-
Medical journals
- Career
INDICATION AND IMPORTANCE OF RECONSTRUCTIVE SURGERIES OF FACIAL SKELETON IN MAXILLOFACIAL SURGERY: REVIEW
Authors: R. Pink 1,4; Z. Dvořák 1,2,3; P. Heinz 1,4; P. Michl 1,4; P. Tvrdý 1,4
Authors‘ workplace: University Hospital Olomouc, Department of Oral and Maxillofacial Surgery, Olomouc, Czech Republic 1; St. Anne’s University Hospital, Department of Plastic and Aesthetic Surgery, Brno, Czech Republic 2; Masaryk University, Faculty of Medicine, Brno, Czech Republic 3; Palacký University Olomouc, Faculty of Medicine and Dentistry, Olomouc, Czech Republic 4
Published in: ACTA CHIRURGIAE PLASTICAE, 62, 1-2, 2020, pp. 29-39
INTRODUCTION
Carcinomas of the oral cavity and the oropharynx belong among 10 malignancies most frequently occurring in the human population. There are approximately 500,000 newly diagnosed cases worldwide every year. However, only approximately one third of the patients survives 5 years after the diagnosis. The proportional representation of these tumors in the total number of malignancies ranges around approximately 2% in the Czech Republic (3% in the USA, 35–40% in the countries of southeast Asia).1
Currently, the incidence of oropharyngeal malignancies in younger generation increases (frequently occurring carcinomas in the third or fourth decade of life) as well as the malignancy percentage in women and the number of distant metastases from extraoral primary tumors.2
In 90% of cases, these represent malignant epithelium tumors, most often spinocellular (epidermoidal) carcinomas. The remaining 10% consist of adenoid cystic carcinomas, mucoepidermoid and other salivary gland adenocarcinomas, malignant lymphomas, rarely sarcomas. On the skin of the face, we most often find basocellular and spinocellular carcinomas, melanomas, and as an exception, some other rare malignancies, such as the Merkel cell carcinoma. On the other hand, malignant mesenchymal tumors (except for haematological malignancies) are much rarer in the oropharyngeal region.3
The prognosis of malignant tumors of the oral cavity and oropharynx is determined primarily by the degree of invasiveness of the primary tumor and the extent of the metastatic involvement of regional and distant nodes. It is significantly affected by a timely determination of the diagnosis, which is in turn strongly affected by clinical experience and oncological awarness of both physicians and layman population.4 In the postoperative period of time, especially in case of more voluminous tumors, the prognosis is affected by their complete removal, which is easier to achieve if the resection is not limited by the possibilities of the subsequent reconstruction.5
At present times, free flap techniques (radial forearm flap, latissimus dorsi flap, anterolateral thigh flap, free fibula flap, rectus abdominis muscle flap) are used primarily in reconstructive oncosurgery of the head and neck. Reconstruction of bones and large defects of the orofacial region is the main advantage of these osteomyocutaneous and myocutaneous flaps. A certain limitation of these flaps lies in the performance of vascular anastomosis, which can be difficult in these oncological patients because of frequent sclerosis of the vessels caused by smoking and poor regime. In older, polymorbid patients, we use pedicled flap techniques (pectoralis major flap, submental flap, supraclavicular flap, facial artery musculomucosal flap – FAMM), in which the procedure is shortened by omitting the microsurgical vascular anastomosis, and therefore, the total operational stress on the patient is decreased. In addition, the texture and color of the skin are similar to facial skin in case of these regional flaps located above the level of clavicula, which contributes to an aesthetically pleasing result. Osseous and voluminous cranially located defects represent the limitations of the use of pedicled flaps because of the limited rotational arc and the insufficient volume of the necessary tissue.
EVALUATION OF PROBLEMS
Principles of surgical treatment and tissue defect reconstruction
In head and neck oncology, apart from prolongation of life, more and more emphasis is being put on the quality of life of the patient while keeping in mind the preservation of functionality of the orofacial system (mastication, swallowing, breathing) and an acceptable aesthetic result without a significant negative impact upon the socioeconomic status of the patient. Interdisciplinary cooperation between a maxillofacial surgeon, otolaryngologist, reconstructive surgeon, oncologist, speech therapist and a psychologist is important to achieve the best possible result. In surgical procedures of this difficulty, the importance of paramedical personnel in presurgical and especially postsurgical care cannot be forgotten. Before the surgical procedure, several criteria have to be considered and the best possible relation and balance between them has to be found. Complete removal of the tumor is an absolute priority and it must not be affected by the complexity of the defect reconstruction. Therefore, two viewpoints are important in the reconstructive surgery: functionality and aesthetics. From the viewpoint of function conservation, we evaluate the ability to articulate, masticate and swallow. The best aesthetic result is achieved by a defect reconstruction using the surrounding tissues with similar color, thickness and skin texture.
