ApoA1 concentrations were found in our studied subjects. When the subjects were divided into 3 groups according to the levels of HDL-C and ApoA1, it was shown, that subjects with decreased levels of both HDL-C a ApoA1 have the lowest PON1 activity, whereas subjects with normal levels of both HDL-C and ApoA1 the highest PON1 activity among the stu-died subjects. It could be hypothesize, that changes in composition of HDL influence the activity and function of PON1.
Several mechanisms are supposed to decrease PON1 activity, not only the changes in HDL composition. It was shown, that increased oxidative stress connected with elevated levels of oxidized LDL cause inactivation of PON1. Oxidized LDL appears to inactivate PON1 through interactions between the enzyme’s free sulfhydryl group and oxidized lipids, which are formed during LDL oxidation [26]. Also in our study levels of markers of lipid peroxidation (CD/LDL) were increased in subjects with decreased PON1 activity.
Other reason for the decrease in PON1 activity could be the glycation of the enzyme, which takes place as was shown in diabetes mellitus [27]. The acute phase response could also lead to the decreased activities of PON1 which are caused by the down-regulation of liver PON1 mRNA [28].
This study has shown that the levels of PON1 are extensively affected by the concentration of HDL-C and ApoA1. PON1 activity is depressed and CD/LDL levels are increased mainly in subjects who fulfill all five criteria of MetS.
Acknowledgement: This study and preparation of this manuscript was supported by the research project NT13199 of Ministry of Health of the Czech Republic
Do redakce došlo: 1. 2. 2016
Adresa pro korespondenci
RNDr. Lucie Vávrová, Ph.D.
Laboratoř pro výzkum aterosklerózy
1.LF UK Praha
Na Bojišti 3
120 00 Praha 2
e-mail: vavrova3@seznam.cz
Sources
1. Flekač, M., Škrha, J., Novotný, Z. Faktory ovlivňující aktivitu a koncentraci antioxidačního enzymu paraoxonáza 1. Klin. Biochem. Metab., 2006, 14(35), p. 33-39.
2. Sorenson, R. C., Bisgaier, C. L., Aviram, M., Hsu, C., Billecke, S., La Du, B. N. Human serum paraoxonase/arylesterase’s retained hydrophobic N-terminal leader sequence associates with HDLs by binding phospholipids: apolipoprotein A-I stabilizes activity. Arterioscler. Thromb. Vasc. Biol., 1999, 19, p. 2214-25.
3. Davies, H. G., Richter, R. J., Keifer, M., Broomfield, C. A., Sowalla, J. and Furlong, C. E. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet., 1996, 14, p. 334–336.
4. Aviram, M., Billecke, S., Sorenson, R. et al. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler. Thromb. Vasc. Biol., 1998a, 18(10), p. 1617-24.
5. Billecke, S., Draganov, D., Counsell, R. et al. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos., 2000, 28, p. 1335–1342.
6. Rajkovic, M.G., Rumora, L., Barisic, K. The parao-xonase 1, 2 and 3 in humans. Biochem. Med. (Zagreb), 2011, 21(2), p. 122-30.
7. Jakubowski, H. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J. Biol. Chem., 2000, 275, p. 3957–3962.
8. Mackness, M. I., Arrol, S., Durrington, P. N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett., 1991, 286, p. 152–154.
9. Mackness, M. I., Arrol, S., Abbot, C., Durrington, P. N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis, 1993, 104, p. 129–135.
10. Aviram, M., Rosenblat, M., Bisgaier, C. L., Newton, R. S., Primo-Parmo, S. L., La Du, B. N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for parao-xonase. J. Clin. Invest., 1998b, 101, p. 1581–90.
11. Précourt, L. P., Amre, D., Denis, M. C. et al. The three-gene paraoxonase family: physiologic roles, acti-ons and regulation. Atherosclerosis, 2011, 214(1), p. 20-36.
12. Güzel, S., Seven, A., Satman. I, Burçak, G. Comparison of oxidative stress indicators in plasma of recent-onset and long-term type 1 diabetic patients. J. Toxicol. Environ. Health A, 2000, 59, p. 7-14.
13. Alberti, K.G., Zimmet, P., Shaw, J. The metabolic syndrome-a new worldwide definition. Lancet, 2005, 366, p. 1059-1062.
14. Eckerson, H. W., Wyte, C. M., La Du, B. N. The human serum paraoxonase/arylesterase polymorphism. Am. J. Hum. Genet., 1983, 35, p. 1126-1138.