The condition, volume and mobility of the surrounding soft tissues and bones represent the critical factors for the decision on the type of defect closure (direct suture vs. flap) after primary tumor removal.
The reconstruction of both maxilla and mandibula does not depend only on the volume of the resected tissue, but also on its location. Apart from localization of the defect, the mobility of the surrounding soft tissues, which differs in individual anatomic locations, is also important. Removal of important structures (nerves, blood vessels and cartilage) can also affect the functionality and quality of life of the patient.
In small, well-localized tumors, we usually perform a primary closure of the defect using a direct suture due to the great mobility of the surrounding soft tissues. In larger tumors, direct closure of the defect using a suture is not possible, and that is why we have to perform the reconstruction using local or regional or free flap techniques.
Local flaps
Local flaps with random vascularization
We use local random pattern flaps to close defects, which could not be closed using a direct suture and which are surrounded by a sufficient amount of mobile tissue. The defects are differentiated to mucosal defects and skin defects in accordance with their location. Tongue flap, pharyngeal flap and palatal flap are being used in reconstructive surgeries to cover mucosal defects. 6,7,8,9,10,11,12 The first two mentioned can be used in reconstruction of defects of the oropharynx and pharynx, but their disadvantages lie in the fixation of the tongue or narrowing of the pharynx, and that is why they are considered as flaps of second choice. On the other hand, palatal flaps, which can be used without any unacceptable impairment of the swallowing and breathing functions, are still used to close smaller defects at present. Well-perfused orofacial system offers a wide spectrum of local skin flaps, which are used primarily in smaller skin defects, often with a great visual and functional result. In principle, these are advancement, rotational or transposition flaps. The most commonly used local flaps in the head and neck region are nasolabial flap (superficial random pattern flap), glabellar flap, rhomboid flap and bilobed flap. Nasolabial flap can also be used as an axial flap, which is nourished through the angular or lateral nasal branch of the facial artery, and as such, it can be used to cover intraoral defects (Figure 1–4).13,14,15,16 The principle of the rhomboid flap described by Limberg 17 lies in the removal of the lesion of the aforementioned shape and in an elevation of the rhombic flap in the adjacent pliable area with its subsequent transposition. Bilobed flap (first described by Esser) can be used not only for surface defect reconstruction, but also for facial defects communicating into the oral cavity.18
1. Condition after a radical excision of a malignant lesion on an ala nasi (author’s archive) 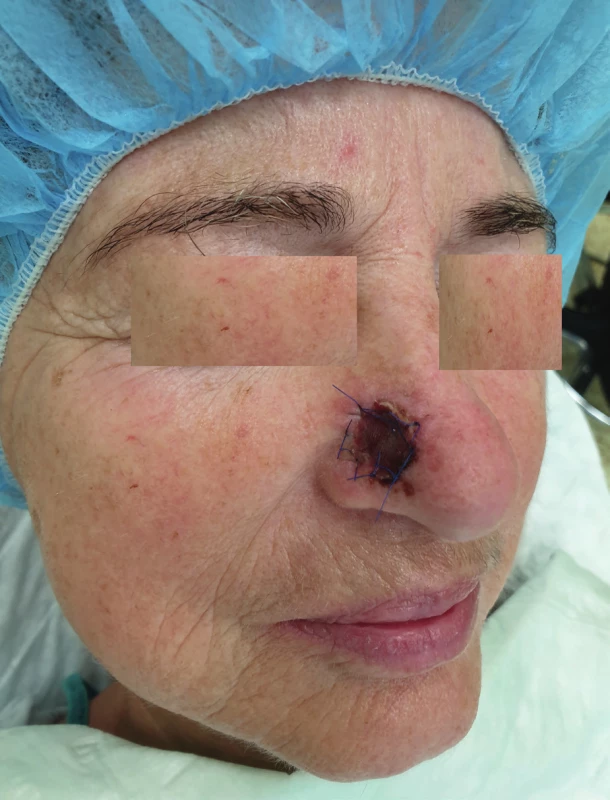
2. Elevation of the nasolabial flap (author’s archive) 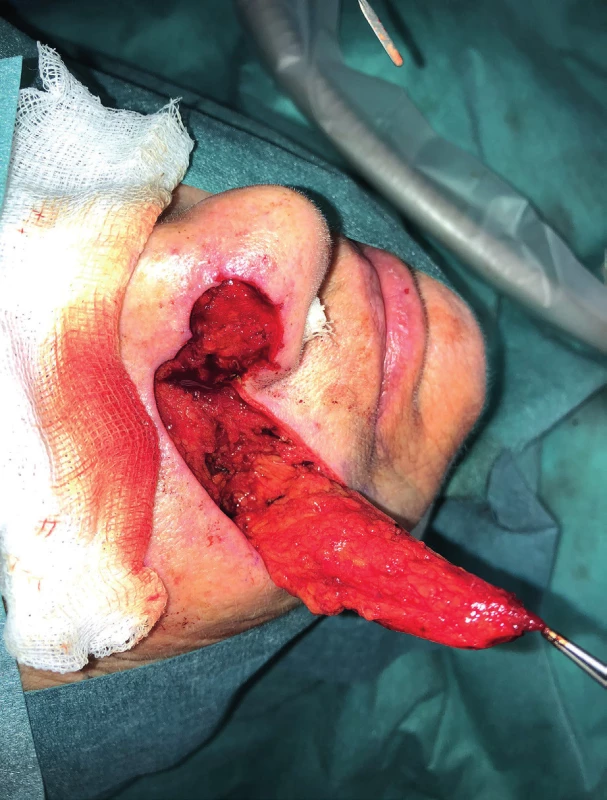
3. Condition after insertion of the nasolabial flap into the defect (author’s archive) 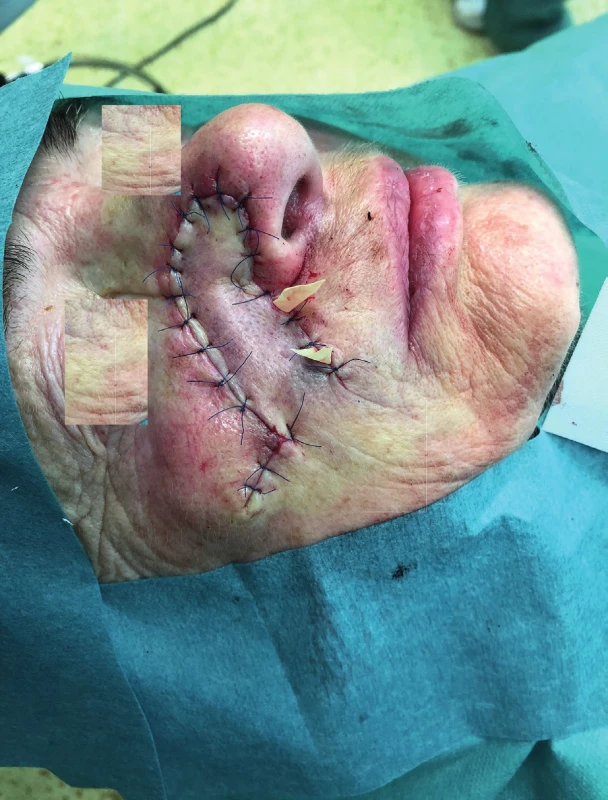
4. Condition 1 week after healing of the nasolabial flap (author’s archive) 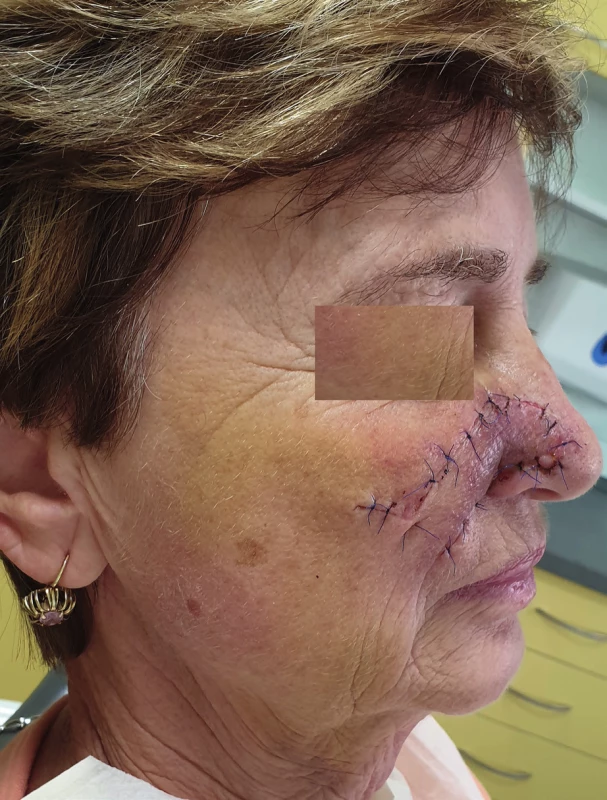
Local flaps with defined vascularization
Regional pedicled (axial) flaps have been used since 1970s. Their principle lies in the elevation of tissue with a nutritional vascular pedicle in the extent of the dermatosome. Therefore, their vascularization is clearly defined. From regional flap techniques, the pectoralis major, submental, supraclavicular and temporalis flaps are used most commonly for the reconstruction of defects in the oropharyngeal region. Forehead flap, trapezoidal flap and m. latissimus dorsi pedicled flap19–31 are used less often. Facial artery musculomucosal flap (FAMM) has to be mentioned within this group of flaps as well.32,33 Lower technical difficulty and the related shortening of the surgery time is one of the advantages of pedicled flaps, which do not require microsurgical anastomosis. These are mostly indicated in older and high-risk patients. Possible limitations include smaller radius and extent of flaps, possible inferior perfusion in comparison to free flaps and practical usability only in soft tissues reconstruction. Admittedly, the pedicled pectoralis flap can be elevated with a rib and the pedicled musculus latissimus dorsi flap can be elevated with the scapula or the rib, but from the implantological point of view, it represents a bone material of insufficient quality, which is acquired for the price of significant morbidity and complicated healing of the donor site. They may seem less aesthetically acceptable due to the larger number of post-surgery scars, but on the other hand, if these are flaps above the level of clavicles, then they usually retain more natural color and texture for the head and neck region and they are less conspicuous than free flaps. Especially the submental (Figure 5–7) and supraclavicular flaps are thin, pliable and easy to dissect flaps with good cosmetic and functional results. Their advantage also lies in the minimal morbidity of the donor site. Some authors already consider the supraclavicular flap to be the gold standard for reconstructions of head and neck soft tissue defects34.
5. Elevation of the submental flap (author’s archive) 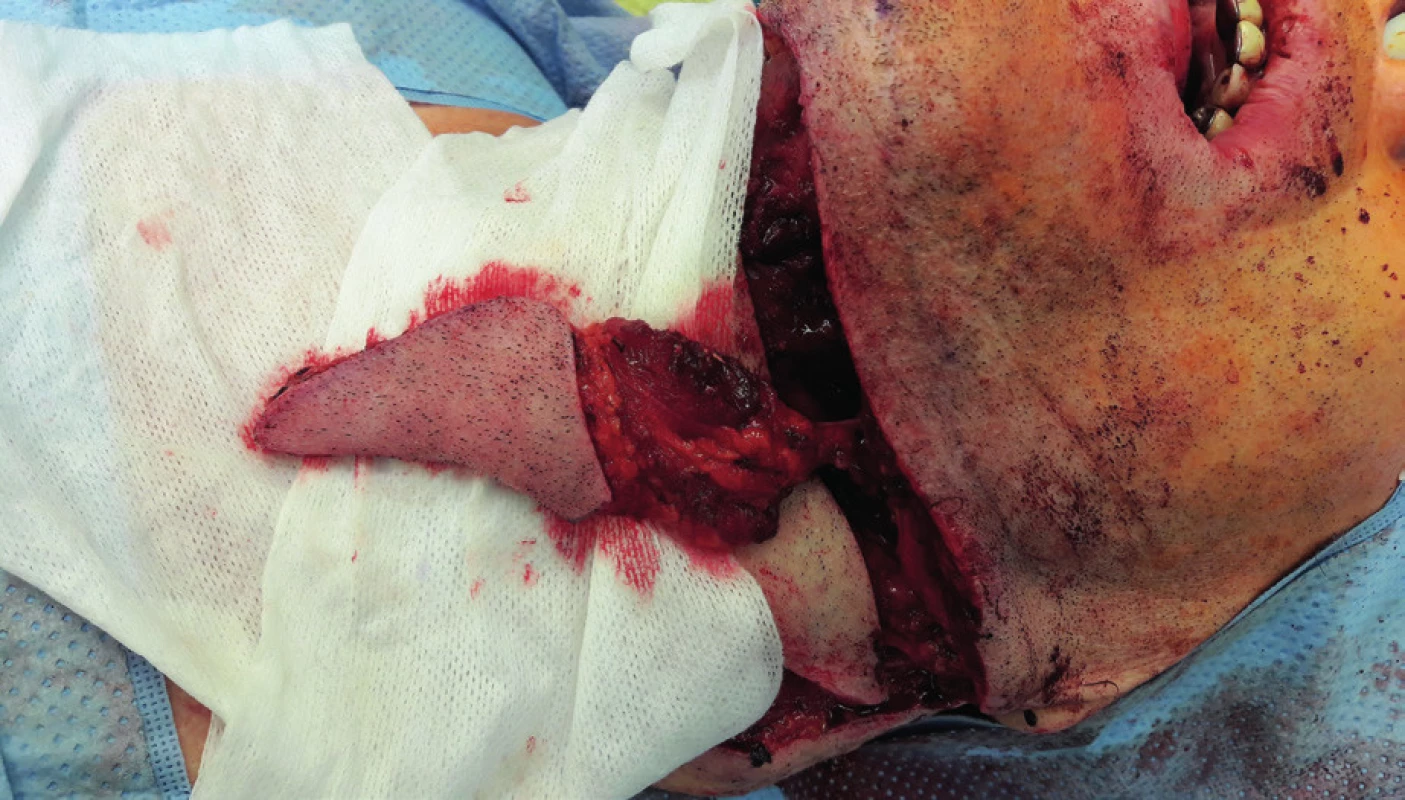
6. Condition afterinsertion of the submental flap into the defect of the tongue (author’s archive) 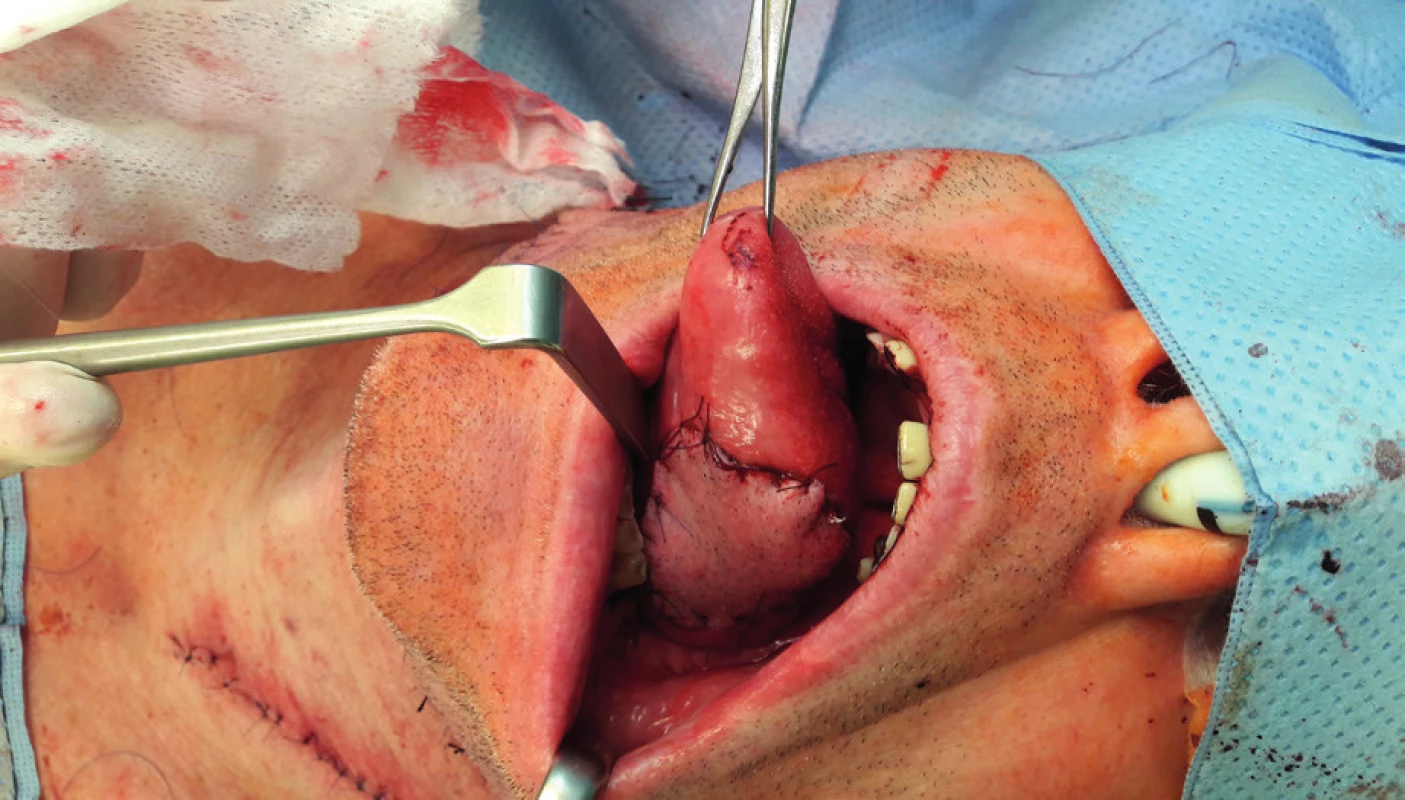
7. View of the submental flap donor site (author’s archive) 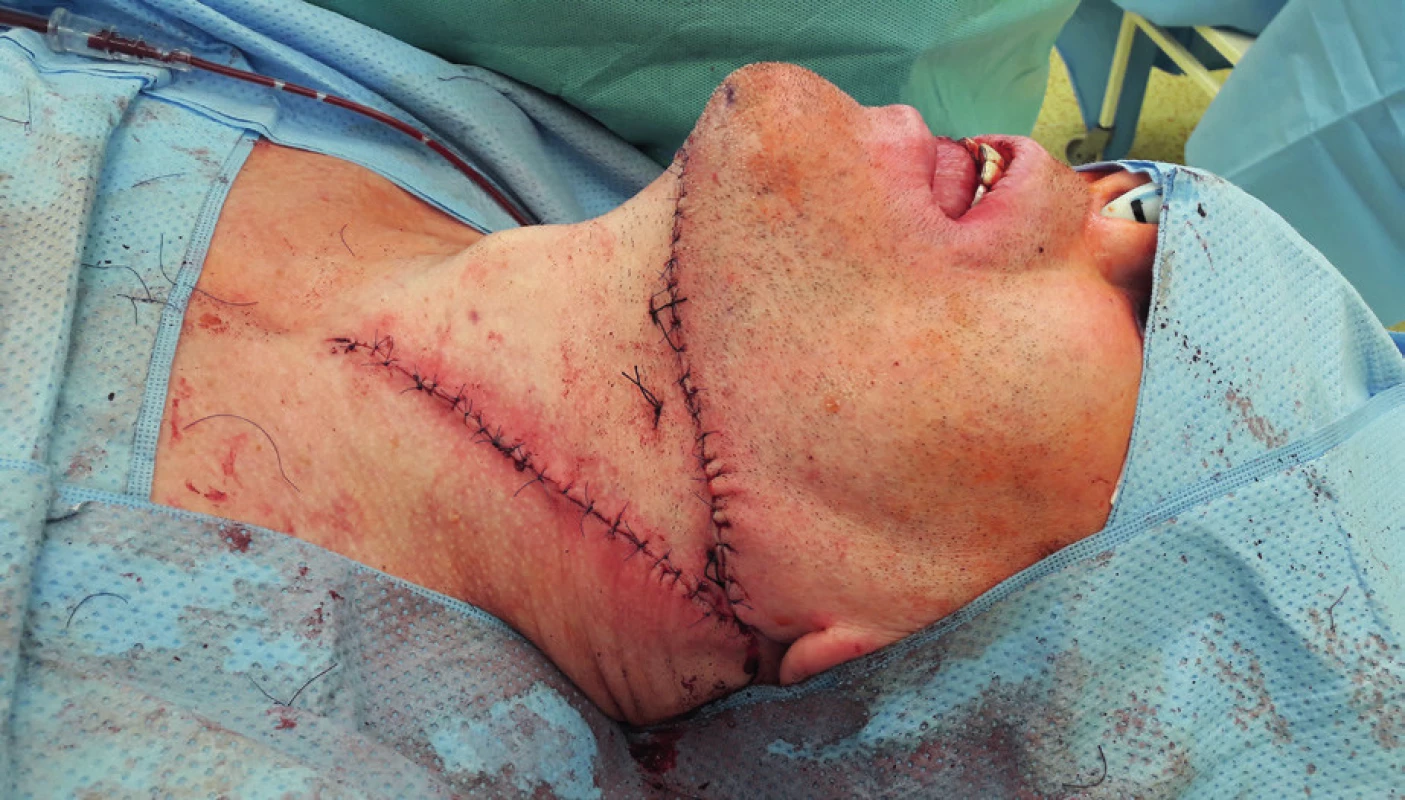
Distant flaps with defined vascularization – free flaps
In the past 50 years, free flap techniques have created a new dimension in the reconstructive surgery. The free microvascular flap technique was used for the first time by Seidenberg in 1959, at which time he used a vascularized jejunum flap to reconstruct a pharyngeal defect.35 The first transfer of a vascularized fibula flap is dated into 1973, when Ueba and Fujikawa used this flap on an 11-year-old boy to reconstruct an ulnar defect after a removal of a neurofibroma.36 Two years later, Taylor used the same flap to reconstruct a tibial defect.37
As regards the orofacial region, vascularized fibula flap was used for the first time by Hidago in 1989 to repair a mandibular defect38, and five years later, Nakayama used it to reconstruct a maxilla.39 The year 1981 was an important milestone, because Shaw reconstructed a nasal defect40 using a forearm flap in this year. Later, in 1980s, an intense development of microvascular reconstructive surgery in the maxillofacial region has begun.
Almost any defect can be reconstructed using these techniques, but a certain professional restraint and humility should be maintained. Careful dissection of the vascular pedicle and a responsibly performed microanastomosis is a prerequisite of a successful healing of these flaps. The main complication of these flaps is a failure of satisfactory blood supply. Funds to pay for the surgical microscope, instrument kit, training and leasing curve of the microsurgeon represent other difficulties. That is why these procedures should be performed by a surgeon knowledgeable in this field, who performs these procedures often, and if possible, regularly. In accordance with the tissue content, free flaps can be divided to cutaneous, myocutaneous and osteocutaneous or osteomyocutaneous. In the oropharyngeal region, the most commonly used are radial forearm flap41, anterolateral thigh flap42, musculus latissimus dorsi flap43, musculus rectus abdominis flap44, vascularized fibula flap38, vascularized hip bone45 and scapular skin flap.46
In the pre-operative planning, it is important to plan the actual harvest of the free flap very well and to consider the morbidity of the donor site, because it should correspond with the tissue, which is being reconstructed as much as possible in terms of shape and aesthetics. That is why a good communication between the surgeon performing the destructive phase and the reconstructive surgeon is a must. In cases where we cannot achieve satisfactory functional and aesthetic result using local and regional flap techniques, free flaps represent the first choice in head and neck reconstruction. In maxillofacial oncosurgery, these most often concern reconstructions of jawbones, full thickness facial defects and defects in other parts of the oral cavity. The procedural benefit and health risks of the patient have to be evaluated before the procedure. We especially focus on comorbidities such as diabetes mellitus, hypertension, cardiovascular diseases and last but not least the blood vessel permeability. That is why the greatest attention is paid to senior patients suffering from atherosclerosis, in whom we consider mandibular reconstruction with a vascularized bone.