15. Kodydková, J., Vávrová, L., Zeman, M. et al. Antioxidative enzymes and increased oxidative stress in depressive women. Clin. Biochem., 2009, 42, p. 1368-74.
16. Ahotupa, M., Ruutu, M., Mantyla, E. Simple methods of quantifying oxidation products and antioxidant potential of low density lipoproteins. Clin. Biochem.,1996, 29, p. 139-144.
17. Wieland. H., Seidel, D. A. Simple specific method for precipitation of low density lipoproteins. J. Lipid Res., 1983, 24, p. 904-909.
18. Vogeser, M., König, D., Frey, I., Predel, H. G., Parhofer, K. G., Berg, A. Fasting serum insulin and the homeostasis model of insulin resistance (HOMA-IR) in the monitoring of lifestyle interventions in obese persons. Clin. Biochem., 2007, 40, p. 964-8.
19. Vavrova, L., Kodydkova, J., Zeman, M. et al. Altered Activities of Antioxidant Enzymes in Patients with Metabolic Syndrome. Obesity Facts, 2013, 6(1), p. 39-47.
20. Hashemi, M., Kordi-Tamandani, D. M., Sharifi, N. et al. Serum paraoxonase and arylesterase activities in metabolic syndrome in Zahedan, southeast Iran. Eur. J. Endocrinol., 2011 164(2), p. 219-22.
21. Kappelle, P. J., Bijzet, J., Hazenberg, B. P., Dullaart, R. P. Lower serum paraoxonase-1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch. Med. Res., 2011, 42(3), p. 219-25.
22. Martinelli, N., Micaglio, R., Consoli, L. et al. Low le-vels of serum paraoxonase activities are characteristic of metabolic syndrome and may influence the metabolic-syndrome-related risk of coronary artery disease. Exp. Diabetes Res., 2012, Dostupný z www:
23. Tabur, S., Torun, A. N., Sabuncu, T., Turan, M. N., Celik, H., Ocak, A. R., Aksoy, N. Non-diabetic metabolic syndrome and obesity do not affect serum parao-xonase and arylesterase activities but do affect oxidative stress and inflammation. Eur. J. Endocrinol., 2010, 162, p. 535–541.
24. Yilmaz, H., Sayar, N., Yilmaz, M. et al. Serum paraoxonase 1 activity in women with metabolic syndrome. Kardiol. Pol., 2010, 68(11), p. 1219-24.
25. Lagos, K. G., Filippatos, T. D., Tsimihodimos, V. et al. Alterations in the high density lipoprotein phenotype and HDL-associated enzymes in subjects with metabolic syndrome. Lipids, 2009, 44(1), p. 9-16.
26. Aviram, M., Rosenblat, M., Billecke, S. et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic. Biol. Med., 1999, 26, p. 892–904.
27. Hedrick, C. C., Thorpe, S. R., Fu, M. X. et al. Glycation impairs high-density lipoprotein function. Diabetologia 2000, 43 : 312–320.
28. Deakin, S. P., James, R. W. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin. Sci. (Lond.), 2004, 107(5), p. 435-47.
Labels
Clinical biochemistry Nuclear medicine Nutritive therapist
Article was published inClinical Biochemistry and Metabolism

2016 Issue 2-
All articles in this issue
- Čipy a kapilární elektroforéza v imunoanalýze
- Ověření použitelnosti poměru expresí genů NPHS2 a SYNPO při diagnostice fokální segmentální glomerulosklerózy a minimálních změn glomerulů
- Aktivita fosfomanomutázy 2 u pacientů s podezřením na dědičnou poruchu glykosylace
- Kvalita, kontrola a validace glukometrů a CGM systémů. Přehled stavu.
- Postanalytická fáze a interpretace laboratorního testu (post-postanalytická fáze)
- Changes in Paraoxonase 1 activity and concentration of conjugated dienes in connection with number of metabolic syndrome components
- Clinical Biochemistry and Metabolism
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Kvalita, kontrola a validace glukometrů a CGM systémů. Přehled stavu.
- Postanalytická fáze a interpretace laboratorního testu (post-postanalytická fáze)
- Aktivita fosfomanomutázy 2 u pacientů s podezřením na dědičnou poruchu glykosylace
- Ověření použitelnosti poměru expresí genů NPHS2 a SYNPO při diagnostice fokální segmentální glomerulosklerózy a minimálních změn glomerulů
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