Mandibular reconstruction
In order to achieve a satisfactory mandibular reconstruction, we have to take several viewpoints into consideration – functional, anatomical and aesthetic. From the functional viewpoint, satisfactory mastication and phonation should be guaranteed, the swallowing reflex should be preserved as well as the permeability of the upper respiratory tract. From the anatomical viewpoint, we evaluate restoration of the occlusal plane and the correct position of the TMJ (temporomandibular joint) head on an OPG (orthopantomogram) image. From the aesthetic viewpoint, the important factors include facial symmetry, chin prominence, height of the lower third of the face and frequency and visibility of the scars.38
The following circumstances have to be taken into consideration before the mandibular reconstruction:
– overall state of health of the patient (cardiovascular diseases, ischemic heart disease, diabetes mellitus, toxonutritive hepatopathy, vessel permeability) and their capability to undergo a long surgical procedure)
– age of the patient (the calendar age of the patient does not have to correspond to the biological age)
– whether this is a primary or a secondary surgical procedure (irradiated terrain, blood vessel wall quality and the number of blood vessels usable for microanastomosis)
– location of the defect on the mandibula (lateral vs. anterior)
– volume and biological character of the tumor
– donor site morbidity after flap harvest
– the wishes of the patient and their standpoint and motivation, willingness to cooperate
– alcohol addiction, which represents a strong limiting factor in accordance with our experiences
In case of a positive decision, we approach the reconstruction type selection. At the moment, we have several modalities at our disposal (Table 1):
1. Mandibular reconstruction options 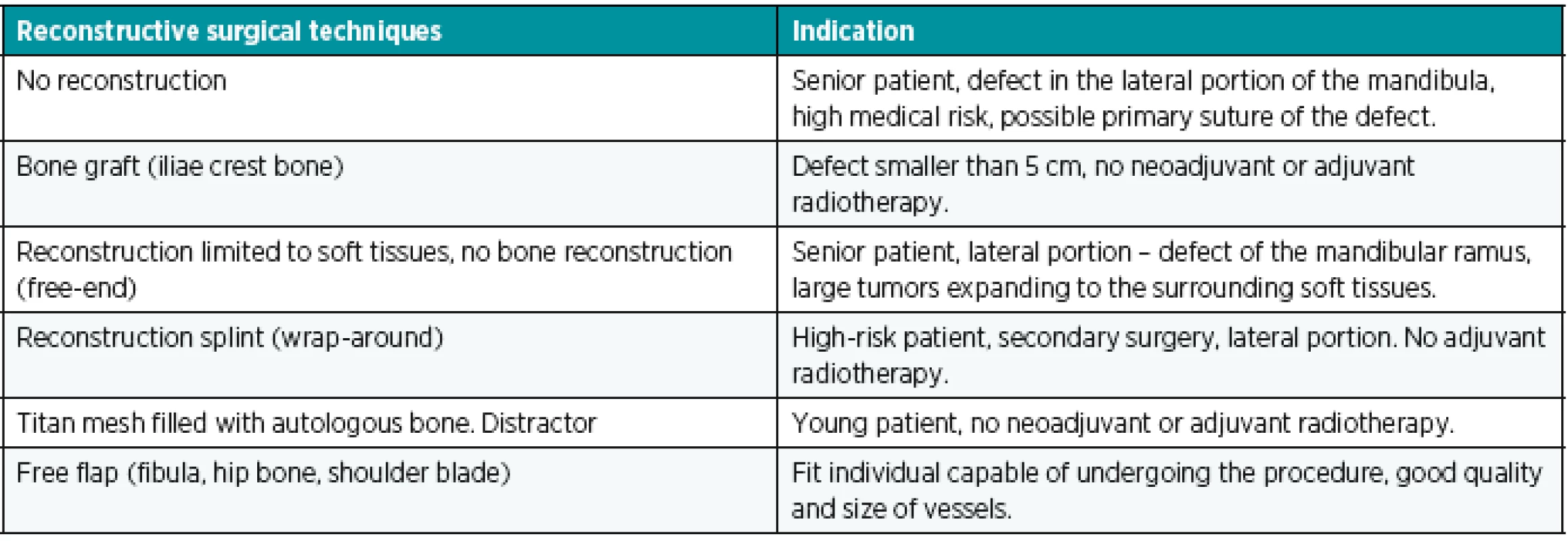
If the overall state of health of the patient allows, free flap is currently the first-choice method for reconstruction of extensive defects. Alternatively, we can reconstruct the mandibula using a splint and a muscle or a musculocutaneous flap, which covers the splint (wrap-around technique).47 In senior patients suffering from smaller tumors located in the lateral portion of the mandibula, the defect can be closed using a primary suture and the mandibula can be kept without any reconstruction. In larger defects, without any option of primary suture, we only reconstruct soft tissues using a musculocutaneous flap (free or regional) and we keep the resected bone stubs free (free-end). On the contrary, in young patients without the security of radiotherapy, in which we resect the mandibula up to 5 cm, we can use a bone graft harvested from the hip bone for the reconstruction.48 Other options include mandibula distraction49 and mandibula reconstruction with a titanium mesh of size and shape corresponding to the mandibula, filled with milled autologous bone.
We have to realize that even after a free fibula flap is properly healed into the defect, it is often followed by radiotherapy, which can cause its post-radiation osteonecrosis. That is why an interval of at least 5 weeks after the surgery has to be kept and the reconstruction splint has to be shielded as much as possible during the radiotherapy.50
Free flaps most often used for mandibula reconstruction
Vascularized fibula flap
At present, free fibula flap is used most commonly for mandibula reconstruction, because it provides a bone of good quality and the right length (maximum harvest of 24 cm) (Figure 8, 9, 10).51, 52 The morbidity of the donor location is acceptable after the defect is healed, the patient is able to walk fast and ride a bicycle53. The skin island flap is segmentally nourished by minor perforators, which lead from arteria peronaea towards the skin, which allows us to perform several osteotomies with subsequent shaping of the fibula into the desired shape and a harvest of bone with a skin island in order to cover soft tissue defects54. The flap harvest is a rather demanding procedure for the patient and it requires good technical hinterland and excellent knowledge and skill on the part of the surgeon. The actual shaping of the flap is performed either “free-hand” or using a previously engineered 3D model.55 We affix this model to the fibula and we perform several osteotomies under the selected angle (ideally using a piezo-surgical device to protect the perforators) and we “fold” the individual fragments of fibula into the desired shape of the mandibula even before detaching the flap from the pedicle. It is a very good technique, which decreases the period of ischaemia, blood loss and the blood vessel damage risk, which the patients face in case of shaping of the vascularized fibula by “free hand”. The length and quality of the fibula allows replacement of virtually the entire mandibula, which no other free flap allows. The option to operate in two teams with a significant shortening of the surgery time is another advantage of this procedure.
8. Elevated fibula flap after modelling along the shape of the reconstructive splint, hanging perfused on a lower limb (author’s archive) 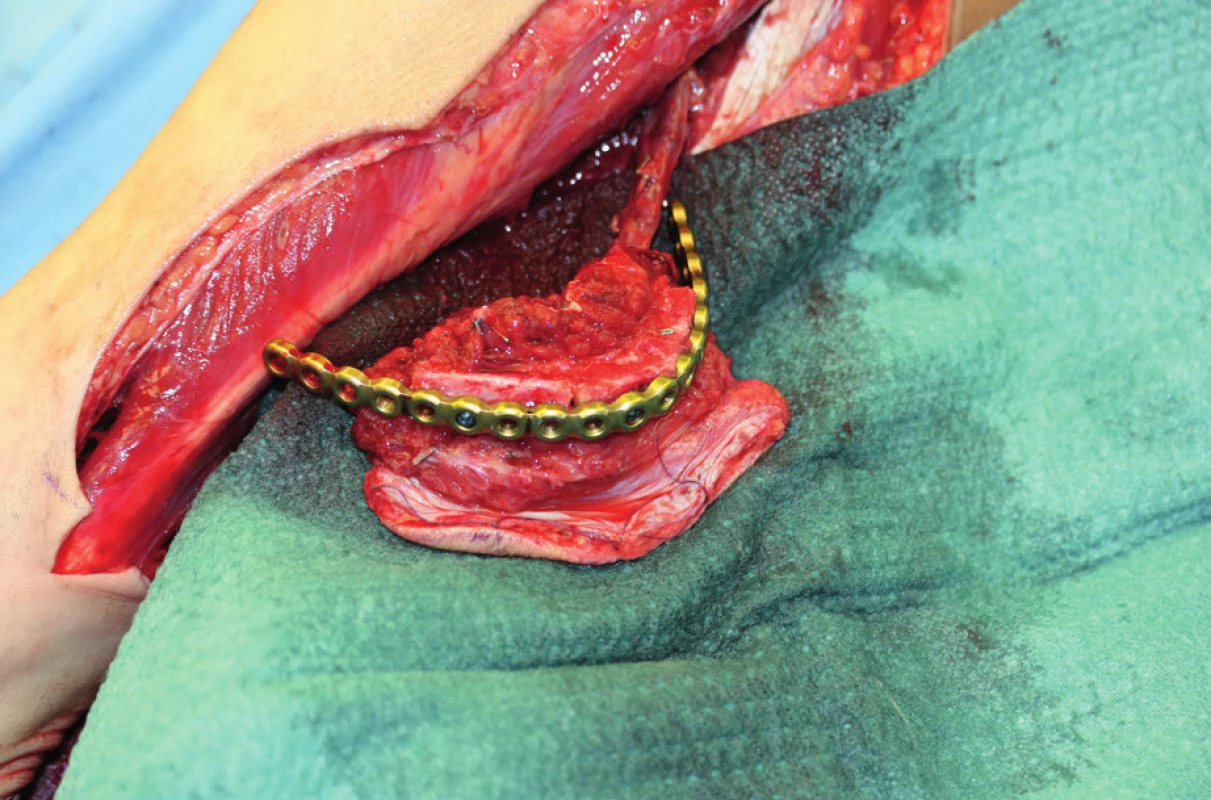
9. Defect after resection of the mandibula and the base of the oral cavity (author’s archive) 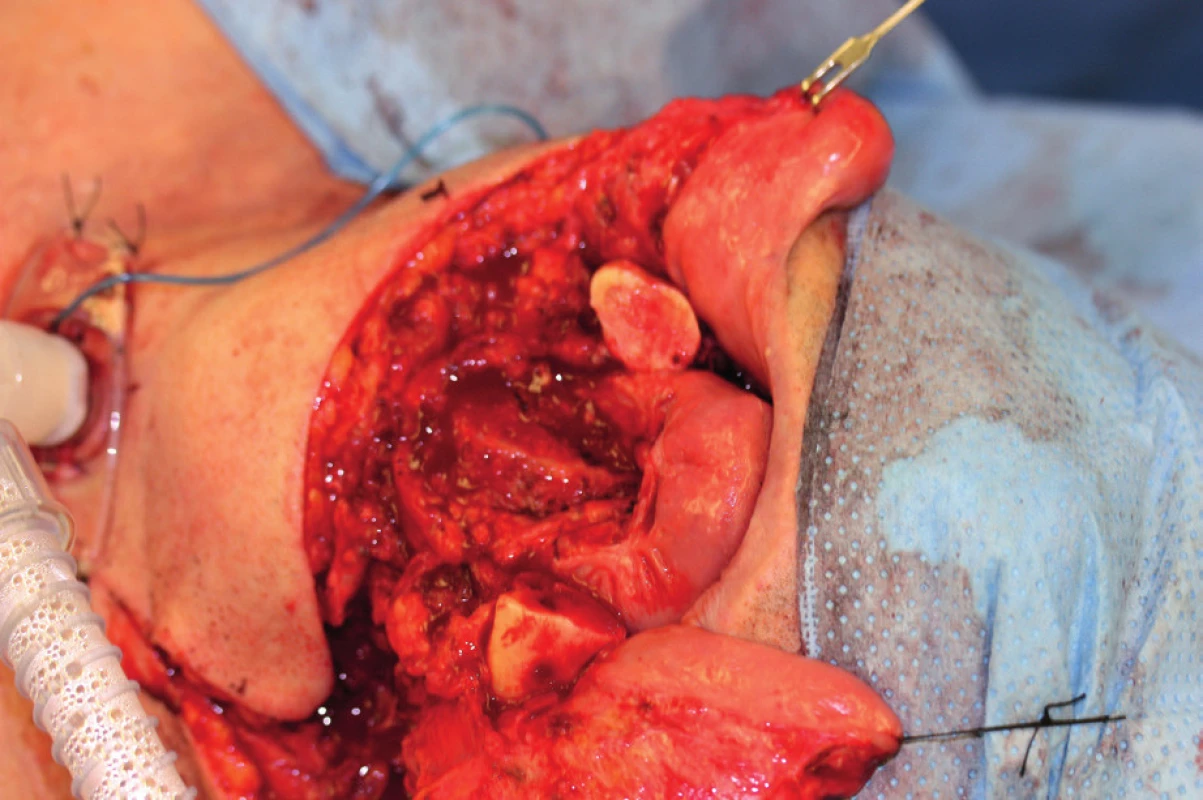
10. View of the oral cavity after healing of the fibula flap with a skin island (author’s archive) 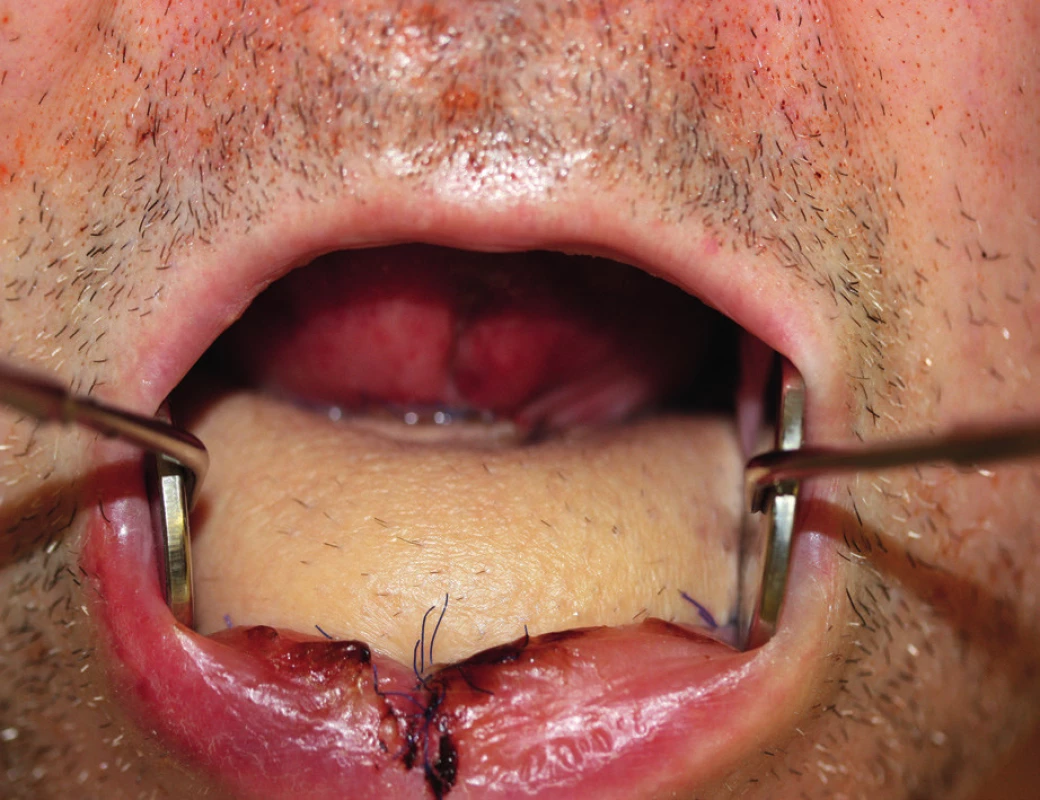
Vascularized iliac crest bone
Mandibula replacement with a hip bone graft is limited by the small size of the harvested bone, relatively short vascular pedicle and the larger ratio of spongiosa over corticalis. In addition, the morbidity of the donor site is larger in comparison to the fibula and patients often suffer from post-operative hernia, severe pain and haematoma formation. Vascularization is not segmental and soft tissues are voluminous, inflexible and poorly pliable.56 Nevertheless, despite all the aforementioned disadvantages and risks, the hip bone flap is preferred by some authors because of the volume of bone tissue usable for reconstruction, especially in the lateral sections and in the mandibular angle.57,58
Vascularized angle of the scapula
Mandibular replacement with a scapula is suitable for reconstruction of larger soft tissue defects and smaller bone defects59. The bone quality is good, but its disadvantage lies in the insufficient thickness and the length limited to 14 cm (60). Vascularization is not segmental and therefore the bone shaping with osteotomies is considered to be risky. The necessity of positioning the patient on the side during the harvest and therefore the inability to operate in two teams and the related significant extension of the surgery length is another disadvantage of this approach. The morbidity of the donor site is not very significant, but in some patients the limitation of the movements in the shoulder joint can occur61. Replacement of the lateral portion of the mandibula and the surrounding soft tissues in the submandibular and facial region is the ideal example of utilization of this flap.
Forearm flap with a part of the radius
The forearm flap is a flap with excellent vascularization of soft tissues, flexibility and elasticity.62 Nevertheless, the vascularization of the harvested bone is not very rich and the morbidity of the donor site is significant.63,64 The patients are facing the risk of a possible fracture of the thinned radius, the long-term healing of soft tissues with a risk of a rigid scar and a limited range of movements of the wrist. In addition, the bone volume and length are limited, and that is why the use of this flap is very limited (almost exclusively to the replacement of corticalis after an alveolotomy).
Maxillary and palatal reconstruction
The goal of the maxillary and palatal reconstruction is not only the separation of the maxillar cavity and nose from the oral cavity, but also the rehabilitation of mastication, pronunciation, swallowing and appearance of the patient. The question of maxillary and palatal reconstruction is not as unambiguous as it is in the case of the mandibula, and apart from surgical techniques, we also use prostheses. Similarly to the mandibular reconstruction, we should consider several circumstances before the procedure:
a) Overall state of health of the patient
b) Defect size and location
c) Whether this is a primary or a secondary reconstruction
d) The wishes of the patient.
The size and location of the defect are important criteria when deciding between reconstruction with a surgical technique and a prosthetic rehabilitation. From the aforementioned surgical techniques (Table 2), we use local flap transposition in smaller defects not involving the alveolus and dentition. In large defects with smaller demands upon dental prosthesis retention, we use myocutaneous or osteomyocutaneous flaps, whose advantage lies not only in reconstruction of soft tissues, but also bone, which is important for the eventual introduction of dental implants in fixed or hybrid replacements.65,66 On the other hand, large defect reconstruction using a myocutaneous flap with an expected replacement of the missing teeth with a removable prosthesis does not have to be the best solution. Admittedly, we will seal the defect hermetically using the myocutaneous flap, but we will rarely achieve the original gothic relief of the palate, which is important for the replacement retention67. The maxillary defect classification in accordance with Okey, who divides the defects into three groups according to their size, seems clear and well usable in practice to us68.
2. Surgical techniques used for maxillary and palatal reconstruction 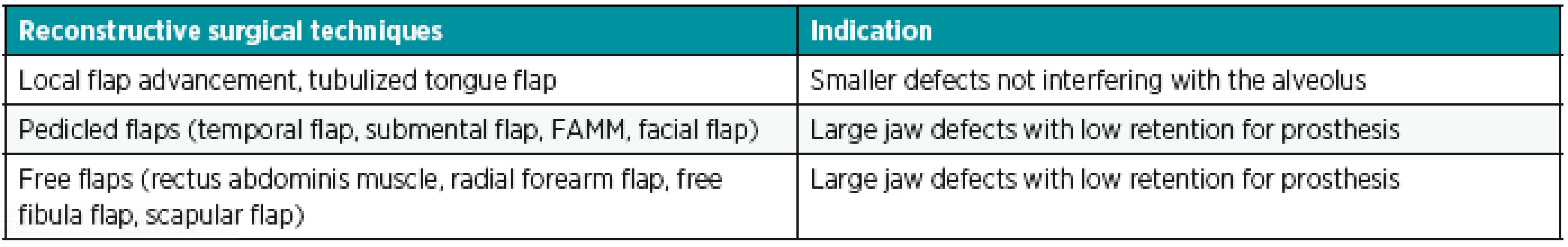
Ia. class defects
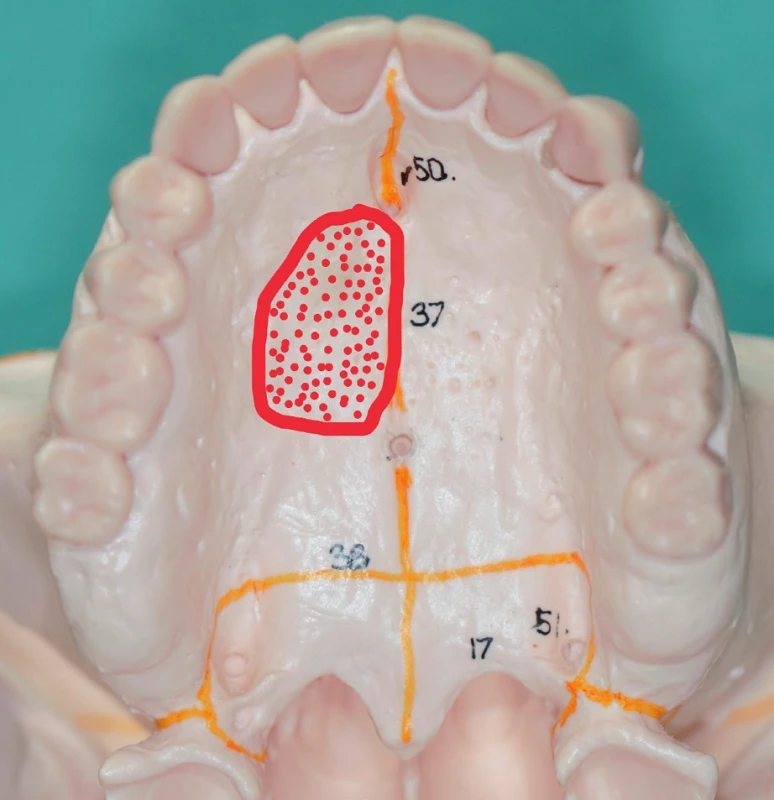
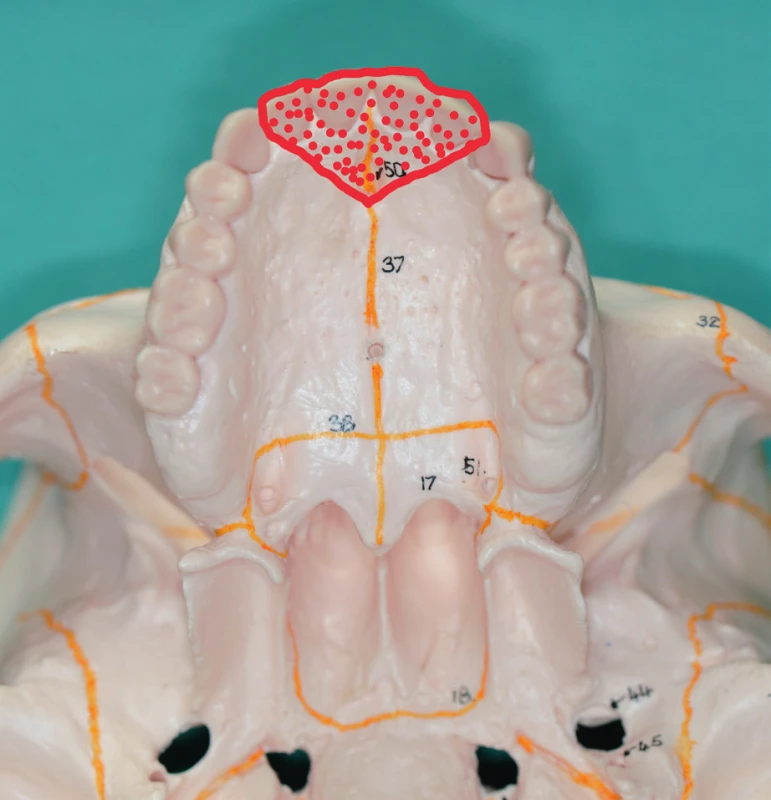
Ia. class defects are located on the hard palate (Figure 11). Because they do not interfere with the alveolus and the dentition, we mostly manage them with a prosthesis (with a palatal plate or an obturator). According to the wishes of the patient or in case of prosthesis intolerance, we use local flap transposition from the available surgical techniques.
Ib. class defects
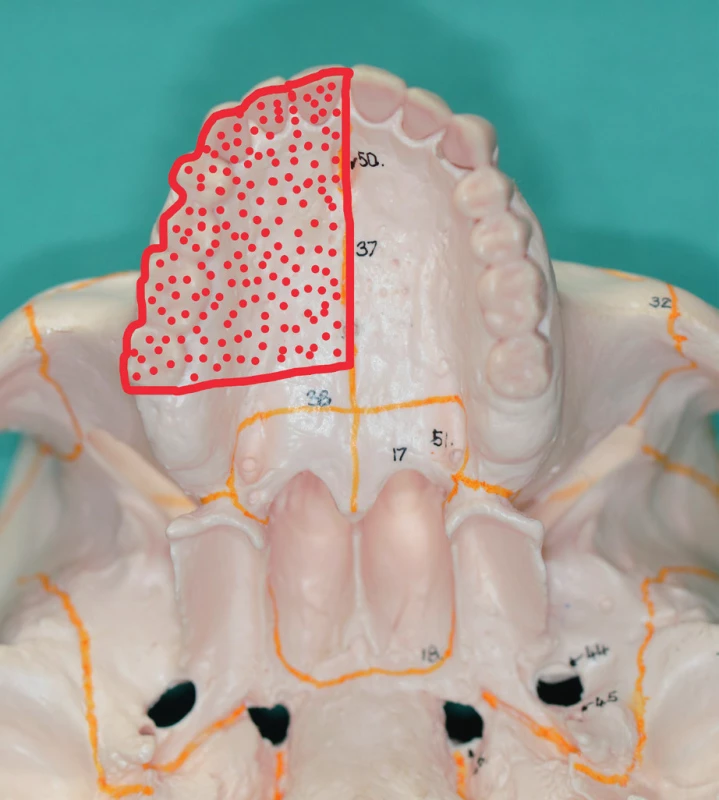
Ib. class defects interfere with the alveolar process and the dentition in the mesial direction from the canine (premaxilla) (Figure 12A), or in the distal direction from the canine (Figure 12 B). We mostly manage these defects using prosthetic replacements (an obturator with an anchoring element – continuous cast clip) and we use myocutaneous or osteomyocutaneous flaps from the available surgical techniques (Figure 13, 14, 15).
13. Example of Ib. class defect after spinocellular carcinoma extirpation – 8 years after surgery (author’s archive) 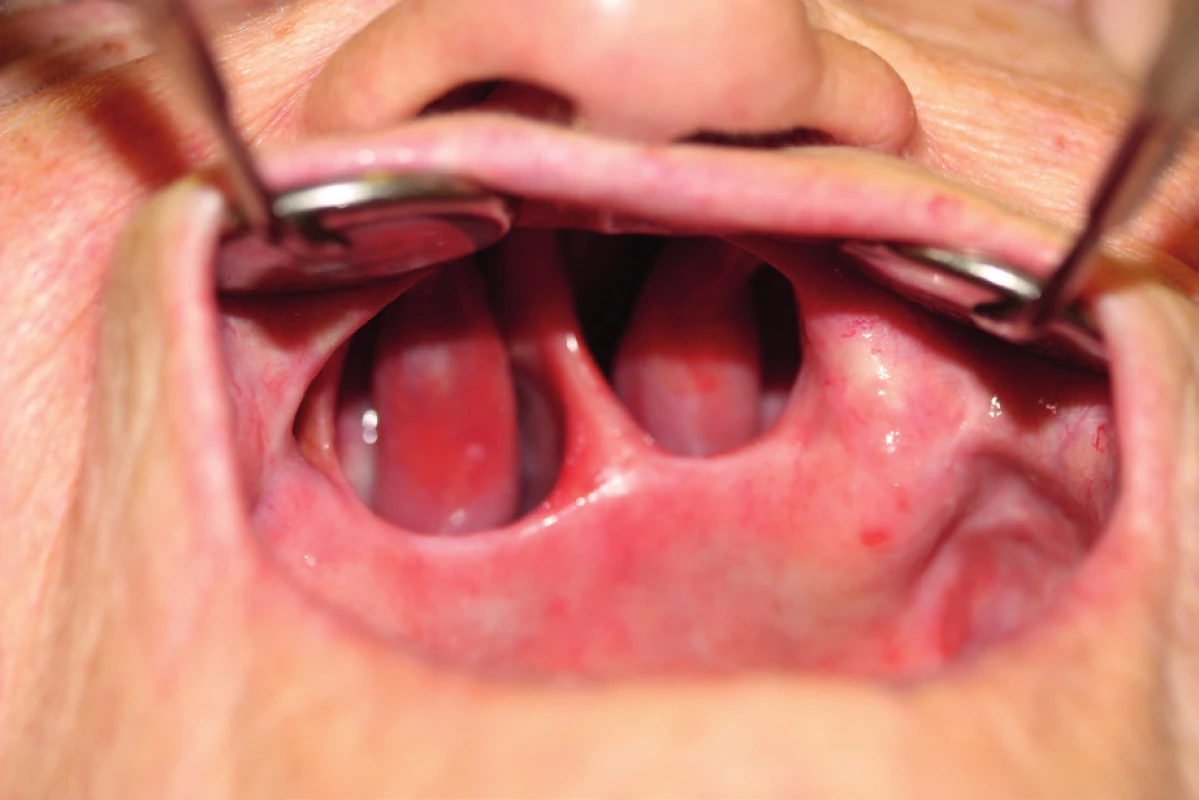
14. An obturator covering Ib. class defect of the maxilla and palate (author’s archive) 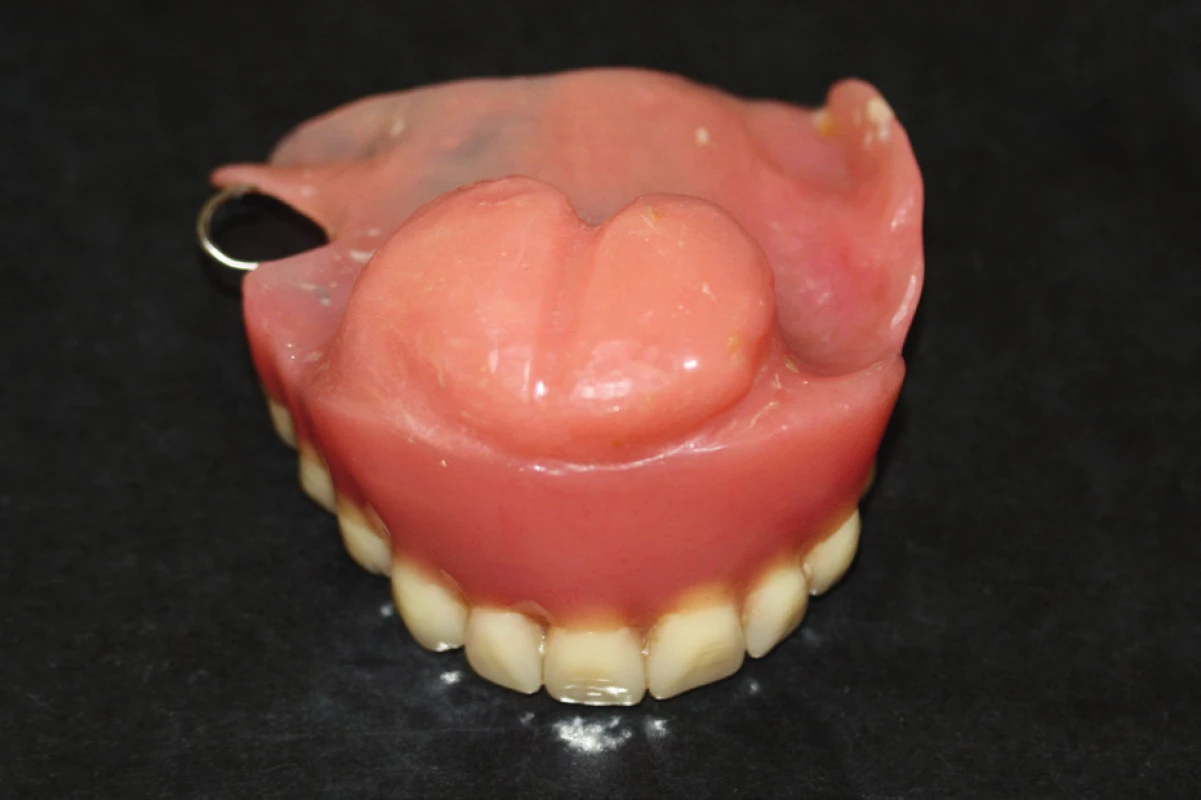
15. Prosthetic rehabilitation of a maxilla defect with an obturator in a female senior patient (author’s archive) 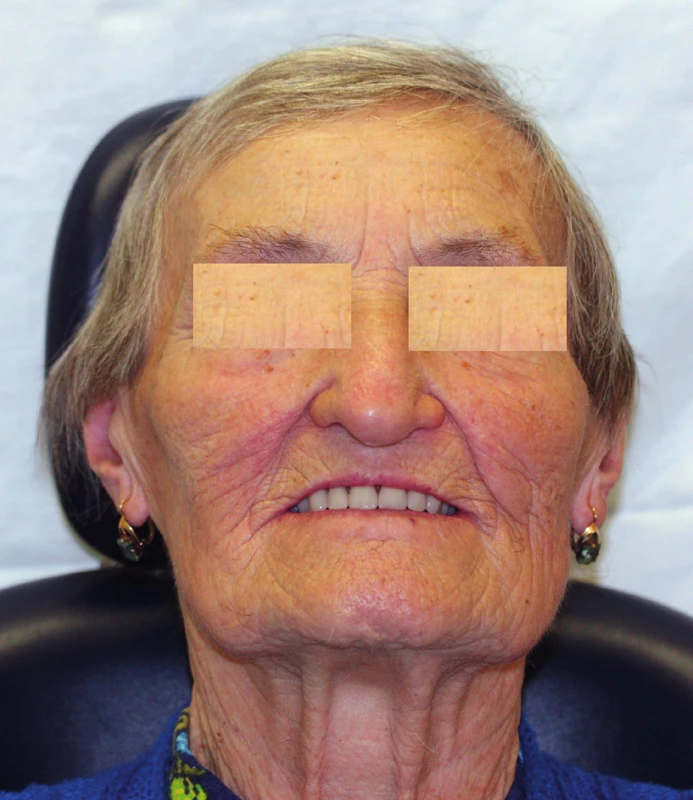
II. class defects
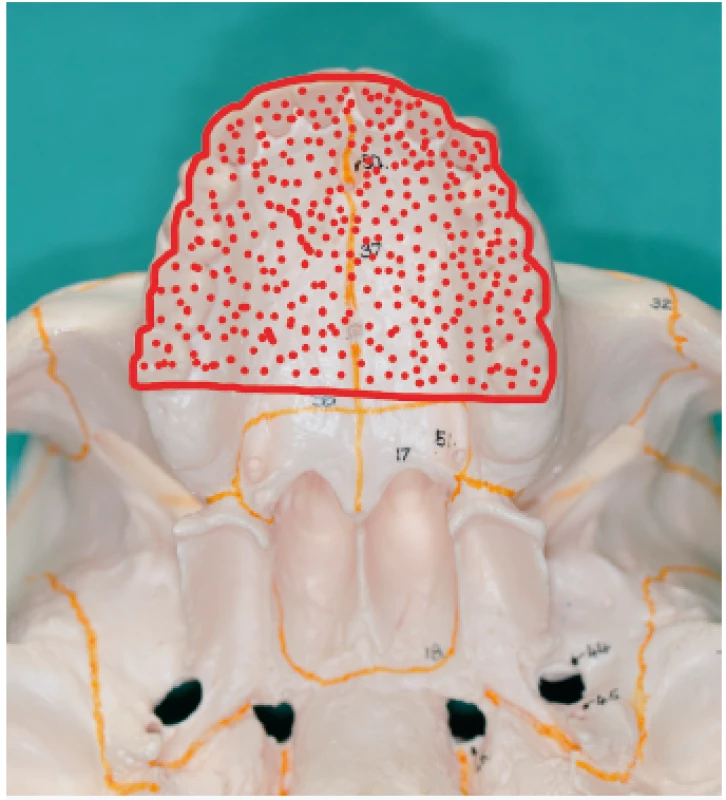
II. class defects involve less than a half of the hard palate in the sagittal (Figure 16 A) or transversal direction (Figure 16 B). In case of preservation of teeth with good biological factor in the surroundings of the defect, we can use a prosthesis (an obturator with anchoring elements).
In toothless jaws with a low alveolar process, we reconstruct defects using free or regional flaps.
III. class defects
III. class defects involve more than half of the palate, leave small number of the remaining teeth and minimum palatine bone (Figure 17 A, 17 B). The retention rate of removable obturator prostheses is low in these defects, and that is why we reconstruct them using free or regional flaps, if the overall state of health allows.
CONCLUSION
According to our experiences, pedicled flaps are mainly represented in intraoral defects of soft tissues of older, polymorbid patients. Supraclavicular flap, which is excellently pliable, is suitable for reconstruction of smaller defects of the tongue and the caudal portions of the face. In case of the palatal and oropharyngeal defects, we prefer submental flaps on upper pedicle over the supraclavicular flap because of its limited radius. Extensive intraoral defects are reconstructed using pectoralis major flaps for their large radius and volume of tissue.
Free flap techniques are indicated in mandibular defects, especially in its anterior portion. They are also suitable in cranial facial defects located out of the radius of pedicled flaps. We prefer these flaps especially in younger patients without serious internal comorbidities.
Flap plastic surgeries are a step forward, they allow larger surgical radicality and if correctly indicated, they provide better quality of life and longer survival.
Role of authors: R. Pink, Z. Dvořák – writing the text, composition of the entire paper. P. Heinz – creation of tables, figures and diagrams. P. Michl, P. Tvrdý – research in literature.
Conflict of interest: The authors have no conflicts of interest to declare.
Corresponding author:
Zdeněk Dvořák, MD, PhD
Department of Plastic and Aesthetic Surgery
St. Anne’s University Hospital
Berkova 34, 612 00 Brno
Czech Republic
E-mail: zdenek.dvorak@fnusa.cz
Sources
1. Moore SR., Johnson NW., Pierce AM., Wilson DF. The epidemiology of mouth cancer: a review of global incidence. Oral Dis. 2000 March, 6 : 65–74.
2. Dušek L. et al. Cancer incidence and mortality in the Czech Republic. Klin Onkol. 2014, 27 : 406–23.
3. Pazdera J. Základy orofaciální onkologie. In: Pazdera J. Základy ústní čelistní chirurgie. 3rd ed. Olomouc: Univerzita Palackého v Olomouci (UPOL); 2013. p 225–82.
4. Woolgar JA., Rogers S., West CR., Errington RD., Brown JS., Vaughan ED. Survival and patterns of recurrence in 200 oral cancer patients treated by radical surgery and neck dissection. Oral Oncol. 1999, 35, 1999 : 257–65.
5. Pink P., Molitor M., Tvrdy P., Michl P., Pazdera J., Zboril V., Zalesak B. Reconstructive procedures in maxillofacial oncusurgery. Biomed Pap. 2016, 160 : 153–7.
6. Houseman ND., Taylor GI., Pan WR. The angiosomes of the head and neck: anatomic study and clinical applications. Plast Reconstr Surg. 2000, 105 : 2287–313.
7. Chicarilli ZN. Sliding posterior tongue flap. Plast Reconstr Surg. 1987, 79 : 697–700.
8. Chambers RG., Jaques DA., Mahoney WD. Tongue flaps for intraoral reconstruction. Am J Surg. 1969, 118 : 783–6.
9. Kim YK., Yeo HH., Kim SG. Use of the tongue flap for intraoral reconstruction: a report of 16 cases. J Oral Maxillofac Surg. 1998, 56 : 716–9.
10. Lore JM Jr., Klotch DW., Lee KY. One-stage reconstruction of the hypopharynx using myomucosal tongue flap and dermal graft. Am J Surg. 1982, 144 : 473–6.
11. Genden EM., Lee BB., Urken ML. The palatal island flap for reconstruction of palatal and retromolar trigone defects revisited. Arch Otolaryngol Head Neck Surg. 2001, 127 : 837–41.
12. Ducic Y., Herford AS. The use of palatal island flaps as an adjunct to microvascular free tissue transfer for reconstruction of complex oromandibular defects. Laryngoscope. 2001, 111 : 1666–9.
13. Cohen IK., Edgerton MT. Transbuccal flaps for reconstruction of the floor of the mouth. Plast Reconstr Surg. 1971, 48 : 8–10.
14. Cohen IK., Theogaraj SD. Nasolabial flap reconstruction of the floor of the mouth after extirpation of oral cancer. Am J Surg. 1975, 130 : 479–80.
15. Elliott RA Jr. Use of nasolabial skin flap to cover intraoral defects. Plast Reconstr Surg. 1976, 58 : 201–5.
16. Rose EH. One-stage arterialized nasolabial island flap for floor of mouth reconstruction. Ann Plast Surg. 1981, 6 : 71–5.
17. Lindbergh AA. Mathematical principles of local plastic procedures on the surface of the human body Clin Plast Surg. 1976, 3 : 481–94.
18. Esser JFS. Die Rotation der Wang. Leipzig; Vogel Verlag, 1918.
19. Pink R., Dvořák Z., Heinz P., Michl P., Tvrdý P., Azar B. Pedicled flaps for reconstruction of head and neck region [Stopkované laloky jako jedna z možností rekonstrukce hlavy a krku] Klinicka Onkologie. 2018, 31 : 59–65.
20. Dvořák Z., Pink R., Michl P., Heinz P., Tvrdý P. Pedicled pectoralis major flap in head and neck reconstruction – technique and overview. Acta chirurgiae plasticae. 2018, 60 : 14–21.
21. Dvořák Z., Pink R., Michl P., Heinz P., Tvrdý P. Pedicled pectoralis major flap in head and neck reconstruction – our experience. Acta chirurgiae plasticae. 2018, 60 : 30–4.
22. Martin D., Baudet J., Mondie JM., Peri G. The submental island skin flap. A surgical protocol. Prospects of use. Ann Chir Plast Esthet. 1990, 35 : 480–4.
23. Martin D., Pascal JF., Baudet J., Mondie JM., Farhat JB., Athoum A, et al. The submental island flap: a new donor site. Anatomy and clinical applications as a free or pedicled flap. Plast Reconstr Surg. 1993, 92 : 867–73.
24. Lamberty BG. The supra-clavicular axial patterned flap. Br J Plast Surg. 1979, 32 : 207–12.
25. Pallua N., Machens HG., Rennekampff O., Becker M., Berger A. The fasciocutaneous supraclavicular artery island flap for releasing postburn mentosternal contractures. Plast Reconstr Surg. 1997, 99 : 1878–84–6.
26. Chen W-L., Zhang D., Yang Z., Huang Z., Wang J., Zhang B, et al. Extended supraclavicular fasciocutaneous island flap based on the transverse cervical artery for head and neck reconstruction after cancer ablation. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2010, 68 : 2422–30.
27. Seidenberg B., Rosenak SS., Hurwitt ES., Som ML. Immediate reconsruction of the cervical esophagus by a revascularized isolated jejunal segment. Ann Surg. 1959, 149 : 162–171.
28. Granzow JW., Suliman A., Roostaeian J., Perry A., Boyd JB. The supraclavicular artery island flap (SCAIF) for head and neck reconstruction: surgical technique and refinements. Otolaryngol Head Neck Surg. 2013, 148 : 933–40.
29. Wilson JS., Yiacoumettis AM., O’Neill T. Some observations on 112 pectoralis major myocutaneous flaps. Am J Surg. 1984, 147 : 273–9.
30. Milenović A., Virag M., Uglesić V., Aljinović-Ratković N. The pectoralis major flap in head and neck reconstruction: first 500 patients. J. Craniomaxillofac. Surg. 2006 Sep;34(6):340–4.
31. Liu R., Gullane P., Brown D., Irish J. Pectoralis major myocutaneous pedicled flap in head and neck reconstruction: retrospective review of indications and results in 244 consecutive cases at the Toronto General Hospital. J Otolaryngol. 2001, 30 : 34–40.
32. Pribaz JJ. et al: A new intraoral flap: facial artery musculomucosal (FAMM) flap. Plast Reconstr Surg. 1992, 90 : 421–9.
33. Dupoirieux L., Plane L., Gard C., Penneau M. Anatomical basis and results of the facial artery musculomucosal flap for oral reconstruction. Br J Oral Maxillofac Surg. 1999, 37 : 25–8.
34. Kim RJ., Izzard ME., Patel RS. Supraclavicular artery island flap for reconstructing defects in the head and neck region. Curr Opin Otolaryngol Head Neck Surg. 2011, 19 : 248–50.
35. Seidenberg B., Rosenak SS., Hurwitt ES., Som ML. Immediate reconsruction of the cervical esophagus by a revascularized isolated jejunal segment. Ann Surg. 1959, 149 : 162–71.
36. Ueba Y., Fujikawa S. Nine years follow-up of a free vascularized fibula graft in neurofibromatosis - A case report and literature review. Jap J Orthop Trauma Surg. 1983, 26 : 595–600.
37. Taylor IG., Miller GDH., Ham FJ. The Free Vascularized Bone Graft. Plastic and Reconstructive Surgery. 1975, 55 : 533–44.
38. Hidalgo DA. Fibula Free Flap: A New Method of Mandible Reconstruction. Plastic and Reconstructive Surgery. 1989, 84 : 71–9.
39. Nakayama B., Matsuura H., Hasegawa Y., Ishihara O., Hasegawa H., Torii S. New reconstruction for total maxillectomy defect with a fibula osteocutaneous free flap. Br J Plast Surg. 1994, 47 : 247–9.
40. Shaw W. Microvascular reconstruction of the nose. Clin Plast Surg. 1997, 50 : 162–5.
41. Yang G. et al: Forearm free skin flap transplantation. Nat Med J China. 1981, 61 : 139–41.
42. Shaw RJ. et al. The anterolateral thigh flap in head and neck reconstruction: “pearls and pitfalls”. British Journal of Oral and Maxillofacial Surgery. 2010, 48 : 5–10.
43. Quillen CG., Shearin JC Jr., Georgiade NG. Use of the latissimus dorsi myocutaneous island flap for reconstruction in the head and neck area. Plast Reconstr Surg. 1978, 62 : 113–7.
44. Kroll SS. et al. Comparison of the rectus abdominis free flap with the pectoralis major myocutaneous flap for reconstructions in the head and neck. Am J Surg. 1992, 164 : 615–8.
45. Taylor GI., Townsend P., Corlett R. Superiority of the deep circumflex iliac vessels as the supple for free groin flaps. Clinical work. Plast Reconstr Surg. 1979, 64 : 745–59.
46. Thoma A. et al: The free medial scapular osteofasciocutaneous flap for head and neck reconstruction. Br J Plast Surg. 1991, 44 : 477–82.
47. Liu R., Gullane P., Brown D., Irish J. Pectoralis major myocutaneous pedicled flap in head and neck reconstruction: retrospective review of indications and results in 244 consecutive cases at the Toronto General Hospital. J Otolaryngol. 2001, 30 : 34–40.
48. Montoro JR., Tavares MG., Melo DH., Franco Rde L., Mello-Filho FV., Xavier SP., Trivellato AE., Lucas AS. Mandibular ameloblastoma treated by bone resection and imediate reconstruction. Brazilian Journal of Otorhinolaryngology. 2008, 74 : 155–7.
49. Schöning H., Emshoff R. Primary temporary reconstruction of mandibular defects using AO reconstruction plates: a retrospective analysis of 51 cases. 36, 1998, p. 222–223.
50. Deutsch M., Kroll SS., Ainsle N., Wang B. Influence of radiation on late complications in patients with free fibular flaps for mandibular reconstruction. Ann Plast Surg. 1999, 42 : 662–4.
51. Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989, 84 : 71–9.
52. Hidalgo DA., Rekow A. A review of 60 consecutive fibula free flap mandible reconstructions. Plast Reconstr Surg. 95, 1995, 585–96.
53. Hidalgo DA. Aesthetic improvements in free-flap mandible reconstruction. Plast Reconstr Surg. 1991, 88 : 574–85.
54. Schusterman MA., Reece GP., Miller MJ. The osteocutaneous fibula free flap: Is the skin paddle reliable? Plast Reconstr Surg. 1992, 90 : 787–93.
55. Leon BR., Carrillo FJ., Gonzalez HM., Franco JL. Mandibular reconstruction with the free vascularized fibular flap: utility of three-dimensional computerized tomography. J Reconstr Microsurg. 1999, 15 : 83–90.
56. Shpitzer T., Neligan PC., Gullane PJ, et al: The free iliac crest and fibula flaps in vascularized oromandibular reconstruction: comparison and long-term evaluation. Head Neck. 1999, 21 : 639–47.
57. Franklin JD. et al: Single-stage reconstruction of mandibular and soft tissue defects using a free osteocutaneous groin flap. Am J Surg. 1980, 140 : 492–8.
58. Riediger D. Restoration of masticatory function by microsurgically revascularized iliac crest bone grafts using enosseous implants. Plast Reconstr Surg. 1988, 81 : 861–77.
59. Rosenthal E., Couch M., Farwell DG., Wax MK. Current concepts in microvascular reconstruction. Otolaryngol Head Neck Surg. 2007, 136 : 519–24.
60. Swartz WM et al: The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast Reconstr Surg. 1986, 77 : 530–45.
61. Silverberg B., Banis JC Jr., Acland RD. Mandibular reconstruction with microvascular bone transfer. Series of 10 patients. Am J Surg. 1985, 150 : 440–6.
62. Soutar DS., McGregor IA: The radial forearm flap in intraoral reconstruction: the experience of 60 consecutive cases. Plast Reconstr Surg. 78, 1986, p. 1–8.
63. Boyd JB., Gullane PJ., Rotstein LE., Brown DH., Irish JC. Classification of mandibular defects. Plast Reconstr Surg. 1993, 92 : 1266–75.
64. Soutar DS., Widdowson WP. Immediate reconstruction of the mandible using a vascularized segment of radius. Head Neck Surg. 1986, 8 : 232-46.
65. Valentini V., Gennaro P., Torroni A., Longo G., Aboh IV., Cassoni A., Battisti A., Anelli A. Scapula free flap for complex maxillofacial reconstruction. J Craniofac Surg. 2009, 20 : 1125–31.
66. Granick MS., Ramasastry SS., Newton ED. et al. Reconstruction of complex maxillectomy defects with the scapular free flap. Head Neck Surg. 1990, 12 : 377–85.
67. Bernhart BJ., Huryn JM., Disa J. et al. Hard palate resection, microvascular reconstruction, and prosthetic restoration: a 14-year retrospective analysis. Head Neck. 2003, 25 : 671–80.
68. Okay D., Genden E., Buchbinder D., Urken M. Prosthodontic guidelines for surgical reconstruction of the maxilla. J Prosthet Dent. 2001, Oct;86(4):352–63.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2020 Issue 1-2-
All articles in this issue
- AN OVERVIEW AND OUR APPROACH IN THE TREATMENT OF MALIGNANT CUTANEOUS TUMOURS OF THE HAND
- ENHANCED RECOVERY PROTOCOL FOLLOWING AUTOLOGOUS FREE TISSUE BREAST RECONSTRUCTION
- SKIN SUBSTITUTES IN RECONSTRUCTION SURGERY: THE PRESENT AND FUTURE PERSPECTIVES
- GUNSHOT INJURIES OF THE OROFACIAL REGION
- INDICATION AND IMPORTANCE OF RECONSTRUCTIVE SURGERIES OF FACIAL SKELETON IN MAXILLOFACIAL SURGERY: REVIEW
- Editorial
- CURRENT OPTIONS IN PHARMACOLOGICAL INTERVENTIONS FOR MICROVASCULAR ANASTOMOSIS PATENCY: REVIEW
- ACCIDENTAL FINDING OF SYNCHRONOUS BILATERAL DUCTAL CARCINOMA IN SITU IN A YOUNG MAN REFERRED TO MASTECTOMY DUE TO GYNECOMASTIA – AND WHAT IF LIPOSUCTION HAVE BEEN USED? CASE REPORT
- RED BREAST SYNDROME (RBS) ASSOCIATED TO THE USE OF POLYGLYCOLIC MESH IN BREAST RECONSTRUCTION: A CASE REPORT
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- RED BREAST SYNDROME (RBS) ASSOCIATED TO THE USE OF POLYGLYCOLIC MESH IN BREAST RECONSTRUCTION: A CASE REPORT
- AN OVERVIEW AND OUR APPROACH IN THE TREATMENT OF MALIGNANT CUTANEOUS TUMOURS OF THE HAND
- GUNSHOT INJURIES OF THE OROFACIAL REGION
- SKIN SUBSTITUTES IN RECONSTRUCTION SURGERY: THE PRESENT AND FUTURE PERSPECTIVES
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career